Abstract
The small GTPase, Ras-related protein 17 (Rab17), a member of the Rab family, plays a critical role in the regulation of membrane traffic in polarized eukaryotic cells. However, the role of Rab17 in hepatocellular carcinoma (HCC) is not clear. Clinical speciments reveal that Rab17 was present in 15 of 20 (75.0%) paraneoplastic tissues and 7 of 20 (35.0%) HCC samples (P = 0.0248). To elucidate the tumourigenic role of Rab17 in HCC, we generated two Rab17 low-expressing HCC cell lines (Hep3B and Huh-7). The results showed that Rab17 down-regulation significantly promoted the tumourigenic properties of HCC cells in vitro and in vivo, as demonstrated by enhanced cell proliferation, colony formation, invasion and migration, decreased G1 arrest, and increased tumour xenograft growth and angiogenesis. However, the enhanced tumourigenic properties of HCC cells by Rab17 down-regulation was significantly inhibited by PD980592, the inhibitor of the Erk pathway, indicating that the Erk pathway plays a critical role in Rab17 down-regulation-induced enhanced tumourigenic properties of HCC cells. Our data provide a new insight into the essential role of Rab17 in HCC carcinogenesis and suggest that Rab17 expression might be tumor suppressor gene and might provide a new interventional therapeutic target for this common malignancy.
Keywords: Rab17, small GTPase, hepatocellular carcinoma cells, interventional therapy, signaling pathway, cell polarization
Introduction
In humans, hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide [1]. Although HCC accounts for nearly 90% of primary malignant hepatic tumours in adults, the prognosis of HCC is poor, and approximately 40% of the patients are eligible for potential curative treatments (for example, resection, transplantation, or local ablation) and 20% of the patients are eligible for chemoembolization [2-4]. According to the US Centers for Disease Control and Prevention, more than 600,000 new cases of HCC will occur annually, equalling the number of HCC-related deaths, and the 5-year survival rate is less than 9% [5,6]. Most frequently, HCC develops slowly with chronic liver injury, such as chronic hepatitis infection and fibrosis [7-10]. Recently, although HCC interventional therapy has demonstrated breakthroughs in the molecular-targeted therapy and has achieved clinically significant response rates [11], the molecular mechanism of liver tumourigenesis at the genetic level is only beginning to emerge.
Cell polarization, which is a well-known biological process, is involved in a variety of malignant tumours, including HCC. Rab-type small GTPases are conserved membrane trafficking proteins and are spatially and functionally associated with cell polarization. They mediate various steps in membrane trafficking, including apical recycling and transcytosis pathways in polarized epithelial cells. Recent studies have proposed that the Rab GTPase family might correlate with the aggressiveness and tumourigenesis of cancer [12-15].
Rab17 is a recently discovered Rab subfamily of small GTPases. In humans, the predicted location of Rab17 gene is in chromosome 2q32-q37 or 18q21-q22 [16]. Unlike other Rab proteins equally expressed in polarized and nonpolarized cells, Rab17 shows epithelial cell specificity. Northern analysis revealed that Rab17 mRNA is predominantly expressed in the kidney, liver, and intestine but not in organs lacking epithelial cells [17]. In epithelial cells, Rab17 localizes to recycling endosomes and regulates transcellular traffic through apical recycling endosomes [18]. A study by Mori et al demonstrated that Rab17 plays a role in the regulation of dendritic morphogenesis and postsynaptic development of hippocampal neurons [19]. An analysis of the Erk pathway in tumour cell migration has suggested that Rab17 plays an inhibitory role in the invasive behaviour of cancer cells [20]. This conclusion implies that Rab17 may be a tumour suppressor in the aggressiveness and tumourigenesis of cancer.
To date, there are no reports concerning the biological function of Rab17 in HCC or in other human tumours. In the present study, we evaluated the hypothesis that Rab17 plays an inhibitory role in tumourigenic properties of HCC cells via the Erk pathway.
