Abstract
Background and Objective: Ovarian cancer is among the most lethal of all malignancies in women. While chemotherapy is the preferred treatment modality, chemoresistance severely limits treatment success. Because transforming growth factor-beta (TGF-β) could increase survival of ovarian cancer cells in the presence of cisplatin, we conducted a preclinical study of the antitumor effects of the TGF-β type I (TβRI) and type II (TβRII) kinase inhibitor LY2109761 in combination with cisplatin. Methods: SKOV3, OV-90 and SKOV3DDP cells were treated with LY2109761, and/or cisplatin, and cell viability, apoptosis mRNA and protein expression levels were then evaluated. Furthermore, the efficacy of LY2109761 combined with cisplatin was further examined in established xenograft models. Results: LY2109761 was sufficient to induce spontaneous apoptosis of ovarian cancer cells. Combination with LY2109761 significantly augmented the cytotoxicity of cisplatin in both parental and cisplatin resistant ovarian cancer cells. LY2109761 significantly increased apoptotic cell death in cisplatin-resistant cells. Combination treatment of LY2109761 and cisplatin showed antiproliferative effects and induced a greater rate of apoptosis than the sum of the single-treatment rates and promoted tumor regression in established parental and cisplatin resistant ovarian cancer xenograft models. Conclusions: Chemotherapeutic approaches using LY2109761 might enhance the treatment benefit of the cisplatin in the treatment of ovarian cancer patients.
Keywords: Ovarian cancer, chemotherapy, transforming growth factor-beta, LY2109761
Introduction
Epithelial ovarian cancer (EOC), accounting for more than 85% of human ovarian cancer, is the fifth leading cause of death in female cancer patients and has the highest mortality rate of all gynecological cancers worldwide [1]. The overall 5-year survival rate of ovarian cancer patients diagnosed at an advanced stage is less than 30% [2]. The poor survival is mainly attributed to the high resistance of EOC to current chemotherapeutic regimens [3]. Cisplatin and its analogues are first-line chemotherapeutic agents for the treatment of human ovarian cancer [4,5]. Cisplatin promotes its cytotoxicity by forming DNA-protein cross-links, DNA mono-adducts, and intrastrand DNA cross-links, which all trigger apoptosis [6,7]. To circumvent cisplatin resistance various attempts using combinational therapy have been tried. However, most trials were not very promising. Therefore discovering new therapeutic targets to overcome cisplatin resistance is urgent and essential task for treatment of EOC.
Recent reports demonstrated that activation of several biochemical pathways induces acquired drug resistance during drug treatment [8-10]. Thus exploration of kinome for sensitization of ECO cells to cisplatin can be useful tool to isolate new target kinases. Recent developments in small molecule inhibitors for numerous protein kinases have enabled us to explore the protein kinase targets in ECO cells. Through the screening a series of protein kinase inhibitors (PKIs), we found that several PKIs show synergism in combination with cisplatin in ECO [11-15]. Among these newly found PKIs, a TβRI/II kinase selective inhibitor LY2109761, exhibited substantial potency in reducing drug resistance [16-18].
Transforming growth factor β (TGF-β) is a family of dimeric polypeptide growth factors that initiate cell signaling by dimerizing the TGF-β type I (TβRI) and type II (TβRII) serine/threonine kinase receptors. This dimerization allows for the constitutively active TβRII kinase to transphosphorylate and activate the TβRI kinase which, in turn, propagates the signal by activating downstream Smad-dependent and Smad-independent pathways [19,20]. The tumor suppressor function of TGF-β signaling is well established. However, TGF-β signaling also plays a key role in tumor-promoting effects in advanced disease [21]. TGFβ is a potent regulator of cell proliferation and differentiation [22]. TGFβ preferentially binds to TβRII then the ligand-bound TβRII forms heteromeric receptor complex with TβRI, which triggers downstream signals [23].
The overexpression of TGFβ ligands has been reported in various malignant entities including ECO [24-29]. In patients with ECO, enhanced expression of TGF-beta I and TGF-beta3, as well as the loss of expression of T beta R-I and T beta R-III, contribute to ovarian carcinogenesis and/or tumor progression [29]. These multiple roles of TGFβ in ECO initiation and progression have promoted the development of therapeutic agents based on the inhibition of the TGFβ pathway [30,31]. LY2109761, a novel TβRI/II kinase inhibitor, has shown a SMAD2-selective inhibitory profile with antitumor activity in various tumor models such as colorectal cancer [32], pancreatic cancer [33], and hepatocellular carcinoma [34]. LY2109761 was also as the radiosensitizers to improve the treatment of glioblastoma [35].
In the present study, we investigated the combination effects of cisplatin and LY2109761 in established human EOC cell lines in vitro and in vivo in a subcutaneous and an orthotopic tumor model. Our data indicate that LY2109761 is an effective treatment approach alone and augments the cisplatin treatment response in ECO cells.
