Abstract
Crocin, a representative of carotenoid compounds, exerts a spectrum of activities including radical scavenger, anti-microbial and anti-inflammatory properties. To investigate the protective effect of crocin on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. ALI was induced in mice by intratracheal instillation of LPS (1 mg/kg). The mice received intragastric injection of crocin (50 mg/kg) 1 h before LPS administration. Pulmonary histological changes were evaluated by hematoxylineosin stain and lung wet/dry weight ratios were observed. Concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and nitric oxide (NO), and myeloperoxidase (MPO) activity were measured by enzymelinked immunosorbent assay. Expression of inducible nitric oxide synthase (iNOS) in lung tissues was determined by Western blot analysis. Crocin pretreatment significantly alleviated the severity of lung injury and inhibited the production of TNF-α and IL-1β in mice with ALI. After LPS administration, the lung wet/dry weight ratios, as an index of lung edema, and MPO activity were also markedly reduced by crocin pretreatment. Crocin pretreatment also reduced the concentrations of NO in lung tissues. Furthermore, the expression of iNOS was significantly suppressed by crocin pretreatment. Croncin potently protected against LPS-induced ALI and the protective effects of crocin may attribute partly to the suppression of iNOS expression.
Keywords: Crocin, LPS, Acute lung injury, iNOS
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are well defined and readily recognized clinical disorders caused by many clinical insults to the lung or because of predispositions to lung injury [1]. Although the causes of ALI and ARDS are numerous, endotoxin is thought to be the most pathogen that leads to the development of ALI and ARDS. Endotoxin or lipopolysacchride (LPS), derived from the cell wall of gram-negative bacteria, induces a sepsis syndrome accompanied by key features of ALI, including the recruitment of inflammatory cells into the lung with subsequent increases in capillary permeability and alveolar edema [2]. Despite significant advances in intense care research and diverse therapeutic trials made in the past few decades, ALI/ARDS remains a severe disease and still presents the high mortality rate of approximately 40% [3,4].
Inducible nitric oxide synthase (iNOS), an inducible enzyme, is transcriptionally regulated by pro-inflammatory products and cytokines, and results in sustained and elevated release of nitric oxide (NO). This iNOS-derived NO contributes to the pathophysiologic features of ALI [5,6]. It has been shown that mice deficient in iNOS gene were more resistant to lung injury than were wild-type mice [7,8]. Therefore, suppression of the induction and activity of iNOS has been considered a new paradigm in inflammation therapy.
Crocins, a major component of saffron, are a group of hydrophilic carotenoides that are either mono- or di-glycosyl polyene esters of crocetin in which D-glucose and/or D-gentiobiose occur as carbohydrate residues. It has been showed that crocin has a wide range of activities, including antidepressant, anticarcinogenic, antiatherosclerotic, antioxidant, and antihyperlipidemic [9-12]. Recent studies have demonstrated the anti-inflammatory effect of crocin [13,14]. In neuroinflammatoty diseases, crocin protected oligodendrocytes exposed to cytotoxic supernatants derived from Syncytin-1-expressing astrocytes and NO-mediated oligodendrocytotoxicity [15]. But to our knowledge, the anti-inflammatory effect of crocin in ALI has not been well established. Therefore, the aim of this study is to investigate the protective effects of crocin on LPS-induced ALI.
Materials and methods
Animals and reagents
Male BALB/C mice weighing 20-25 g were purchased from the Animal Center of the Fourth Military Medical University (Xian, China). All animals were allowed to take food and tap water ad libitum. All procedures were in accordance with the Declaration of Helsinki of the World Medical Association. The protocols were also approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical University Tangdu Hospital. Crocin was purchased from Sigma Chemical Co. (Missouri, USA). LPS (Escherichia coli lipopolysaccharide, 055:B5) was obtained from Sigma Chemical Company (St. Louis, MO., USA). Enzyme-linked immunosorbent assay (ELISA) kits of TNF-α, IL-1β, myeloperoxidase (MPO) and nitric oxide (NO) were purchased from R&D Corporation (R&D Systems Inc. Minneapolis, MN, USA). Antibodies specific for iNOS and β-actin were obtained from the Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Cell lysis buffer was the product of Beyotime Biotechnology (Beijing, China). BCA protein assay kit was obtained from Thermo Scientific Pierce Protein Research Products (Rockford, IL., USA).
