Abstract
Intestinal injury is a key feature in sepsis. Heparanase, a heparin sulfate-specific glucuronidase, mediates the onset of organ injury during early sepsis. Heparin has the function to attenuate inflammation and injury induced by multiple factors; however, whether unfractionated heparin (UFH) can attenuate the intestinal injury induced by sepsis as well as the underlying mechanism is still unknown. In the present study, the function of UFH in intestinal injury induced by sepsis was explored. Results of our study showed that after CLP operation, the inflammatory response and expression of heparanase were increased and NF-κB and MAPK P38 signaling pathways were activated. However, pretreatment with UFH will inhibit the expression and activation of heparanase, and reverse the activation of NF-κB and MAPK P38 signaling pathways, thus attenuating inflammatory responses induced by sepsis. These results suggest that UFH may be a promising therapeutic drug for intestinal injury caused by sepsis.
Keywords: Unfractionated heparin, heparanase, intestinal injury, sepsis, NF-κB, MAPK
Introduction
The mortality rate of patients with sepsis has remained high in spite of significant investigative efforts [1], which is partly because of an incomplete understanding of the underlying pathophysiology. Information suggests that the small intestine plays a central role in the pathophysiology of sepsis and it has been referred to as the “motor” of systemic inflammatory response syndrome (SIRS) [2]. Pathologic inflammation in the gastrointestinal tract and breakdown of the gut barrier function are key hallmarks in sepsis [3,4]. Excessive inflammation may cause tissue damage and even organ failure. Failure to maintain intestinal epithelial barrier integrity could promote the translocation and invasion of bacteria and their toxins.
Heparanase is an endogenous glucuronidase capable of degrading both heparin sulfate (HS) and heparin glycosaminoglycan chains [5]. HS is ubiquitous polysaccharides found at the cell surface and in the extracellular matrix (ECM), playing important roles in ECM integrity and barrier function [6]. Heparanase was induced in several inflammatory condition, associated with degradation of HS, remodeling of the ECM, facilitation of inflammatory cells migration towards the injury sites and release of chemokines anchored within the ECM network and cell surfaces [7]. Sepsis is associated with activation of pulmonary heparanase, leading to degradation of the pulmonary endothelial glycocalyx with consequent endothelial dysfunction and inflammatory lung injury [8]. In chronic inflammatory bowel disease, such as ulcerative colitis, and Crohn’s disease, heparanase was also up-regulated [9,10].
Unfractionated heparin (UFH), as a potent anticoagulant, is a linear polysaccharide and composed of a polymer of alternating derivatives of D-glucosamine and uronic acid connected by glycosidic linkages, which is related to HS but has higher N- and O-sulfate contents [11]. Systematic reviews suggest that heparin may be beneficial in sepsis [12,13]. Interestingly, besides being an effective anticoagulant, there is mounting evidence that heparin is also a potent modulator of inflammation. Heparin inhibits the expression and function of adhesion molecules, such as P-selectin and L-selectin [14,15]. Moreover, heparin can directly affect proinflammatory mediators, such as tumor necrosis factor-α (TNF-α) through the nitric oxide system [16]. Our previous report demonstrated that UFH attenuated coagulation and inflammation in endotoxemic mice [17]. However, whether UFH has a relieving function on intestinal injury caused by sepsis as well as the underlying mechanism is not yet clear. In the present study, effect of UFH on intestinal injury induced by sepsis was explored and the results indicate that UFH is a promising drug for sepsis intestinal injury treatment.
Materials and methods
Animals
6-8 week-old male C57BL/6 mice weighing 22-25 g were obtained from the Experimental Animal Centre of China Medical University (Shenyang, China). They were housed in plastic cages containing wood shaving and maintained in a room with a 12-h light cycle with free access to food and water. The animal study protocol was reviewed and approved by the Animal Experimental Committee of China Medical University.
Sepsis model
Mice were subject to cecal ligation and puncture (CLP) according to the method of Rittirsch et al [18], but with some changes. Mice were anesthetized with 10% chloral hydrate, and a 1 cm ventral midline abdominal incision was made aseptically through the skin and linea alba. The cecum was located, exteriorized, and ligated with 4 silk just distal to the ileocecal valve, and was then punctured with a 23G needle. The cecum was gently squeezed to extrude a small amount of stool and then replaced into the abdomen, which was then closed in layers. Sham mice were treated identically, except that the cecum was neither ligated nor punctured. Animals were humanely killed at 4 h and 24 h for functional studies.