Material and methods
Cell culture and reagents
Human epithelial ovarian cancer cell lines SKOV3 and OV-90 was obtained from American Type Culture Collection, cisplatin (DDP) resistant SKOV3 cell line (SKOV3DDP) was obtained from Yiyeqi. cc (Shanghai, China). They were all routinely cultured in RPMI 1640 (Life Technologies, Inc., Shanghai, China), supplemented with 10% heat-inactivated FBS. All cells were cultured in a 5% CO2 humidified atmosphere at 37°C. LY2109761 is an orally active TβRI/II kinase dual inhibitor, which was purchased from Selleck (Houston, TX, USA). Test compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted with culture medium [DMSO final concentration, 0.1% (v/v)]. Stock DDP solution was prepared in DMSO (330 mM), stored as aliquots at 20°C, and used within 2 weeks. DDP was further diluted in medium before adding to the cells.
MTT assay
SKOV3, OV-90 and SKOV3DDP cells were seeded at a density of 3.5 × 103 per well in 96-well microtiter culture plates. After overnight incubation, medium was removed and replaced with fresh medium containing different concentrations of LY2109761 (0.5-10 μM) for 72 h. On completion of incubation, viability was assessed after adding 50 μL trypan blue solution (0.4% in PBS) in culture medium. After 1 to 2 min, the number of dead cells, which retained the dye, was compared with the total number to calculate the percentage of viable cells. Cell growth inhibition was also detected using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. To determine the effect of drug combination, SKOV3, OV-90and SKOV3DDP cells were plated onto 96-well plates. After 72 h treatment with DDP (0.15-3.0 μM) and/or LY2109761 (0.5-10 μM), cell viabilities were measured using MTT assays.
Detection of apoptosis by ELISA
The cell death detection ELISA kit was used for assessing apoptosis according to the manufacturer’s protocol. Briefly, cells were treated with LY2109761 or/and DDP for different periods of time. After treatment, the cells were lysed and the cell lysates were overlaid and incubated in microtiter plate modules coated with anti-histone antibody for detection of apoptosis.
Western blotting
Western blot was used to measure the expression levels of proteins. Cells cultured with LY2109761 were harvested and the proteins in total cell extracts were generated using RIPA buffer supplemented with protease inhibitors. LY2109761 treated cells were prepared using protein Extraction Kit (Affymetrix, Santa Clara, Guangzhou, China) according to the manufacturer’s protocol. Protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) then transferred to polyvinylidene fluoride (PVDF) membrane. Anti-phospho (p)-SMAD2 and total SMAD2 (Cell Signaling) were used as primary antibodies. An anti-β-actin monoclonal antibody (Sigma) as internal loading control. Anti- rabbit IgG peroxidase antibodies (Sigma, St. Louis, MO, USA) were used for secondary antibody and enhanced chemiluminescence (ECL) solution (Santa Cruz Biotechnology, Inc.) was used for detection.
RT-PCR
Total cellular RNA was extracted with TRIzol reagent (Invitrogen), and reverse-transcribed with a reverse transcription kit (Invitrogen). Primer pairs designed to amplify p-SMAD2 and β-actin were synthesized at Shanghai PharmNet Biotechnologies. The sequences used were: p-SMAD2 primer, sense, 5’-AGCCACGAGAAACAGGAAGCAGG-3’; antisense, 5’-TCGAAAGAACGTCACTGACTTTA-3’; β-actin primer, sense, 5’-GGGCGCCCCAGGCACCA-3’, antisense, 5’-CTCCTTAATGTCACGCACGATTT-3’. Extracted RNA (2 µL) was subjected to PCR using 1 µL of sense and antisense primers, 1 µL of β-actin primers, 2.5 µL of MgCl2 (25 mmol/L), 1 µL of deoxynucleotide triphosphates, 2.5 µl of 10× PCR buffer, 5 units of Taq DNA polymerase, and 14 µL of double distilled water in a 25 µL reaction volume. The cycling variables were: one 4-minute cycle at 94°C, 35 cycles at 94°C for 52 seconds, 55°C for 1 minute, 72°C for 50 seconds; final extension was at 72°C for 10 minutes. PCR products (1.5 µL) were electrophoresed on 1.2% agarose gels and the gray scale ratio of p-SMAD2/β-actin was calculated.