Animal model of ALI
Before the induction of ALI, mice were fasted overnight but allowed water ad libitum. Animals were anesthetized with intraperitoneal pentobarbital (50 mg/kg). LPS (1 mg/kg) was instilled intratracheally to induce ALI.
Experimental protocol
All animals were randomly divided into 4 groups (n=10, each group). Group 1 (control group) treated with intragastric injection of normal saline and 1 h later received an intratracheal injection of normal saline, group 2 (crocin group) treated with intragastric injection of crocin (50 mg/kg) and 1 h later received an intratracheal injection of normal saline, group 3 (LPS group) treated with intragastric injection of normal saline and 1 h later received an intratracheal injection of LPS (1 mg/kg) and group 4 (LPS+crocin group) treated with intragastric injection of crocin (50 mg/kg) and 1 h later received an intratracheal injection of LPS (1 mg/kg). Twelve hours after LPS administration, all animals were sacrificed. Bronchoalveolar lavage (BAL) was performed through the left lung. After the lung vasculature was flushed, the superior lobe of right lung was excised for histopathologic examination. The middle lobe of right lung was excised for analysis of lung wet/dry weight ratio. The lower lobe of right lung was rapidly removed and cut into two parts in same size. A part of the lower lobe was homogenized and frozen in a cold phosphate solution at -80°C for MPO analysis, and the another part was used to extract proteins for Western blot analysis.
BAL
Animals were anesthetized with intraperitoneal pentobarbital (50 mg/kg). A median sternotomy allowed for exposure of both of the lungs. The trachea was exposed and inserted with an intravenous infusion needle. After ligating the hilum of right lung, the left lung was lavaged 5 times with 0.5 ml ice-cold phosphate buffered saline. The recovery ratio of the fluid was about 90%. The BAL fluid (BALF) was immediately centrifuged at 500 × g for 10 min at 4°C, and the cell-free supernatant was stored at -80°C for analysis of cytokines.
Cytokines measurement
Concentrations of IL-1β and TNF-α in BALF were measured by using ELISA kits. All procedures were done in accordance with the manufacturer’s instructions.
MPO and NO assays
To carry out the assays, tissue samples were subjected to three further freeze-thaw cycles and centrifuged at 12 000 × g for 10 min at 4°C. The supernatant was assayed for MPO activity and NO concentrations with ELISA kits. All procedures were done in accordance with the manufacturer’s instructions.
Lung wet/dry weight ratio
As an index of lung edema, the amount of extravascular lung water was calculated. The middle lobe of right lung was excised and the wet weight was recorded. The lobe was then placed in an incubator at 80°C for 24 hours to obtain the dry weight. And the wet/dry weight ratios were calculated by dividing the wet weight by the dry weight.
Pulmonary histopathology
The superior lobe of right lung was harvested at 12 h after LPS administration and fixed with an intratracheal instillation of 1 ml buffered formalin (10%, PH 7.2). The lobe was further fixed in 10% neutral buffered formalin for 24 h at 4°C. The tissues were embedded in paraffin and cut into 5 μm sections. Hematoxylin-eosin stains were performed using standard protocol.
Western blot analysis
The lower lobe of right lung in each mouse was harvested at 12 h after LPS administration and frozen in liquid nitrogen immediately until homogenization. Tissue samples were homogenized in cell lysis buffer. After centrifugation (12 000 × g, 10 min, 4°C), supernatants were aspirated. Protein concentrations were determined by BCA protein assay kit. Samples were separated on a denaturing 12% polyacrylamide gel and transferred to a nitrocellulose membrane. iNOS proteins were detected by chemiluminescence using a rabbit polyclonal antibody according to the manufacturer’s instructions.
Statistical analyses
Data were entered into a database and analyzed using SPSS software, and expressed as means ± S.D. On a preliminary analysis, the Kolmogorov-Smirnov test found that the raw pooled data followed a Gaussian distribution. Thus, statistically significant differences between groups were determined by ANOVA followed by Tukey’s test. Significance was accepted when P < 0.05.