Groups
Forty C57BL/6 mice were randomly divided into four groups (n = 10 mice per group): Sham, CLP, CLP + UFH and CLP + NAH. Mice in the CLP group were treated with CLP operation. Mice in the Sham group were externalized without ligation or puncture. Mice in the CLP + UFH group were intravenously injected with 8 U of UFH (Heparin sodium injection, Qianhong Biopharm, Changzhou, China) diluted in 200 μl sterile saline 0.5 h before and 12 h after CLP. Mice in the CLP + NAH group were subcutaneously injected with 150 μg NAH (ZZStandard, Shanghai, China) diluted in 200 μl sterile saline 2 h before CLP. 4 h and 24 h after CLP, five animals were sacrificed respectively and the blood samples were immediately centrifuged, then serum was stored at -80°C respectively. Intestinal tissue samples in each group were collected for histopathology examination, Immunohistochemistry, cytokines detection. Intestinal tissues were frozen in liquid nitrogen for Western blot and quantitative real time polymerase chain reaction (PCR).
Enzyme linked immunosorbent assay (ELISA)
Concentration of myeloperoxidase (MPO) in intestinal tissue was measured with MPO Detection Kit (JianchengBio, Nanjing, China); tumor necrosis factor-α (TNF-α), iInterleukin-6 (IL-6) and interleukin-1β (IL-1β) in serum were determined with corresponding ELISA kits (Boster, Wuhan, China) and heparanase in serum was determined with Heparanase Detection Kits (USCN, Wuhan, China) according to the manufacturer’s instructions.
Histopathology examination
Intestinal tissues were fixed in 4% paraformaldehyde and embedded in paraffin. 5 μm thick sections were obtained for routine hematoxylin and eosin (H&E) staining and light microscopic examination.
Quantitative real time PCR (RT-PCR)
Total RNA was extracted from intestinal tissue using RNA simple Total RNA Kit (Tiangen, Beijing, China) according to the manufacturer’s instruction. The concentration and purity of the RNA in each sample were determined using a spectrophotometer. Reverse transcription was performed on 1 μg of total RNA from each sample using Super M-MLV (BioTeke, Beijing, China) and oligo(dT)15. Then the mRNA levels of TNF-α, IL-6, IL-1β and heparanase were measured using RT-PCR (SYBR GREEN method) with cDNA as template and primers in Table 1. Mouse β-actin was served as an internal control. The relative mRNA level was calculated using 2-ΔΔCT method [19].
Table 1.
Primers for quantitative real time PCR
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| TNF-α | TTCTACTGAACTTCGGGGTGAT | CACTTGGTGGTTTGCTACGA |
| IL-6 | ACTTCCATCCAGTTGCCTTCTT | TCATTTCCACGATTTCCCAGA |
| IL-1β | TTTGAAGTTGACGGACCCC | ATCTCCACAGCCACAATGAGTG |
| heparanase | AAGCGTGAGTCCCTCGTTC | GGCTCAGACCTGCAAATATC |
| β-actin | CTGTGCCCATCTACGAGGGCTAT | TTTGATGTCACGCACGATTTCC |
Western blotting
Protein in each sample was extracted using RIPA lysis buffer (Beyotime, Shanghai, China) or Nucleoprotein Extraction Kit (Beyotime). After measurement of protein concentration, equal amount of protein was loaded onto a sodium dodecyl sulfate polyacrylamide gel for electrophoresis after the concentration of total protein was adjusted. The separated protein was transferred to polyvinylidene fluoride (PVDF) membranes. After blockade, the membranes were incubated with primary antibody against heparanase (1:300, Bioss, Beijing, China), P38, p-P38, NF-κB P65, IκB, Lamin A, β-actin (1:1000, Wanleibio, Shenyang, China) overnight at 4°C. After washing with Tris buffered saline with 0.05% Tween-20 (TBST), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000, Beyotime) at 37°C for 45 min. The membranes were washed three times with TBST and detected using Enhanced ECL Detection System (Beyotime).