Materials and methods
Patients and tissue samples
Human HCC samples (n = 20) were randomly collected from surgery specimens at the Department of Pathology, the First Hospital Affiliated to the Xinxiang Medical University (Xinxiang, China) between 2013 and 2014. As the matched adjacent non-cancerous tissues (n = 20), paraneoplastic tissues were taken from the non-cancerous tissue 5 cm away from the tumor margin at the same time. All tissue samples were fixed in 10% formalin and embedded in paraffin for immunohistochemical analyses. None of the patients received radiotherapy or chemotherapy prior to surgery. The diagnoses were all verified by pathologists in our hospital. All samples were obtained with informed consent, and the protocol for this study was approved by the ethics committee of the hospital.
Cell culture and generation of stable cell lines
Human HCC cell lines (Huh-7 and Hep3B) were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and were grown in Dulbecco’s minimum essential medium (DMEM) high glucose (Gibco, Grand Island, NY, USA) with 10% foetal bovine serum (FBS) (Hyclone, Logan, UT, USA) at 37°C in 5% CO2 cell culture incubator.
To generate the Rab17 low-expressing stable cell HCC line, the Rab17 shRNA (19-base target site: 5’-TGCTGCGCTCCTGGTTTAT-3’) was cloned into pSliencer 4.1-CMVpuro vector. The cells were transfected with the recombinant plasmid using Effectene Transfection Reagent (Qiagen Inc., Alameda, CA, USA). After 72 h, the cells were selected with 2 µg/ml puromycin.
Real-time polymerase chain reaction (RT-PCR)
The total RNA of cells was extracted with Trizol reagent (Invitrogen, Carlsbad, CA) and the first-strand complementary DNA (cDNA) was rever-sely transcribed from RNA using the Reverse Transcription System kit (Promega, Madison, WI). RT-PCR was performed using a Light Cycler (Roche, Mannhein, Germany). The mRNA expression levels, which were normalized against glyceraldehyde phosphate dehydrogenase (GAPDH), were calculated and expressed as 2-ΔΔCT. The primer sequences were as follows: Rab17 sense, 5’-GTGGGCAACAAGACGGACCTCAG-3’; Rab17 antisense, 5’-CTCGCG GGCCCCTTGTTCAG-3’; GAPDH sense, 5’-GCACCGTCAAGGCTGAGAAC-3’; and GAPDH antisense, 5’-TGGTGAAGAC GCCAGTGGA-3’.
Western blot assay
The cells were lysed on ice with liver homogenization buffer. Protein concentrations were measured using the BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). The protein extracts (50 μg) were separated using SDS-polyacrylamide gel electrophoresis (PAGE) and were transferred onto polyvinylidene fluoride (PVDF) membranes. The following antibodies were used: human Rab17 antibody (final dilution 1:1000) (Sigma), human phospho-Erk1/2 and Erk1/2 antibodies (final dilution 1:1000) (Cell Signaling Technology). Tubulin antibody (final dilution 1:1000) (Cell Signaling Technology) was used as the control. The bands were detected using the enhanced chemiluminescence (ECL) kit (GE Healthcare) and were visualized using the ChemiDoc XRS system (Bio-Rad).
Cell proliferation assay
The cells were plated into 96-well microplates (103 cells/well). Cell proliferation was detected using cell proliferation kit Ι (MTT) (Roche Diagnostics Corporation, Indianapolis, IN) according to standard procedures. The absorbance value (A) at 570 nm was read using the Benchmark Microplate Reader (Bio-Rad Laboratories, Hercules, CA, USA).
Colony-forming assay
The colony-forming assay was modified as previously described [21]. The cells were seeded onto a 10-cm dish (3 × 102 cells) and cultured in a 37°C, 5% CO2 incubator and for 14 days, were fixed with cold methanol for 10 min, and were subsequently stained using crystal violet.