In vivo tumor model
All animal experiments were carried out according to the Chinese Laws of animal welfare and were approved by the Ethics Commission of the Weifang Medical College. Four-week-old female ICR-SCID mice were obtained from Weifang Medical College. Briefly, the female ICR-SCID mice were injected s.c. with 106 SKOV3 or SKOV3DDP cells in 100 µL of PBS at a single dorsal site. When the tumors reached a size of about 50-100 mm3 (10-15 days after transplantation), mice were treated with LY2109761. LY2109761 was administered orally at 50 mg/kg twice daily (days 1-5 of each week) until the end of observation (4 weeks). During which, the DDP (5 mg/kg) was administered i.p at 5 mg/kg once week for 4 weeks. Tumor volume for the subcutaneous experiment was determined 1 times/4 days by direct measurement with calipers (V = 1/2 × (L × W2).
Immunohistochemistry
Five-um-thick sections of paraffin-embedded, formalin-fixed s.c. tumor tissues were stained with H&E for histological examination. Ki67 and p-SMAD2 detection was performed as the manufacture’s instruction.
TUNEL assay
Apoptosis induction in the tumor was measured using the TUNEL kits. The TUNEL staining was performed with an in situ apoptotic cell detection kit following the manufacturer’s protocol. The slides were observed under an Olympus fluorescence microscope attached to a charge-coupled device camera. The images were acquired under × 40 objectives using the Image-Pro software. Apoptotic index was determined by calculating the average number of nuclei apoptotic cells in 5 high-power microscopic fields (× 400) selected from a central region in viable tumor areas, avoiding areas containing necrosis.
Statistical analysis
All in vivo experiments included at least 10 mice per group. Results consisting of three or more groups were analyzed using single-factor ANOVA. Analysis of results containing two groups was done using the Student’s t test, assuming unequal variance. Values of P < 0.05 were considered statistically significant.
Results
Effects of LY2109761 on phospho (p)-SMAD2 expression
We first examined the effect of LY2109761, a SMAD2-selective inhibitory profile on phospho (p)-SMAD2 (p-SMAD2) and total SMAD2 in SKOV3, OV-90 and SKOV3DDP cells. SKOV3, OV-90 and SKOV3DDP cells were treated with LY2109761 at concentration of 0.5-10 μM for 24 hours. p-SMAD2 and total SMAD2 was detected by western blot assay. The results showed that LY2109761 inhibited p-SMAD2 mRNA (Figure 1A) and p-SMAD2 protein (Figure 1B) expression in a dose and time-dependant way. Treatment with 10 μM for 12 hours, p-SMAD2 mRNA was completely inhibited in the three cells, and p-SMAD2 protein was almost inhibited at 24 hours. LY2109761 did not have significant effect on total SMAD2 (data not shown).
Figure 1.
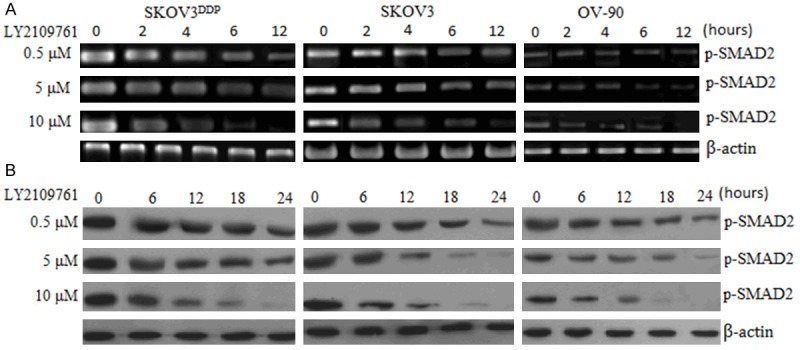
Effect of LY2109761 on p-SMAD2. A, RT-PCR assay; B, Western blot assay.
Effects of LY2109761 on cell growth
The baseline expression of p-SMAD2 was determined in SKOV3 and OV-90 cell lines. The results showed that p-SMAD2 was overexpressed in the two cell lines (Figure 1). Next, we examined the growth inhibitory effects of LY2109761 using the MTT assay in SKOV3 and OV-90. The treatment of OC cells for 1-3 days with 0.5, 5 and 10 µM of LY2109761 resulted in cell growth inhibition in a dose- and time-dependent manner in 2 OC cell lines (Figure 2A, 2B). Next, we examined whether the inhibition of cell growth was also accompanied by the induction of apoptosis induced by LY2109761. ELISA analysis was employed to investigate the degree of apoptosis induced by LY2109761.
Figure 2.