Immunohistochemistry
Intestinal tissues were fixed, processed for embedding in paraffin, and cut into 5 μm sections. The sections were dewaxed in xylene and hydrated, and antigen retrieval was done by sodium citrate treatment at 40°C for 10 min. After inactivation of endogenous peroxidase by 3% H2O2 and blockade with goat serum, the sections were incubated with primary antibody against heparanase (1:100, Bioss) and heparin sulfate (HS) (1:100, Boster) overnight at 4°C. After thoroughly washed with phosphate buffered saline (PBS), the corresponding secondary antibody (biotin labeled) and avidin (HRP labeled) were applied and incubated at room temperature for 30 min. Reaction products were visualized by incubation with 3,3’-diaminobenzidine (DAB) and then counterstained with hematoxylin.
Data analysis
The results were presented as means ± SD. Differences between groups were conducted using a one-way analysis of variance (ANOVA) and Bonferroni’s Multiple Comparison. The processing of the data and figures was performed using Graphpad Prism 5.0 software. P < 0.05 was considered to be significant.
Results
UFH attenuated intestinal injury in mouse model of sepsis
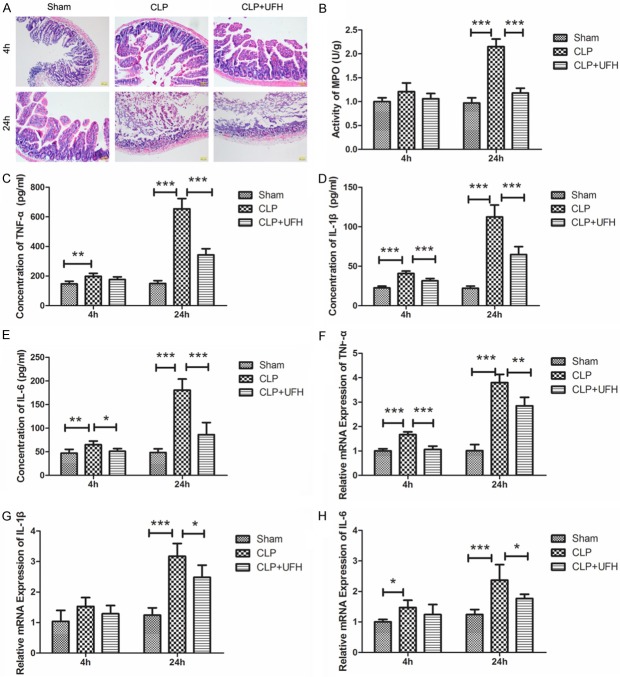
Acute intestinal injury occurred early in sepsis. To explore the effect of UFH, a histopathology examination was performed. As shown in Figure 1A, in the Sham group, the intestinal tissue showed a normal structure and epithelium under a light microscope. However, in the CLP group, intestinal tissue lost its normal structure, and widespread epithelium destruction and inflammatory cell infiltration was discovered at 24 h after CLP. In CLP + UFH group, the histopathology changes of the intestine were attenuated compared to those in the CLP group. These results suggested that UFH could attenuate intestinal injury in mouse model of sepsis. To estimate the extent of neutrophil infiltration into the intestine, MPO in intestinal tissue was detected. After CLP operation, the concentration of MPO was increased significantly (Figure 1B, P < 0.001). Whereas, pretreated with UFH reversed the upheaval of MPO concentration. These results demonstrated that pretreatment with UFH attenuated intestinal injury in mouse model of sepsis.
Figure 1.
UFH attenuates intestinal injury induced by CLP. A. H&E staining for intestinal tissues of each group. B. MPO activity in intestinal tissues of each group. C-E. The concentrations of TNF-α, IL-1β and IL-6 in serum of each group were measured by ELISA. F-H. The relative mRNA levels of TNF-α, IL-1β and IL-6 in intestinal tissues of each group were detected by RT-PCR. The results were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Typical results are presented.
To further validate whether UFH could attenuate intestinal injury in mouse model of sepsis, inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, were detected by ELISA and RT-PCR. Consistent with the results of histopathology, after CLP operation, the expression levels of TNF-α, IL-6 and IL-1β in intestinal tissue and serum were both increased. However, pretreated with UFH, the expression levels of TNF-α, IL-6 and IL-1β in intestinal tissue and serum were both lower than that of CLP group (Figure 1C-H). These results further demonstrated that UFH could attenuate intestinal injury.