Transwell migration assay
The transwell migration assays were performed with 6.5-mm diameter cell culture inserts (8-µm pore size) (Corning, Life Sciences, NY, USA) in 24-well culture plates. The transwell inserts were coated with Matrigel (80 μg/well) (BD Biosciences, MD, USA) for the invasion assays. The cells (5 × 104 per well) were added to the upper chamber in 200-µl serum-free DMEM high-glucose medium with 0.1% BSA. The cells were subsequently placed into 24-well plates in DMEM high-glucose medium with 10% FBS. After a 24-h incubation, the non-migrated cells were removed from the upper surface using cotton tips; the cells that had migrated to the lower surface were fixed by cold methanol, were stained using crystal violet, and were microscopically counted.
Scratch-wound assays
The migration ability of HCC cells was evaluated using scratch-wound assays. The cells were seeded onto 6-well plates (5 × 105 per well) after transfection. Twenty-eight hours after seeding, the cell monolayer was wounded by manually scratching with a pipette tip. The exfoliated cells were removed by washing them with PBS. After washing, fresh medium was added, and the cells were incubated for 24 hours. Migration activity was calculated as the percentage of wound closure using the initial scratch width as 100%. The experiments were performed three times.
Xenograft model
For xenograft experiments, nude mice (5- to 6-week old; male) were received with ultrasound guided subcutaneous injections of 1 × 107 cells in the flank area in a volume of 100 µl PBS. The tumour volume was measured every week, and the mice were sacrificed when the max tumour was approximately 4 cm3. The volume of tumours was determined using the following formula: volume = (length × width2)/2.
Immunohistochemistry
Immunohistochemistry was performed as previously described [22]. Briefly, paraffin-embedded tissue sections were stained with Rab17 polyclonal antibodies (BD Biosciences, San Jose, CA, USA) and CD34 monoclonal antibody (Santa Cruz, CA, USA), followed by incubation with FITC-labeled goat anti-mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) as a secondary antibody.
Microvascular density counting
CD34 positive endothelial cells were counted using Weidner’s method [23]. Any CD34 positive endothelial cell or endothelial cell cluster clearly separated from adjacent microvessels. Three separate sections were screened and five areas with most intense neovascularization (where the highest number of discrete microvessels was stained) in each sample were chosen in low magnifications (100 ×) under a light microscope (Leica, Germany). Subsequently, microvessels were counted in each section in high magnification (200 ×). Final counts were expressed as the average of all the three sections examined.
Cell cycle analysis
For cell cycle assays, the cells were seeded at a density of 1 × 105 cells on a 6-cm dish and were synchronized in G1-phase by serum starvation for 12 h. After a 24 h incubation, the cells were washed with cold PBS and were fixed with 70% cold alcohol at 4°C overnight. Subsequently, the fixed cells were collected, washed using PBS, and stained using propidium iodide (PI) (Sigma, St. Louis, MO, USA) in the presence of RNAase (Sigma). Flow cytometry (FCM) (Becton Dickinson, San Jose, CA, USA) analysis was used to determine the cell cycle of the cells. The cell cycle was analysed using ModFit software.
Apoptosis assay
To evaluate cell apoptosis, flow cytometry was performed using the Annexin-V fluorescein isothiocyanate (FITC) Early Apotosis Detection Kit (Cell Signaling Technology, Boston, MA, USA). The cells were washed and resuspended with the binding buffer, followed by incubation using the Annexin-V FITC and PI buffers for 15 min at 4°C in the dark, and were subsequently analysed using flow cytometry.
Statistical analysis
SPSS 17.0 software (SPSS, Chicago, IL, USA) was used to perform the statistical analysis. The data are presented as the mean ± standard error of the mean (SEM) for at minimum three repeated individual experiments for each group. The significant differences were determined using ANOVA and Student’s t-test. Statistical significance was accepted at the level of P < 0.05.