Results
Effect of crocin on the pulmonary histopathological changes of mice with ALI
Lung tissues from the control and crocin groups showed a normal structure and no histopathological changes under a light microscope (Figure 1A and 1B). In LPS group, the lungs stained with hematoxylin-eosin indicated widespread alveolar wall thickness caused by edema, severe hemorrhage in the alveolus, alveolus collapse and obvious inflammatory cells infiltration (Figure 1C). In LPS+crocin group, the histopathological changes of lung were minor compared with those in LPS group, especially in inflammatory cells infiltration (Figure 1D).
Figure 1.
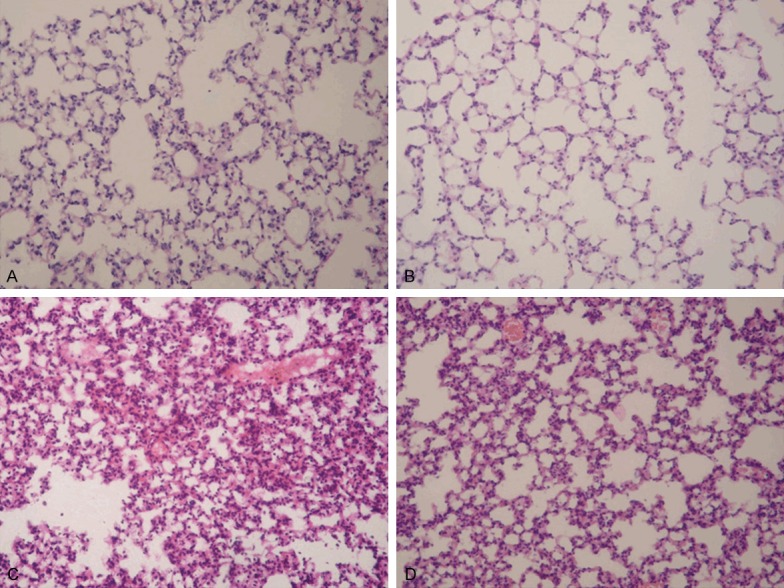
Effect of crocin on the pulmonary histopathological changes of mice with ALI. Lung sections stained with hematoxylin-eosin from 12 h after LPS administration revealed pulmonary histopathological changes (original magnification ×200). A. Control group: normal structure. B. Crocin group: same as control group. C. LPS group: alveolar wall thickness, hemorrhage, alveolus collapse and obvious inflammatory cells infiltration. D. LPS+crocin group: minor histopathological changes compared with LPS group.
Effect of Crocin on MPO activity and NO concentrations in lung tissues of mice with ALI
After LPS administration, the MPO activity in lung tissues was significantly increased compared with the control and crocin groups (Figure 2A). In addition, the concentrations of NO were also significantly increased after LPS administration (Figure 2B). However, crocin pretreatment markedly decreased the MPO activity and NO concentrations (Figure 2A and 2B).
Figure 2.
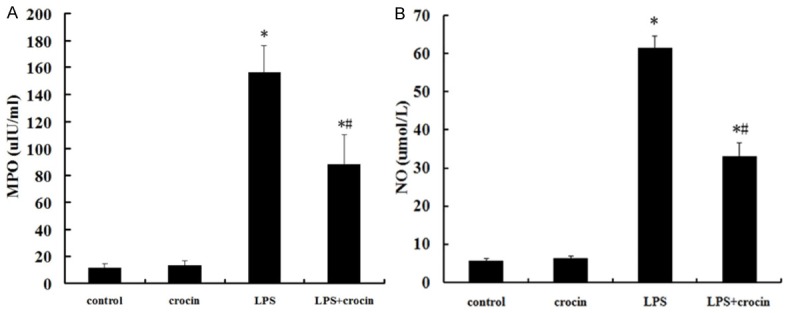
Effect of crocin on MPO activity and NO concentrations in lung tissues of mice with ALI. A. The MPO activity in lung tissues after LPS administration and the effect of crocin pretreatment. B. The concentrations of NO in lung tissues after LPS administration and the effect of crocin pretreatment. Data are expressed as mean±S.D., *P<0.05 vs. control and crocin groups; #P<0.05 vs. LPS group.
Effect of crocin on the concentrations of IL-1β and TNF-α in BALF of mice with ALI
The concentrations of IL-1β and TNF-α in BALF were significantly increased at 12 h after LPS administration (Figure 3A and 3B). Crocin pretreatment efficiently reduced the production of IL-1β and TNF-α (Figure 3A and 3B).