Heparanase was increased after CLP operation
Heparanase plays an important role in degradation of extracellular matrix and it was reported to be important to the formation of injury in multiple organs. The expression level of heparanase in intestinal tissue was detected by immunohistochemistry. As shown in Figure 2A, the expression of heparanase was increased within endothelium and epithelium of intestine after CLP operation comparing with the Sham group. Similar result was also discovered in serum heparanase level detected by ELISA: after CLP operation, the heparanase level in serum was also significantly increased (Figure 2B, P < 0.001). These results demonstrated that heparanase level, both in intestinal tissue and in serum, was increased after CLP operation.
Figure 2.
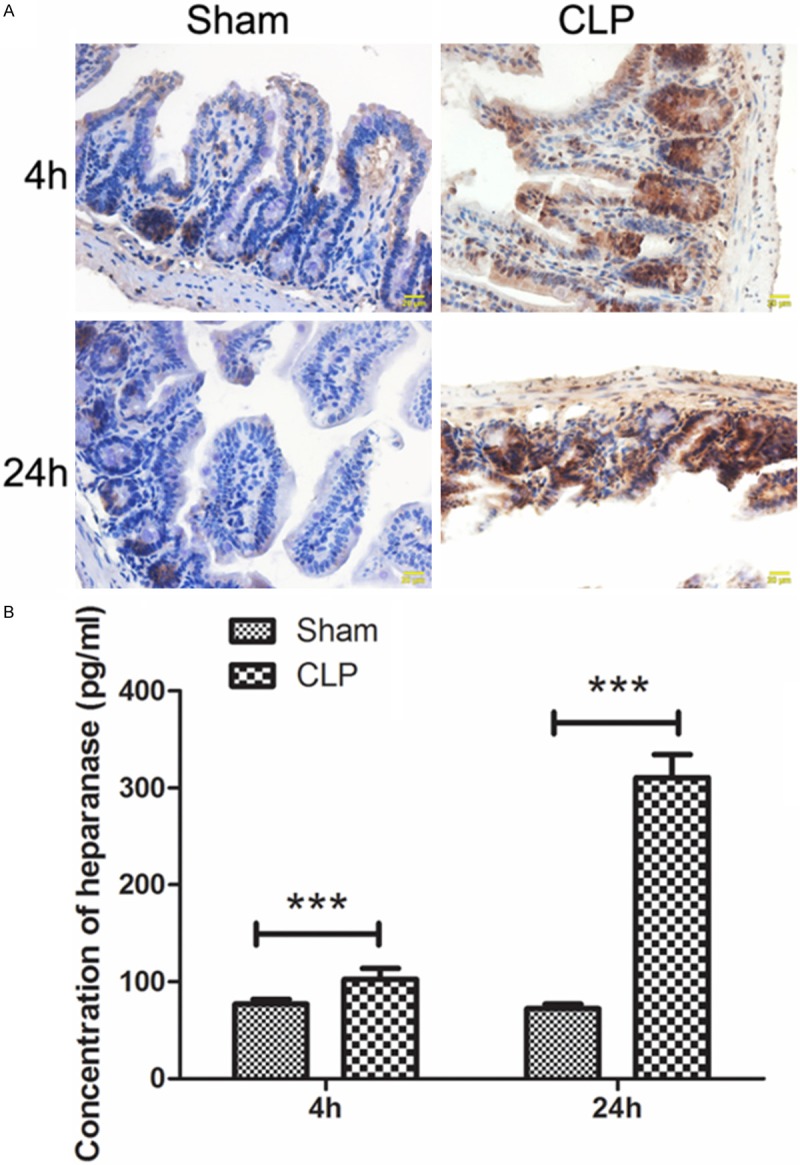
Heparanase is increased by CLP operation. A. The heparanase levels in intestinal tissues of each group were detected by immunohistochemistry. B. The concentration of heparanase in serum of each group was detected by ELISA. The results were presented as mean ± SD. ***P < 0.001. Typical results are presented.
UFH inhibited the up-regulation of heparanase expression and activity in mouse model of sepsis
The expression of heparanase in intestinal tissue was detected by RT-PCR and Western blot. As shown in Figure 3, after CLP operation, heparanase expression level was increased both in mRNA level and in protein level. However, pretreated with UFH, the up-regulation of heparanase was inhibited (Figure 3A-C). As HS degradation is a marker of heparanase activity, the level of HS in intestinal was detected using immunohistochemistry. Coincident with the increased expression level of heparanase in intestinal after CLP operation, the HS level was decreased significantly after CLP. Whereas, pretreated with UFH, the level of HS in intestinal was increased compared with that of CLP group (Figure 3D), which indicated the inhibition of heparanase activity. These results demonstrated that pretreatment with UFH inhibited the up-regulation of heparanase expression and activity induced by CLP.