Results
Down-regulation of Rab17 promotes tumourigenic properties of HCC cells
To investigate the characteristics of Rab17 protein expression in paraneoplastic tissues and HCC, 20 paraneoplastic tissues samples and HCC samples were immunohistochemically analyzed. We found that Rab17 was present in 15 of 20 (75.0%) paraneoplastic tissues and 7 of 20 (35.0%) HCC samples (P = 0.0248) (Figure 1A). Next, we generated two Rab17 low-expressing HCC cell lines (Hep3B and Huh-7), and the knockdown efficiencies of Rab17 in HCC cell lines were first assessed using RT-PCR. The Rab17 low-expressing cells showed a 36.4% reduction of Rab17 mRNA levels in Hep3B cells and a 44.9% reduction in Huh-7 cells compared with control cells (Figure 1B). Moreover, western blot demonstrated that Rab17 shRNA transfection significantly decreased the Rab17 protein expression in both cell lines (Figure 1C).
Figure 1.
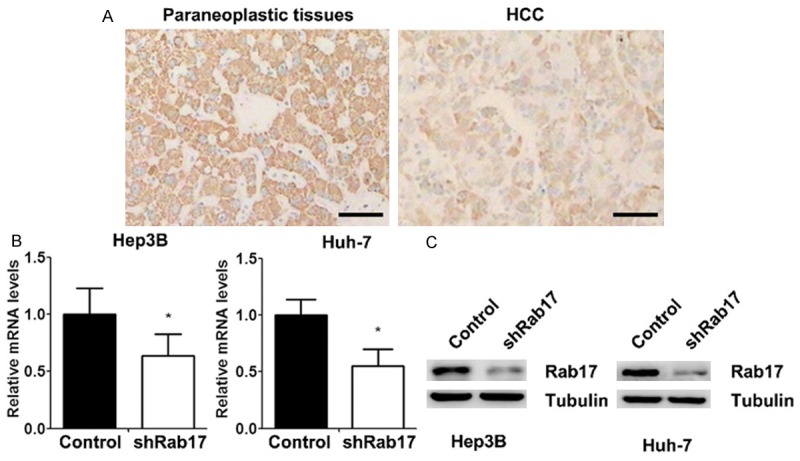
Generation of Rab17 low-expressing HCC cell lines (Hep3B and Huh-7). A. Expression of Rab17 in paraneoplastic tissues and HCC revealed by immunohistochemistry (scale bar = 100 μm). B. The mRNA expressions of Rab17 were measured using RT-PCR in HCC cells. GAPDH was used as an internal reference. C. Western blot analysis verified the knockdown of Rab17 in HCC cells. Tubulin was analysed as a loading control. The data represent the mean ± SEM values; *P < 0.05 vs. control; n = 5. Control, control cells. shRab17, Rab17 low-expressing cells.
We examined the effect of Rab17 down-regulation on the tumourigenic properties of HCC cells. The MTT assay showed that the knockdown of Rab17 significantly accelerated the growth of cells, which increased with time (Figure 2A). Similarly, the ability of single cells to form colonies was increased by 64.1% in Hep3B cells and 63.1% in Huh-7 cells (Figure 2B, 2C). The invasive number of cells was boosted on the down-regulation of Rab17 in the chamber of the transwell plate, which correlated with 60.1% enhancement in Hep3B cells and 83.1% enhancement in Huh-7 cells (Figure 2D, 2E). Additionally, the knockdown of Rab17 improved the migratory ability of HCC cells by 66.9% in Hep3B cells and 60.7% in Huh-7 cells (Figure 2F, 2G). These results validated that Rab17 is important to the biological function of HCC and that the knockdown Rab17 promotes the tumourigenic properties in vitro.
Figure 2.