Figure 3.
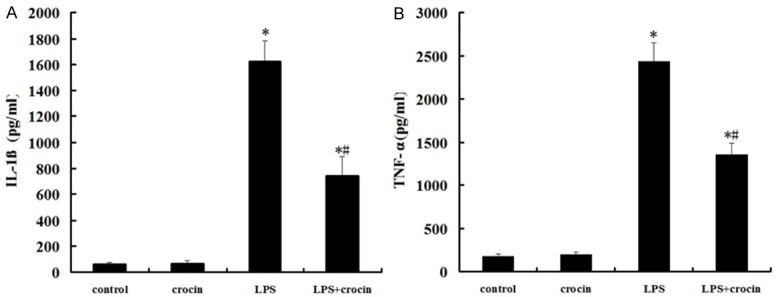
Effect of crocin on the concentrations of IL-1β and TNF-α in BALF of mice with ALI. A. The concentrations of IL-1β in BALF after LPS administration and the effect of crocin pretreatment. B. The concentrations of TNF-α in BALF after LPS administration and the effect of crocin pretreatment. Data are expressed as mean±S.D., *P<0.05 vs. control and crocin groups; #P<0.05 vs. LPS group.
Effect of crocin on the lung edema of mice with ALI
Compared with the control and crocin groups, the lung wet/dry weight ratios were significantly increased after LPS administration. The increase of the lung wet/dry weight ratios was significantly reduced by crocin administration (Figure 4).
Figure 4.
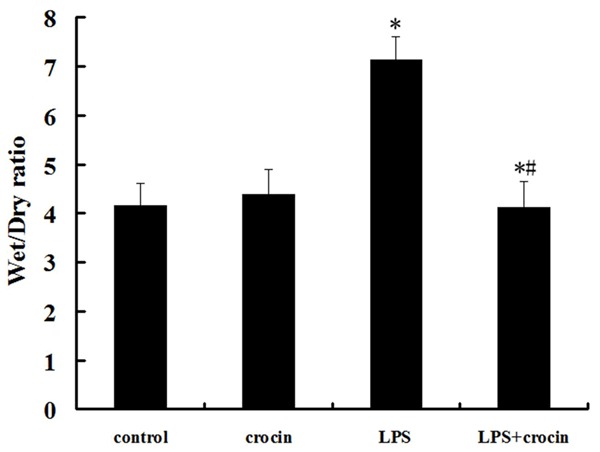
Effect of crocin on the lung edema of mice with ALI. After LPS administration, pretreatment of crocin decreased lung wet/dry ratios markedly. Data are expressed as mean±S.D, *P<0.05 vs. control and crocin groups; #P<0.05 vs. LPS group.
Effect of crocin on the expression of iNOS in lung tissues of mice with ALI
After LPS administration, the expression of iNOS in lung tissues markedly increased (Figure 5). However, the pretreatment of crocin significantly suppressed LPS-induced activation of iNOS (Figure 5). There were no significant changes in the expression of iNOS in control and crocin groups (Figure 5).
Figure 5.
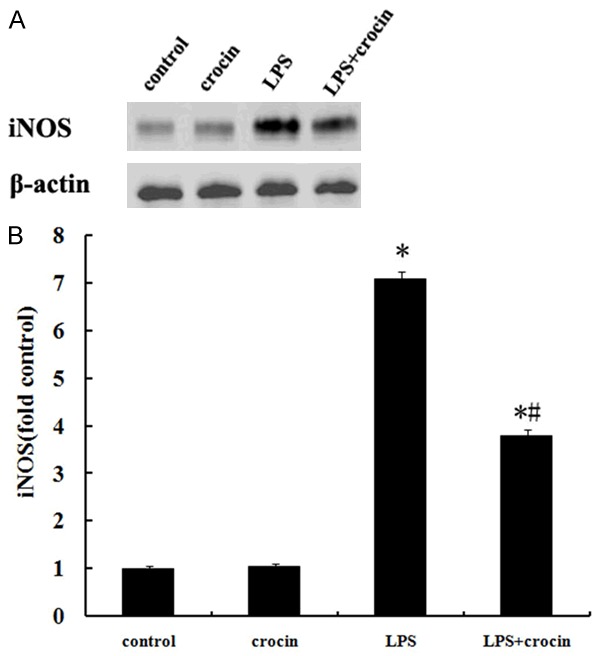
Effect of crocin on the expression of iNOS in lung tissues of mice with ALI. A. A representative Western blot showed the expression of iNOS in lung tissues in different groups. B. iNOS optical densitometry from different groups. Pretreatment of crocin significantly repressed the expression of iNOS after LPS administration. Data are expressed as mean±SD, *P<0.05 vs. control and crocin groups; #P<0.05 vs. LPS group.