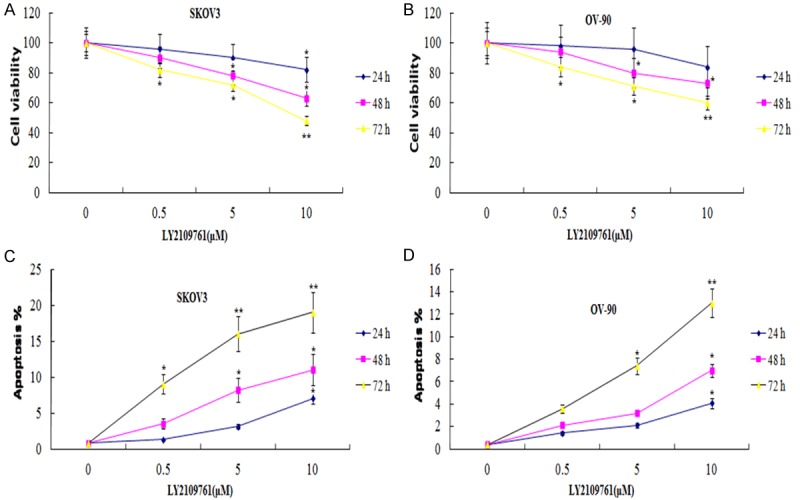
Effects of LY2109761 on cell growth and apoptosis. SKOV3 and OV-90 cells were treated with 0.5, 5 and 10 uM of LY2109761 for 1-3 days. The growth inhibitory effects of LY2109761 on cells by MTT assay (A, B). The apoptosis effects of LY2109761on cells by ELISA assay (C, D). Vs control; *P < 0.05, **P < 0.01.
Effects of LY2109761 on cell apoptosis
SKOV3 and OV-90 cells were treated with 0.5, 5.0 and 10 μM LY2109761 for 24-72 hr. After treatment, the degree of apoptosis was measured in 2 cell lines. The induction of apoptosis was found to be dose and time-dependent (Figure 2C, 2D). These results provided convincing data showing that LY2109761 could induce apoptosis in OC cells.
LY2109761 enhance the cytotoxicity of DDP
In order to evaluate the combinatorial effect of LY2109761 with DDP, we measured cell viability after treatment of cells with DDP and LY2109761. Combined treatment of DDP and LY2109761 significantly reduced cell viability of (Figure 3A, 3B). Similarly, combined treatment of LY2109761 with DDP also effectively promoted apoptosis of both cell lines compared to single treatment with DDP or LY2109761 (Figure 3C, 3D).
Figure 3.
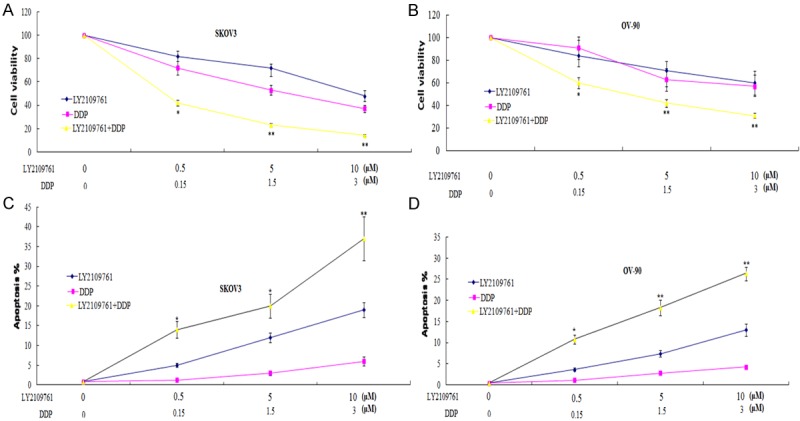
Combination of LY2109761 with DDP efficiently reduces the viability and increases the apoptosis of OC cells. SKOV3 and OV-90 cells were treated with 0.5, 5 and 10 uM of LY2109761 in combination with DDP for 72 h then cell viability was determined by MTT assay and ELISA assay as described in Materials and Methods. Data are expressed as mean ± SD. Student’s t-test was applied for statistical analysis for comparison between DDP treatment and combined treatment. *P < 0.05; **P < 0.01.
LY2109761 abrogate DDP resistance
Since LY2109761 efficiently enhanced the cytotoxicity of DDP, we further evaluated the combinatorial effects in DDP-resistant cells. MTT assay revealed that LY2109761 effectively reduced the cell viability of SKOV3DDP cells in combination with DDP (Figure 4A). Similarly, combined treatment of LY2109761 with DDP also effectively promoted apoptosis of SKOV3DDP cell (Figure 4B).
Figure 4.