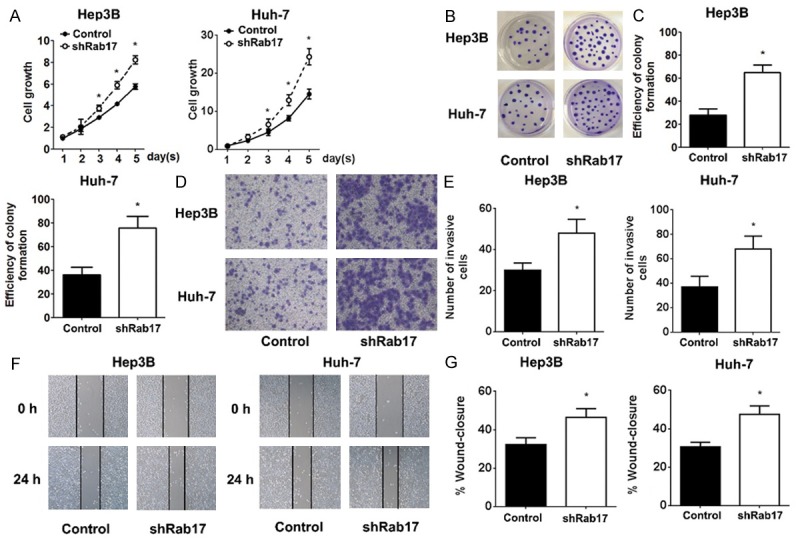
Effect of Rab17 down-regulation on tumourigenic properties in HCC cells in vitro. A. Cell growth was monitored each day using MTT assay. Absorbance on day 1 was assigned a value of 1. B and C. Images and quantification of the number of colonies formed of indicated HCC cells. D. Representative images of transmembrane cells of indicated HCC cells. E. Quantification of the number of transmembrane cells by counting 5 high-power fields of each chamber. F. Representative images were taken at 0 and 24 h to assess the cell migration into the open space. G. Quantification of the percentage of the distance migrated was achieved by measuring these distances in 5 high-power fields. The data represent the mean ± SEM values; *P < 0.05 vs. control; n = 5.
Knockdown of Rab17 reduces the cell cycle G1 and accelerates tumour growth in vivo
To further elucidate the anti-tumourigenic mechanism of Rab17 in HCC cells, we analysed the cell cycle and cell apoptosis. As shown in Figure 3A, knockdown of Rab17 resulted in a significant decrease of cells in G1 phase (from 57.9% to 42.4% in Hep3B cells and from 50.6% to 37.6% in and Huh-7 cells), an obvious increase of cells in S phase (from 34.5% to 49.4% in Hep3B cells and from 42.7% to 55.4% in and Huh-7 cells), compared with controls. However, the apoptosis rates showed no significant difference after the down-regulation of Rab17 expression (Figure 3B).
Figure 3.
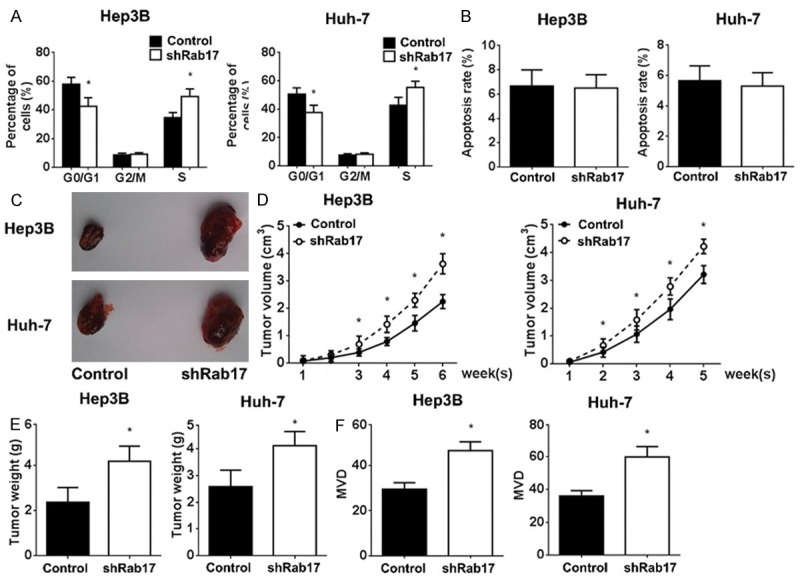
Knockdown of Rab17 decreased G1 phase and accelerated tumour growth in vivo. A and B. The cell cycle and apoptosis were analysed using flow cytometry. The indicated percentages are the average of three independent experiments. The Rab17 low-expressing and control HCC cells were inoculated in nude mice, and tumour volume was monitored. The mice were sacrificed at the indicated time point, and the tumour weight was evaluated. C. Macroscopic xenograft tumours in nude mice by subcutaneously injecting into the HCC cells. D. Growth curves of xenografts generated by Control and shRab17 cells. Tumour volume was measured each week. E. Xenografts weight was measured when the mice were sacrificed. F. The paraffin sections of the excised tumours were observed under microscope and MVD was counted using Weidner’s method. The results represent the mean ± SEM values; *P < 0.05 vs. control; n = 5.