Discussion
LPS are major components of the outer membrane of gram-negative bacteria, and in vivo could trigger potent inflammatory responses. In gram-negative bacteria sepsis and pneumonia, LPS is capable of inducing ALI. The animal models of ALI induced by LPS administration are easy to establish and very reproducible. The intratracheal injection of LPS induces significant increase of proinflammatory cytokines in BALF. In the present study, the concentrations of TNF-α and IL-1β in BALF increased markedly in mice treated by LPS. Elevated levels of TNF-α and IL-1β play a key role in the progression of ALI. Because TNF-α and IL-1β can stimulate production of a host of other cytokines, they have earned a position of prominence at the head of the inflammatory cytokine cascade [15]. Therefore, the inhibition of TNF-α and IL-1β showed the reduction of pulmonary injury in ALI induced by LPS in mice [16,17]. In the present study, crocin pretreatment significantly reduced the concentrations of TNF-α and IL-1β in BALF in mice received intratracheal injection of LPS.
Neutrophils are an important component of the inflammatory response that characterizes ALI and are considered to be the final effector cell responsible for lung injury, due to their ability to express multiple cytotoxic products [18,19]. In endotoxemia-induced ALI, the neutrophils accumulated in the lungs, express proinflammatory cytokines, such as IL-1β and TNF-α, and finally lead to the pulmonary injury [20]. MPO is a major constituent of neutrophil cytoplasmic granules. The total activity of MPO in a tissue is therefore a direct measure of neutrophil sequestration in that tissue [21]. In the present study, we found the MPO activity increased evidently in lung tissues after LPS exposure. As expected, crocin pretreatment significantly decreased the MPO activity in lung tissues. In addition, histopathological study also indicated that crocin pretreatment markedly attenuated the neutrophil infiltration in lungs.
NO is a pleiotropic mediator, which acts in a variety of physiological and pathophysiological processes. Increased levels of NO, measured as nitrite, were present in BALF from LPS-treated rat lungs and LPS-elicited BAL leukocytes produced increased NO in culture [22]. In humans either at risk of ARDS or with confirmed ARDS, the level of NO in their BALF increased significantly [23]. Moreover, the increased NO production is associated with evidence for increased iNOS expression in lung tissue [24,25]. iNOS is calcium-independent and can be induced by pro-inflammatory agents, such as LPS, IL-1β, TNF-α and interferon-γ (INF-γ), in endothelial and smooth-muscle cells, in macrophages and in other cell types [26]. In the present study, the production of NO and the expression of iNOS in lung tissues increased significantly after LPS administration, which were markedly inhibited by crocin pretreatment. It has been demonstrated that iNOS inhibitor prevents the lung injury associated with inflammation [27,28]. Therefore, our results suggest that the attenuation of inflammatory responses in ALI by crocin were partially due to the suppression of iNOS expression by crocin.
Pulmonary edema is a life-threatening condition that frequently leads to acute respiratory failure. Injury to the alveolar epithelium can disrupt the integrity of the alveolar barrier or downregulate ion transport pathways, thus, reducing net alveolar fluid reabsorption and enhancing the extent of alveolar edema [29]. Here, we observed a significant reduction of pulmonary injury and edema in lungs of ALI mice pretreated by crocin. Therefore, crocin possessed the protective effect on ALI, which implied the clinical use of crocin in future.
In conclusion, our study shows that crocin can attenuate the pulmonary histological changes, lung edema, reduce the neutrophils infiltration in lung, and inhibit the release of inflammatory cytokines in BALF. These protective effects of crocin may involve the suppression of iNOS expression. Although crocin exerts its anti-inflammatory effect in our study, further and comprehensive studies are still needed before clinical application.