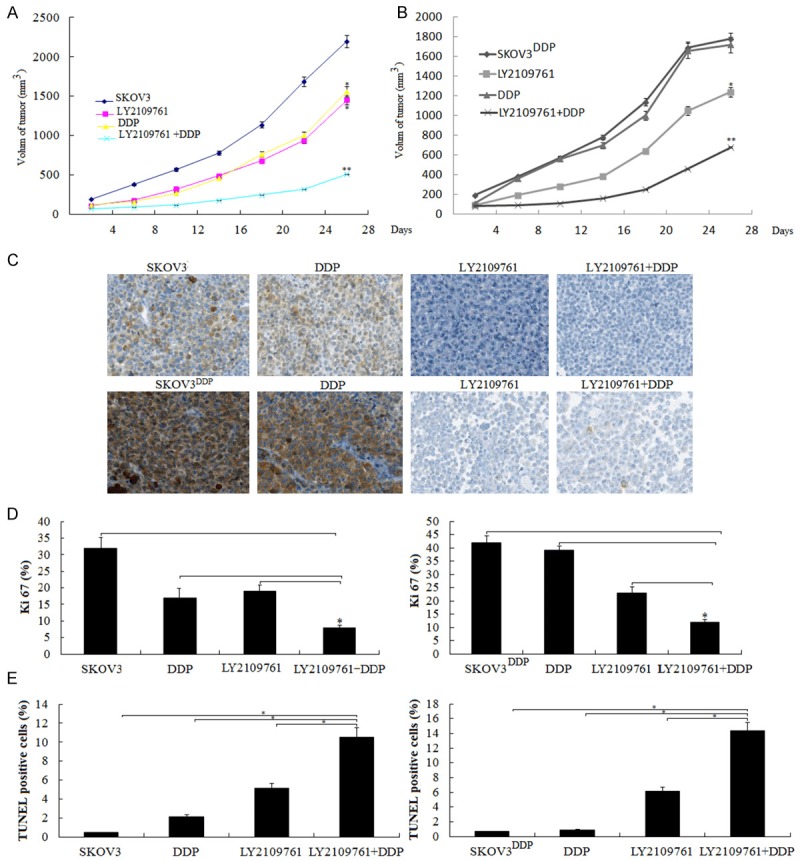
Combination of LY2109761 with DDP on tumorigenicity in nude mice. SKOV3 and SKOV3DDP cells were injected into nude mice, as described in Materials and methods. Tumor volume was determined every 4 days for 28 days. At the end of the experiment, animals were sacrificed and tumors were excised for volume measurement and histological study. A. Growth curve of tumor xenografts in SKOV3 cells; B. Growth curve of tumor xenografts in SKOV3DDP cells; *P < 0.05, **P < 0.01 ,compared to the controls. C. The resected tumors were stained with antibodies against p-SMAD2 Representative images of the staining are shown. D. The resected tumors were stained with antibodies against Ki-67.The percentages of Ki-67-positive cells were indicated. *P < 0.05. E. The resected tumors were stained with TUNEL. The percentages of TUNEL-positive cells were indicated. *P < 0.05.
Effect of LY2109761 on OC growth in vivo
To further determine a role of LY2109761 in progression of OC, we did an in vivo animal experiment. We selected SKOV3 and SKOV3DDP cells in the in vivo study. The female ICR-SCID mice were injected s.c. with 106 SKOV3 or SKOV3DDP cells in 100 µL of PBS at a single dorsal site. When the tumors reached a size of about 0.15 cm3, mice were treated with LY2109761, which was administered orally at 50 mg/kg twice daily (days 1-5 of each week) for 4 weeks. During which, the DDP (5 mg/kg) was administered i.p at 5 mg/kg once week for 4 weeks. We found that SKOV3 and SKOV3DDP tumors administered orally LY2109761 alone formed substantially smaller tumors in nude mice compared with the untreated controls (Figure 4A, 4B). DDP treatment alone also formed substantially smaller SKOV3 tumors, and DDP treatment alone did not increase or decrease tumor volume in SKOV3DDP tumors compared with the untreated controls, however, combination with LY2109761 and DDP significantly decreased the substantial tumor volume in both of the SKOV3 and SKOV3DDP tumors (Figure 4A, 4B).
We then evaluated the histopathological results of p-SMAD2 in response to LY2109761. We showed that high p-SMAD2 expression was found in the SKOV3 and SKOV3DDP tumors, DDP treatment alone did not increase p-SMAD2 expression in the two groups tumors, however, treatment with LY2109761 significantly inhibited p-SMAD2 expression in the two groups tumors (Figure 4C).
We analyzed the number of proliferating cells using the proliferation marker Ki-67. The combined treatment with LY2109761 and DDP presented fewer Ki-67 positive cells, compared with LY2109761 or DDP alone and control (P < 0.01) (Figure 4D). Furthermore, the TUNEL assay showed that the combined treatment with LY2109761 and DDP induced significantly increased apoptosis of tumor cells (Figure 4E). The group receiving combination therapy showed the highest apoptotic index (P < 0.01). These results suggest that combination of LY2109761 and DDP significantly reduced cell proliferation and induced apoptosis in vivo.
Discussion
Chemoresistance is a therapeutic problem that severely limits successful treatment outcomes for many human cancers. This is particularly true of ovarian cancer, where the development of resistance is a common occurrence [4,5,20]. The past few years have seen an enormous growth in our understanding of the mechanisms that regulate drug induced apoptosis, and thus influence chemosensitivity.