Figure 3.
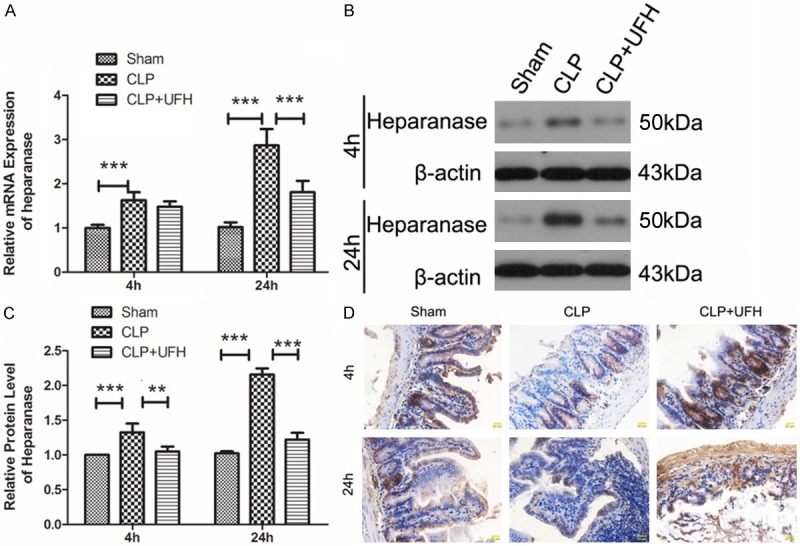
UFH inhibits the up-regulation of heparanase expression and activity induced by CLP. A. The mRNA level of heparanase in intestinal tissues of each group was detected by RT-PCR. The relative mRNA level was calculated using 2-ΔΔCT method. B, C. The protein level of heparanase in intestinal tissues of each group was measured by Western blot using β-actin as internal reference. D. The activity of heparanase in intestinal tissues of each group was evaluated by HS degradation using immunohistochemistry. The results were presented as mean ± SD. **P < 0.01, ***P < 0.001. Typical results are presented.
UFH inhibited the activation of NF-κB and MAPK P38 signaling pathways induced by CLP
The above results indicated that UFH may attenuate intestinal injury by inhibiting heparanase, so we introduced a commercial heparanase inhibitor N-desulfated re-N-acetylated heparin (NAH) which was similar to the hypothetical function of UFH. NF-κB signaling pathway and MAPK P38 signaling pathway have close relationships with inflammation, and the activation of NF-κB and MAPK P38 signaling pathway was detected in the present study. After CLP treatment, the protein level of IκB was decreased (Figure 4A and 4B) and the level of NF-κB in nucleus was up-regulated (Figure 4C and 4D), which indicated the activation of NF-κB signaling pathway. An increase in phosphorylation of P38 was also discovered (Figure 4E and 4F) after CLP, which indicated the activation of MAPK P38 signaling pathway. However, pretreatment with UFH reversed the changes of IκB, nuclear NF-κB and phosphorylation level of P38. These results demonstrated that the activation of NF-κB and MAPK P38 signaling pathways induced by CLP was inhibited by pretreatment with UFH. Coincident results were also discovered in CLP + NAH group. This suggested that the function of UFH was similar to NAH and UFH attenuate CLP-induced intestinal injury by inhibiting heparanase.
Figure 4.

UFH inhibits the activation of NF-κB and MAPK P38 signaling pathways induced by CLP. A, B. The protein level of IκB was detected by Western blot with β-actin as internal reference. C, D. The protein level of NF-κB in nucleus was detected by Western blot. Lamin A was used as an internal reference. E, F. The protein levels of P38 and phosphorylated P38 (p-P38) were detected by Western blot with β-actin as internal reference. The results were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Typical results are presented.
Discussion
In the present study, we explored the effect of UFH on intestinal injury in mouse model of sepsis and found that UFH inhibited the expression and activity of heparanase induced by CLP, inhibited the activation of NF-κB signaling pathway and MAPK P38 signaling pathway, thus attenuating the inflammation of intestines in mouse model of sepsis.