The effect of Rab17 down-regulation on the tumour growth was evaluated by HCC-bearing nude mouse model in vivo. In this study, the Rab17 low-expressing and control cells were subcutaneously injected in the flanks of nude mice. We found that the knockdown of Rab17 could significantly accelerate the growth of tumour compared with controls, which increased with time (Figure 3C-E). Moreover, down-regulation of Rab17 revealed the most significant enhanced tumour angiogenesis activity, as reflected by the MVD (Figure 3F).
Inhibition of Erk pathway can reverse the enhanced tumourigenic properties of silencing Rab17
The Erk pathway plays a critical role in tumour cell migration, invasion, and progression. The western blot analysis demonstrated that the knockdown of Rab17 could markedly elevate the phosphorylation of Erk protein (Figure 4A). To determine whether the enhanced tumourigenic properties by Rab17 down-regulation was associated with the Erk pathway, Rab17 low-expressing cells were treated with the Erk pathway inhibitor PD980592, and simultaneously the tumourigenic properties were evaluated. The growth of the Rab17 low-expressing cells was greatly inhibited after PD980592 treatment, and there was no significant difference between Rab17 low-expressing cells treated with PD980592 and the control cells (Figure 4B). Similar results were obtained in the colony-forming, transwell migration, scratch-wound, cell cycle, xerograft weight and MVD assay (Figure 4C-H). Collectively, the enhanced tumourigenic properties by the Rab17 down-regulation is mediated by the Erk pathway, and inhibition of the Erk pathway can reverse the enhanced tumourigenic properties by the Rab17 down-regulation.
Figure 4.
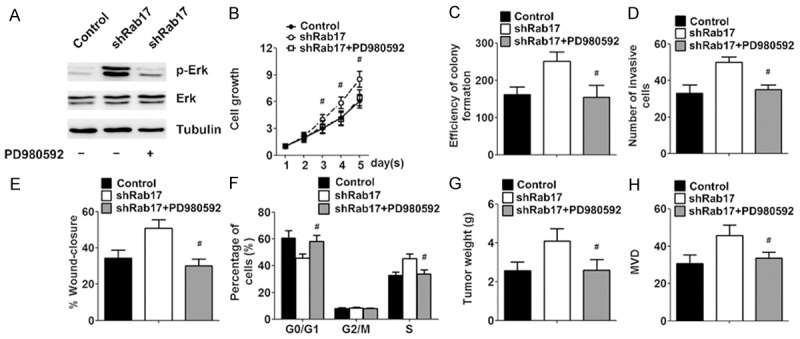
Tumuorigenicity promotive property of silencing Rab17 could be reversed by PD980592. The indicated Huh-7 cells were pretreated with saline or PD980592 (10 μM). A. Western blot analysis of p-Erk and Erk after PD980592 treatment with tubulin as loading control. B. Growth curves of indicated cells treated with medication, as determined by MTT assay. Absorbance on day 1 was assigned a value of 1. C-E. The quantification of colony formation, invasion and migration after PD980592 treatment. F. The cell cycle progression after PD980592 treatment. For xenograft model assay, at one weeks after tumour inoculation, the mice were treated with a negative control (saline), PD980592 (10 μg/kg intraperitoneally, once a week). G. Xenografts weight was measured when the mice were sacrificed. H. MVD of the excised tumours was counted under microscope. The data represent the mean ± SEM values; #P < 0.05 vs. shRab17; n = 5.