TGF-β overproduction is a universal event in cancer cells and is a poor prognostic marker [24-29,36,37]. TGF-β initiates cell signaling by dimerizing the TGF-β type I (TβRI) and type II (TβRII) serine/threonine kinase receptors. Many studies have reported that overexpression of TGF-β signal increased the chemoresistance in cancer cells [38-40], and vice versa [32,35]. LY2109761, TβRI/II kinase inhibitor reduced clonogenicity and increased radiosensitivity in GBM cell lines and cancer stem-like cells, augmenting the tumor growth delay produced by fractionated radiotherapy in a supra-additive manner in vivo. In an orthotopic intracranial model, LY2109761 significantly reduced tumor growth, prolonged survival, and extended the prolongation of survival induced by radiation treatment [18].
In our study, we found that LY2109761 treatment promoted apoptosis in SKOV3 and OV-90 cells , and cisplatin (DDP) resistant SKOV3 cell line (SKOV3DDP). Our observation on potentiating of growth inhibition was consistent with the induction of apoptosis as in EOC cells. To support our in vitro results, an in vivo tumor model was used to assess the anti-tumor activity of LY2109761. Our in vivo results are consistent with in vitro findings showing that the LY2109761 treatment was an effective agent for EOC
Many studies on clinical specimens have shown that TGF-β expression is associated with resistance to chemotherapy or radiation therapy [36-38], and linked to poor prognosis [39-43], suggesting that cancer cells survive with TGF-β. It is tempting to speculate that TGF-β plays a fundamental role in the development of drug resistance in ovarian cancer. In the current study, the anticancer drug cisplatin did not up or down-regulation of TGF-β signals in ovarian cancer cells (data not shown), which suggested that TGF-β signals was not associated with the acquired cisplatin drug resistance in the EOC cells. It is associated with the endogenous drug resistance in the EOC cells. In this study, we observed that inhibition of TβRI/II kinase receptors by LY2109761 combined with cisplatin is much more superior to the single agents in vitro. Our in vivo results are consistent with in vitro findings. Furthermore, abrogation of TGF-β signals by LY2109761 treatment sensitizes resistant SKOV3DDP cells to cisplatin.
In summary, the inhibition of TGF-β signals by LY2109761 could be useful for potentiating the anti-tumor activity of cisplatin in vitro, which appears to be responsible for the observed better anti-tumor activity in vivo. Combination of LY2109761 and cisplatin could be a promising approach for the treatment of human EOC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Sudo T. Molecular-targeted therapies for ovarian cancer: prospects for the future. Int J Clin Oncol. 2012;17:424–429. doi: 10.1007/s10147-012-0461-1. [DOI] [PubMed] [Google Scholar]
- 3.Sueblinvong T, Ghebre R, Iizuka Y, Pambuccian SE, Isaksson Vogel R, Skubitz AP, Bazzaro M. Establishment, characterization and downstream application of primary ovarian cancer cells derived from solid tumors. PLOS One. 2012;7:e50519. doi: 10.1371/journal.pone.0050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeage MJ. New-generation platinum drugs in the treatment of cisplatin-resistant cancers. Expert Opin Investig Drugs. 2005;14:1033–46. doi: 10.1517/13543784.14.8.1033. [DOI] [PubMed] [Google Scholar]
- 5.Tewari K, Mehta R, Burger R, et al. Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs. 2005;10:413–42. doi: 10.1517/14728214.10.2.413. [DOI] [PubMed] [Google Scholar]
- 6.Sherman S, Lippard S. Structural aspects of platinum anticancer drug interaction with DNA. Chem Rev. 1987;87:1153–7. [Google Scholar]
- 7.Hersey P, Zhang X. Overcoming resistance of cancer cells to apoptosis. J Cell Physiol. 2003;196:9–18. doi: 10.1002/jcp.10256. [DOI] [PubMed] [Google Scholar]
- 8.Cui W, Yazlovitskaya EM, Mayo MS, Pelling JC, Persons D. Cisplatin-induced response of c-jun N-terminal kinase 1 and extracellular signal--regulated protein kinases 1 and 2 in a series of cisplatin-resistant ovarian carcinoma cell lines. Mol Carcinog. 2000;29:219–28. [PubMed] [Google Scholar]