Disclosure of conflict of interest
None.
References
- 1.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1565. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 2.Worthen GS, Haslett C, Rees AJ, Gumbay RS, Henson JE, Henson PM. Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis. 1987;136:19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 5.Wang LF, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634–1639. doi: 10.1164/rccm.2110017. [DOI] [PubMed] [Google Scholar]
- 6.Lee RP, Wang D, Kao SJ, Chen HI. The lung is the major site that produces nitric oxide to induce acute pulmonary oedema in endotoxin shock. Clin Exp Pharmacol Physiol. 2001;28:315–320. doi: 10.1046/j.1440-1681.2001.03446.x. [DOI] [PubMed] [Google Scholar]
- 7.Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 8.Genovese T, Cuzzocrea S, Di Paola R, Failla M, Mazzon E, Sortino MA, Frasca G, Gili E, Crimi N, Caputi AP, Vancheri C. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6:58. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, Qin LP. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med. 2010;64:24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 10.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, Shoyama CY, Yuan CS. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–180. [PMC free article] [PubMed] [Google Scholar]
- 11.He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. 2005;77:907–921. doi: 10.1016/j.lfs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;28:2106–2110. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 13.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, Jung WS, Cho KH, Park JH, Kang I, Hong JW, Lee EH. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem. 2009;57:8325–8330. doi: 10.1021/jf901752f. [DOI] [PubMed] [Google Scholar]
- 15.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–35. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Liu X, Shu Q, Li S, Luo F. Ghrelin attenuates lipopolysaccharide-induced acute lung injury through NO pathway. Med Sci Monit. 2008;14:BR141–6. [PubMed] [Google Scholar]
- 17.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 19.Parsey MV, Tuder R, Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1β expression after hemorrhage and endotoxemia. J Immunol. 1998;160:1007–1101. [PubMed] [Google Scholar]
- 20.Chen J, Luo J, Li B, Ran P. E1A has no effect on LPS-induced IL-6 secretion in rat alveolar epithelial cells. Respiration. 2009;78:84–92. doi: 10.1159/000209743. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, He B. The effect of dithiothreitol on chemotactic factors in induced sputum of chronic obstructive pulmonary disease patients. Respiration. 2009;78:217–222. doi: 10.1159/000218713. [DOI] [PubMed] [Google Scholar]
- 22.Li XY, Donaldson K, Macnee W. Lipopolysaccharide-induced alveolar epithelial permeability: The role of nitric oxide. Am J Respir Crit Care Med. 1998;157:1027–33. doi: 10.1164/ajrccm.157.4.9605080. [DOI] [PubMed] [Google Scholar]
- 23.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–10. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 24.Wang LF, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634–9. doi: 10.1164/rccm.2110017. [DOI] [PubMed] [Google Scholar]
- 25.Ermert M, Ruppert C, Gunther A, Duncker HR, Seeger W, Ermert L. Cell-specific nitric oxide synthase-isoenzyme expression and regulation in response to endotoxin in intact rat lungs. Lab Invest. 2002;82:425–41. doi: 10.1038/labinvest.3780436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabó C. Alterations in nitric oxide production in various forms of circulatory shock. New Horiz. 1995;3:2–32. [PubMed] [Google Scholar]
- 27.Dugo L, Marzocco S, Mazzon E, Di Paola R, Genovese T, Caputi AP, Cuzzocrea S. Effects of GW274150, a novel and selective inhibitor of iNOS activity, in acute lung inflammation. Br J Pharmacol. 2004;141:979–87. doi: 10.1038/sj.bjp.0705683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Güzel A, Güzel A, Günaydin M, Alaçam H, Saliş O, Sükrü Paksu M, Murat N, Gacar A, Güvenç T. The role of iNOS inhibitors on lung injury induced by gastrointestinal decontamination agents aspiration. J Mol Histol. 2012;43:351–60. doi: 10.1007/s10735-012-9397-z. [DOI] [PubMed] [Google Scholar]
- 29.Sartori C, Matthay MA. Alveolar epithelial fluid transport in acute lung injury: new insights. Eur Respir J. 2002;20:1299–1313. doi: 10.1183/09031936.02.00401602. [DOI] [PubMed] [Google Scholar]