Heparin is primarily used as an anticoagulant. Recent studies showed that heparin also has an abundance of anti-inflammatory activities [12,13,20], and it is applied in burn injury adjuvant therapy [21,22]. In our study, heparin was also found to attenuate inflammation of intestines induced by CLP and down-regulate the apophysis of inflammatory cytokines. Consistent with our result, heparin was found to prevent acute lung injury induced by sepsis in rats [12], inhibit lipopolysaccharide (LPS) induced inflammation [13] and attenuate intestinal dysfunction caused by ischemia and reperfusion [23].
In the present study, an increase in heparanase level in both intestinal tissues and serum was discovered after CLP operation. Heparanase is an endogenous glucuronidase capable of degrading both heparin sulfate and heparin glycosaminoglycan chains. Heparin sulfate plays important roles in the integrity of extracellular matrix and its barrier function [6], Heparin also regulates the activity of a serious of cytokines and growth factors and has multiple functions in inflammation and disease [5,24,25]. Heparanase mediates renal dysfunction during sepsis [26] and heparanase plays important roles in bowel diseases [10], so heparanase may also play important roles in intestinal injury during sepsis. If so, inhibiting of heparanase will be a promising therapeutic for intestinal injury of sepsis.
The up-regulation of heparanase induced by CLP operation was inhibited by UFH in the present study. These results prompt us to the hypothesis that UFH performed its function to attenuate intestinal injury of sepsis by inhibiting heparanase. NF-κB is an important transcription factor that modulates the generation pro-inflammatory cytokines. Previous studies indicate that LPS-induced NF-κB activation in lung tissue is associated with cytokines and chemokines production and microvascular epithelial permeability changes [13,27]. NF-κB and MAPK P38 signaling pathways are important signaling pathways associated with heparanase function [28-30]. So we introduced a heparanase inhibitor NAH and detected the influence of UFH and NAH on NF-κB and MAPK P38 signaling pathways. Results of our study showed that UFH inhibited the activation of NF-κB and MAPK P38 signaling pathways, which was in accordance with the effect of NAH. This indicates that UFH, acting as an inhibitor of heparanase, inhibited the expression and activity of heparanase, thus inhibiting that activation of NF-κB and MAPK P38 signaling pathways, attenuating intestinal injury induced by sepsis. However, how exactly UFH performed its function of heparanase inhibitor is still unclear and more explorations are needed.
In conclusion, the present study demonstrated that UFH functioned as an inhibitor of heparanase inhibiting the activation of NF-κB and MAPK P38 signaling pathways, thus attenuating intestinal injury induced by sepsis. The protective function of UFH suggests that UFH may be a promising therapeutic drug for intestinal injury caused by sepsis. This study laid theoretical foundation for the development of sepsis therapeutic, however, more explorations about the functions and mechanisms of UFH are still needed.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81301619) and the Shenyang Science and Technology Plan Project (No. F13-220-9-11).
Disclosure of conflict of interest
None.
References
- 1.Angus DC, Pereira CA, Silva E. Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets. 2006;6:207–212. doi: 10.2174/187153006777442332. [DOI] [PubMed] [Google Scholar]
- 2.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak YK, Vikstrom E, Magnusson KE, Vecsey-Semjen B, Colque-Navarro P, Mollby R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect Immun. 2012;80:1670–1680. doi: 10.1128/IAI.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brell B, Temmesfeld-Wollbruck B, Altzschner I, Frisch E, Schmeck B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin reduces Staphylococcus aureus alpha-toxin-induced rat ileum microcirculatory damage. Crit Care Med. 2005;33:819–826. doi: 10.1097/01.ccm.0000159194.53695.7a. [DOI] [PubMed] [Google Scholar]