- 9.Villedieu M, Deslandes E, Duval M, Héron JF, Gauduchon P, Poulain L. Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment. Gynecol Oncol. 2006;101:507–19. doi: 10.1016/j.ygyno.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Feng X, Li L, Jiang H, Jiang K, Jin Y, Zheng J. Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: involvement of apoptosis and autophagy. Biochem Biophys Res Commun. 2014;444:376–81. doi: 10.1016/j.bbrc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 11.Cossa G, Lanzi C, Cassinelli G, Carenini N, Arrighetti N, Gatti L, Corna E, Zunino F, Zaffaroni N, Perego P. Differential outcome of MEK1/2 inhibitor-platinum combinations in platinum-sensitive and -resistant ovarian carcinoma cells. Cancer Lett. 2014;347:212–24. doi: 10.1016/j.canlet.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Itamochi H, Nishimura M, Oumi N, Kato M, Oishi T, Shimada M, Sato S, Naniwa J, Sato S, Kudoh A, Kigawa J, Harada T. Checkpoint kinase inhibitor AZD7762 overcomes cisplatin resistance in clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2014;24:61–9. doi: 10.1097/IGC.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 13.Itamochi H, Oishi T, Shimada M, Sato S, Uegaki K, Naniwa J, Sato S, Nonaka M, Terakawa N, Kigawa J, Harada T. Inhibiting the mTOR pathway synergistically enhances cytotoxicity in ovarian cancer cells induced by etoposide through upregulation of c-Jun. Clin Cancer Res. 2011;17:4742–50. doi: 10.1158/1078-0432.CCR-11-0190. [DOI] [PubMed] [Google Scholar]
- 14.Morgan RJ Jr, Leong L, Chow W, Gandara D, Frankel P, Garcia A, Lenz HJ, Doroshow JH. Phase II trial of bryostatin-1 in combination with cisplatin in patients with recurrent or persistent epithelial ovarian cancer: a California cancer consortium study. Invest New Drugs. 2012;30:723–8. doi: 10.1007/s10637-010-9557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RJ Jr, Leong L, Chow W, Gandara D, Frankel P, Garcia A, Lenz HJ, Doroshow JH. Protein kinase inhibitors emodin and dichloro-ribofuranosylbenzimidazole modulate the cellular accumulation and cytotoxicity of cisplatin in a schedule-dependent manner. Cancer Chemother Pharmacol. 2010;65:427–36. doi: 10.1007/s00280-009-1045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly EC, Saunier EF, Quigley D, Luu MT, De Sapio A, Hann B, Yingling JM, Akhurst RJ. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TβRI/II kinase inhibition with LY-2109761. Cancer Res. 2011;71:2339–49. doi: 10.1158/0008-5472.CAN-10-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Herion TW, Timke C, Han N, Hauser K, Weber KJ, Peschke P, Wirkner U, Lahn M, Huber PE. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-β receptor I kinase inhibitor LY2109761. Neoplasia. 2011;13:537–49. doi: 10.1593/neo.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Kleber S, Röhrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, Lahn M, Huber PE. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 19.Kang SP, Saif MW. Pharmacogenomics and pancreatic cancer treatment. Optimizing current therapy and individualizing future therapy. JOP. 2008;9:251–266. [PubMed] [Google Scholar]
- 20.Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008;99:653–658. doi: 10.1111/j.1349-7006.2008.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 22.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 23.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 24.Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89:251–8. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Jöhnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925s–32s. [PubMed] [Google Scholar]
- 26.Hawinkels LJ, Verspaget HW, van der Reijden JJ, van der Zon JM, Verheijen JH, Hommes DW. Active TGF-beta1 correlates with myofibroblasts and malignancy in the colorectal adenoma-carcinoma sequence. Cancer Sci. 2009;100:663–70. doi: 10.1111/j.1349-7006.2009.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higashi T, Sasagawa T, Inoue M, Oka R, Shuangying L, Saijoh K. Overexpression of latent transforming growth factor-beta 1 (TGF-beta 1) binding protein 1 (LTBP-1) in association with TGF-beta 1 in ovarian carcinoma. Jpn J Cancer Res. 2001;92:506–15. doi: 10.1111/j.1349-7006.2001.tb01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner M, Kleeff J, Friess H, Büchler MW, Korc M. Enhanced expression of the type II transforming growth factor-beta receptor is associated with decreased survival in human pancreatic cancer. Pancreas. 1999;19:370–6. doi: 10.1097/00006676-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Bristow RE, Baldwin RL, Yamada SD, Korc M, Karlan BY. Altered expression of transforming growth factor-beta ligands and receptors in primary and recurrent ovarian carcinoma. Cancer. 