Discussion
The prevalence and mortality rate of HCC are expected to increase worldwide in the next decade. Accordingly, novel therapeutic approaches, such as molecular-targeted therapies, are urgently required to efficiently treat HCC. There was no effective molecular-targeted medication to treat patients with advanced HCC until sorafenib emerged, which represents a breakthrough in the management of this type of carcinoma [11,24]. However, the mechanisms underlying the initiation and progression of HCC remain obscure. Furthermore, the most ambitious approach would be to test novel agents in genetically engineered mice, which may give a rise to specific pathway abnormalities in animals with an underlying risk [25,26].
Membrane polarity plays a vital role in regulating the physiological function of hepatocytes. Loss of membrane polarity has been implicated in the genesis of several types of cancer derived from multiple tissue types, including HCC [27]. Rab GTPases play a critical role in regulating intercellular vesicle trafficking, signal transduction, and receptor recycling, which in turn regulate normal membrane polarity [13]. Recent studies have shown that dysregulation of Rab gene expression and associated regulatory signalling pathway may constitute a generalized component of human HCC [15]. Additionally, the Rab GTPase family, such as Rab23, Rab27A/B, and Rab18, exhibits an elevated expression in HCC, suggesting that Rab GTPases could play an important tumourigenic role in HCC [15,28,29].
Rab17, a small GTPase, is specific for polarised epithelial cells and participates in the regulation of membrane trafficking and transcytosis. Nevertheless, Rab17 is seldom involved in the research of oncobiology, and its role in tumour remains elusive. Our study is the first to reveal decreased expression of Rab17 in HCC. The down-regulation of Rab17 in HCC cells could facilitate all tumourigenic characteristics of HCC cells in vitro or in vivo, such as proliferation, clonogenic survival, invasion, migration and the ability to form tumours in nude mice. The fact that Rab17 exhibited an inhibitory effect on tumourigenic properties was unexpected. Our results are not supportive of the previous findings that Rab GTPases were overexpressed and activated in tumours. However, other evidence is consistent with our viewpoint, implying that Rab17 plays a depressant role in the invasive behaviour of cancer cells and suggesting that Rab17 can be an integrin recycling suppressor whereby the expression must be reduced for cells to efficiently migrate [20]. The present study indicated that Rab17 was associated with the maintenance of a polarized epithelial morphology and that its loss exhibited a drastic invasive capacity in HCC.
We found that the knockdown of Rab17 motivated the activation of the Erk pathway and that the promotive effect on HCC could be reversed by interdiction of the Erk pathway. Recently, much evidence has supported roles for the Erk pathway, cell cycle progression, invasion, and metastasis [30-33]. A recent study has provided direct evidence that the Erk pathway associates with endocytic vesicles and mediates their function by facilitating the translocation of vesicles to the nucleus, implicating Rab GTPases in conducting cell survival signals [34]. It is clear from our study that Rab17 influences hepatoma through the Erk pathway.
Altogether, our results shed light on the function of Rab17 in HCC cells. Identifying Rab17 as essential, small, GTPase-regulated tumourigenic properties of HCC relied on the Erk pathway, which would help to unravel the esoteric link between the Erk pathway and HCC, and proved that Rab17 was a potential tumour suppressor gene in hepatocytes. Manipulating Rab17 might motivate the development of effective interventional therapeutic targets for a subset of HCC.
Disclosure of conflict of interest
None.