- 5.Vlodavsky I, Iozzo RV, Sanderson RD. Heparanase: multiple functions in inflammation, diabetes and atherosclerosis. Matrix Biol. 2013;32:220–222. doi: 10.1016/j.matbio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 7.Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 2009;102:823–828. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, Doviner V, Rubinstein AM, Ishai-Michaeli R, Atzmon R, Sherman Y, Meirovitz A, Peretz T, Vlodavsky I, Elkin M. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;121:1709–1721. doi: 10.1172/JCI43792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermano E, Lerner I, Elkin M. Heparanase enzyme in chronic inflammatory bowel disease and colon cancer. Cell Mol Life Sci. 2012;69:2501–2513. doi: 10.1007/s00018-012-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casu B, Lindahl U. Structure and biological interactions of heparin and heparan sulfate. Adv Carbohydr Chem Biochem. 2001;57:159–206. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]
- 12.Ning F, Wang X, Shang L, Wang T, Lv C, Qi Z, Wu D. Low molecular weight heparin may prevent acute lung injury induced by sepsis in rats. Gene. 2015;557:88–91. doi: 10.1016/j.gene.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zheng Z, Ma X. Unfractionated heparin inhibits lipopolysaccharide-induced inflammatory response through blocking p38 MAPK and NF-kappaB activation on endothelial cell. Cytokine. 2012;60:114–121. doi: 10.1016/j.cyto.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 15.Lazebnik LB, Boldyreva ON, Parfenov AI, Trubitsyna IE, Shcherbakov PL, Khomeriki SG, Kniazev OV, Sagynbaeva VE. [Cell adhesion molecules in evaluation of Crohn’1(1):s disease therapy] . Eksp Klin Gastroenterol. 2013:31–38. [PubMed] [Google Scholar]
- 16.Mu E, Ding R, An X, Li X, Chen S, Ma X. Heparin attenuates lipopolysaccharide-induced acute lung injury by inhibiting nitric oxide synthase and transforming growth factor -beta/Smad signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:810–814. doi: 10.3760/cma.j.issn.2095-4352.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Ding R, Zhao D, Guo R, Zhang Z, Ma X. Treatment with unfractionated heparin attenuates coagulation and inflammation in endotoxemic mice. Thromb Res. 2011;128:e160–165. doi: 10.1016/j.thromres.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Ling Y, Huang M, Yin T, Gou SM, Zhan NY, Xiong JX, Wu HS, Yang ZY, Wang CY. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine. 2015;72:36–42. doi: 10.1016/j.cyto.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Saliba MJ Jr. Heparin in the treatment of burns: a review. Burns. 2001;27:349–358. doi: 10.1016/s0305-4179(00)00130-3. [DOI] [PubMed] [Google Scholar]
- 22.Oremus M, Hanson M, Whitlock R, Young E, Gupta A, Dal Cin A, Archer C, Raina P. The uses of heparin to treat burn injury. Evid Rep Technol Assess (Full Rep) 2006:1–58. [PMC free article] [PubMed] [Google Scholar]
- 23.Ghadie MM, Miranda-Ferreira R, Taha NS, Maroso AS, Moreti RJ, Andraus MP, Zempulski P, Monteiro HP, Simoes MJ, Fagundes DJ, Caricati-Neto A, Taha MO. Study of heparin in intestinal ischemia and reperfusion in rats: morphologic and functional evaluation. Transplant Proc. 2012;44:2300–2303. doi: 10.1016/j.transproceed.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M. Versatile role of heparanase in inflammation. Matrix Biol. 2013;32:234–240. doi: 10.1016/j.matbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meirovitz A, Goldberg R, Binder A, Rubinstein AM, Hermano E, Elkin M. Heparanase in inflammation and inflammation-associated cancer. FEBS J. 2013;280:2307–2319. doi: 10.1111/febs.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, Smith LP, Koyanagi DE, Gandjeva A, Bhargava R, Tuder RM, Faubel S, Schmidt EP. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1:e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Zheng Z, Liu Y, Ma X. Unfractionated heparin suppresses lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human microvascular endothelial cells by blocking Kruppel-like factor 5 and nuclear factor-kappaB pathway. Immunobiology. 2014;219:778–785. doi: 10.1016/j.imbio.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Zheng D, Shen J, Ruan J, Li A, Li W, Xie G, Luo X, Zhao P, Zheng H. Heparanase is involved in the proliferation and invasion of nasopharyngeal carcinoma cells. Oncol Rep. 2013;29:1888–1894. doi: 10.3892/or.2013.2325. [DOI] [PubMed] [Google Scholar]
- 29.Cui H, Shao C, Liu Q, Yu W, Fang J, Ali A, Ding K. Heparanase enhances nerve-growth-factor-induced PC12 cell neuritogenesis via the p38 MAPK pathway. Biochem J. 2011;440:273–282. doi: 10.1042/BJ20110167. [DOI] [PubMed] [Google Scholar]
- 30.Wu W, Pan C, Meng K, Zhao L, Du L, Liu Q, Lin R. Hypoxia activates heparanase expression in an NF-kappaB dependent manner. Oncol Rep. 2010;23:255–261. [PubMed] [Google Scholar]