1999;85:658–68. doi: 10.1002/(sici)1097-0142(19990201)85:3<658::aid-cncr16>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Do TV, Kubba LA, Du H, Sturgis CD, Woodruff TK. Transforming growth factor-beta1, transforming growth factor-beta2, and transforming growth factor-beta3 enhance ovarian cancer metastatic potential by inducing a Smad3-dependent epithelial-to-mesenchymal transition. Mol Cancer Res. 2008;6:695–705. doi: 10.1158/1541-7786.MCR-07-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng JC, Auersperg N, Leung PC. TGF-beta induces serous borderline ovarian tumor cell invasion by activating EMT but triggers apoptosis in low-grade serous ovarian carcinoma cells. PLoS One. 2012;7:e42436. doi: 10.1371/journal.pone.0042436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Cancer Lett. 2009;277:114–20. doi: 10.1016/j.canlet.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–40. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–66. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Kleber S, Ohrich MR, Timke C. Blockade of TGF-β Signaling by the TGFR-I Kinase Inhibitor LY2109761 Enhances Radiation Response and Prolongs Survival in Glioblastoma. Cancer Res. 2011;71:7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Chen L, Helfand BT, Zhu LJ, Kozlowski J, Minn A, Jang T, Yang XJ, Javonovic B, Guo Y, Lonning S, Harper J, Teicher BA, Yu N, Brendler C, Wang J, Catalona WJ, Lee C. Transforming Growth Factor-β-induced DNA methyltransferase contributes to aggressive prostate cancer phenotypes and predicts biochemical recurrence after radical prostatectomy. PLoS One. 2011;6:e25168. doi: 10.1371/journal.pone.0025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vázquez PF, Carlini MJ, Daroqui MC, Colombo L, Dalurzo ML, Smith DE, Grasselli J, Pallotta MG, Ehrlich M, Bal de Kier Joffé ED, Puricelli L. TGF-beta specifically enhances the metastatic attributes of murine lung adenocarcinoma: implications for human non-small cell lung cancer. Clin Exp Metastasis. 2013;30:993–1007. doi: 10.1007/s10585-013-9598-1. [DOI] [PubMed] [Google Scholar]
- 38.Marchini S, Fruscio R, Clivio L, Beltrame L, Porcu L, Fuso Nerini I, Cavalieri D, Chiorino G, Cattoretti G, Mangioni C, Milani R, Torri V, Romualdi C, Zambelli A, Romano M, Signorelli M, di Giandomenico S, D’Incalci M. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer. 2013;49:520–30. doi: 10.1016/j.ejca.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Izumiya M, Kabashima A, Higuchi H, Igarashi T, Sakai G, Iizuka H, Nakamura S, Adachi M, Hamamoto Y, Funakoshi S, Takaishi H, Hibi T. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32:3847–53. [PubMed] [Google Scholar]
- 40.Tabe Y, Shi YX, Zeng Z, Jin L, Shikami M, Hatanaka Y, Miida T, Hsu FJ, Andreeff M, Konopleva M. TGF-β-Neutralizing Antibody 1D11 Enhances Cytarabine-Induced Apoptosis in AML Cells in the Bone Marrow Micro-environment. PLoS One. 2013;8:e62785. doi: 10.1371/journal.pone.0062785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du S, Barcellos-Hoff MH. Tumors as organs: biologically augmenting radiation therapy by inhibiting transforming growth factor β activity in carcinomas. Semin Radiat Oncol. 2013;23:242–51. doi: 10.1016/j.semradonc.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One. 2013;8:e70479. doi: 10.1371/journal.pone.0070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012;72:4119–29. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilbig A, Oettle H. Transforming growth factor beta in pancreatic cancer. Curr Pharm Biotechnol. 2011;12:2158–64. doi: 10.2174/138920111798808356. [DOI] [PubMed] [Google Scholar]
- 45.Dong L, Ge XY, Wang YX, Yang LQ, Li SL, Yu GY, Gao Y, Fu J. Transforming growth factor-β and epithelial-mesenchymal transition are associated with pulmonary metastasis in adenoid cystic carcinoma. Oral Oncol. 2013;49:1051–8. doi: 10.1016/j.oraloncology.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Parviainen H, Schrade A, Kiiveri S, Prunskaite-Hyyryläinen R, Haglund C, Vainio S, Wilson DB, Arola J, Heikinheimo M. Expression of Wnt and TGF-β pathway components and key adrenal transcription factors in adrenocortical tumors: association to carcinoma aggressiveness. Pathol Res Pract. 2013;209:503–9. doi: 10.1016/j.prp.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Divella R, Daniele A, Savino E, Palma F, Bellizzi A, Giotta F, Simone G, Lioce M, Quaranta M, Paradiso A, Mazzocca A. Circulating levels of transforming growth factor-β (TGF-β) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013;33:1491–7. [PubMed] [Google Scholar]
- 48.Kim JY, Jeon TJ, Bae BN, Kwon JE, Kim HJ, Park K, Shin E. The prognostic significance of growth factors and growth factor receptors in gastric adenocarcinoma. APMIS. 2013;121:95–104. doi: 10.1111/j.1600-0463.2012.02942.x. [DOI] [PubMed] [Google Scholar]