References
- 1.Berasain C, Perugorria MJ, Latasa MU, Castillo J, Goni S, Santamaria M, Prieto J, Avila MA. The epidermal growth factor receptor: a link between inflammation and liver cancer. Exp Biol Med (Maywood) 2009;234:713–725. doi: 10.3181/0901-MR-12. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 5.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, screening. Semin Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 7.Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. doi: 10.1038/sj.onc.1209559. [DOI] [PubMed] [Google Scholar]
- 8.Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007;13:74–81. doi: 10.3748/wjg.v13.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markiewski MM, DeAngelis RA, Lambris JD. Liver inflammation and regeneration: two distinct biological phenomena or parallel pathophysiologic processes? Mol Immunol. 2006;43:45–56. doi: 10.1016/j.molimm.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R, Jurisica I, Mills GB, Cheng KW. The emerging role of the RAB25 small GTPase in cancer. Traffic. 2009;10:1561–1568. doi: 10.1111/j.1600-0854.2009.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–2519. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 14.Mitra S, Cheng KW, Mills GB. Rab25 in cancer: a brief update. Biochem Soc Trans. 2012;40:1404–1408. doi: 10.1042/BST20120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YJ, Wang Q, Li W, Huang XH, Zhen MC, Huang SH, Chen LZ, Xue L, Zhang HW. Rab23 is a potential biological target for treating hepatocellular carcinoma. World J Gastroenterol. 2007;13:1010–1017. doi: 10.3748/wjg.v13.i7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurtrie EB, Barbosa MD, Zerial M, Kingsmore SF. Rab17 and rab18, small GTPases with specificity for polarized epithelial cells: genetic mapping in the mouse. Genomics. 1997;45:623–625. doi: 10.1006/geno.1997.4959. [DOI] [PubMed] [Google Scholar]
- 17.Lutcke A, Jansson S, Parton RG, Chavrier P, Valencia A, Huber LA, Lehtonen E, Zerial M. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J Cell Biol. 1993;121:553–564. doi: 10.1083/jcb.121.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunziker W, Peters PJ. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- 19.Mori Y, Matsui T, Furutani Y, Yoshihara Y, Fukuda M. Small GTPase Rab17 regulates dendritic morphogenesis and postsynaptic development of hippocampal neurons. J Biol Chem. 2012;287:8963–8973. doi: 10.1074/jbc.M111.314385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Thun A, Birtwistle M, Kalna G, Grindlay J, Strachan D, Kolch W, von Kriegsheim A, Norman JC. ERK2 drives tumour cell migration in three-dimensional microenvironments by suppressing expression of Rab17 and liprin-beta2. J Cell Sci. 2012;125:1465–1477. doi: 10.1242/jcs.092916. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Zhang X, Bai CX, Chen J, Wei MQ. Inhibition of epidermal growth factor receptor expression by RNA interference in A549 cells. Acta Pharmacol Sin. 2004;25:61–67. [PubMed] [Google Scholar]
- 22.Wang Q, Tan YX, Ren YB, Dong LW, Xie ZF, Tang L, Cao D, Zhang WP, Hu HP, Wang HY. Zinc finger protein ZBTB20 expression is increased in hepatocellular carcinoma and associated with poor prognosis. BMC Cancer. 2011;11:271. doi: 10.1186/1471-2407-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 24.Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2005;21:308–312. doi: 10.1097/01.mog.0000159817.55661.ca. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 26.Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, Odell MM, Bauer RL, Ren HP, Haugen HS, Yeh MM, Fausto N. Platelet-derived growth factor C induces liver fibrosis, steatosis and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espelt MV, Croci DO, Bacigalupo ML, Carabias P, Manzi M, Elola MT, Munoz MC, Dominici FP, Wolfenstein-Todel C, Rabinovich GA, Troncoso MF. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization and in vivo tumor growth. Hepatology. 2011;53:2097–2106. doi: 10.1002/hep.24294. [DOI] [PubMed] [Google Scholar]
- 28.Dong WW, Mou Q, Chen J, Cui JT, Li WM, Xiao WH. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol. 2012;18:1806–1813. doi: 10.3748/wjg.v18.i15.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You X, Liu F, Zhang T, Li Y, Ye L, Zhang X. Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatoma cells. Carcinogenesis. 2013;34:1644–1652. doi: 10.1093/carcin/bgt089. [DOI] [PubMed] [Google Scholar]
- 30.Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, Itohara S, Takishima K. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol. 2006;5:14. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- 33.Ueoka Y, Kato K, Kuriaki Y, Horiuchi S, Terao Y, Nishida J, Ueno H, Wake N. Hepatocyte growth factor modulates motility and invasiveness of ovarian carcinomas via Ras-mediated pathway. Br J Cancer. 2000;82:891–899. doi: 10.1054/bjoc.1999.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]


