Abstract
Background: E6 and E7 of high risk human papillomavirus 16 (HPV16) were reported to correlate with the cervical cancer (CC). And the presence of matrix metalloproteinases (MMPs) has also been indicated to be associated with CC. Methods: The present study investigated the expression of MMPs (MT1-MMP, MMP-2 and MMP-9) in CC cells with HPV16-E6/E7 oncoprotein(s) negative or positive, and then determined the regulation of HPV16-E6/E7 oncoproteins on the expression of MMPs (MT1-MMP, MMP-2 and MMP-9) and the migration of cervical cancer Caski and SiHa cells with RNAi technology. Results: It was demonstrated that the overexpression or the knockdown of HPV16-E6/E7 promoted or reduced MT1-MMP, MMP-2 and MMP-9 in CC cells. And the HPV16-E6, -E7 or -E6E7 influenced the migration of CC cells. The overexpression or the knockdown of them promoted or inhibited the migration of C33A or Caski/SiHa cells. Moreover, the chemical inhibition of MMP-2 or MMP-9 significantly reduced the migration of CC Caski or SiHa cells. Conclusions: Our results demonstrated that the E6-HPV16 or E7-HPV16 promoted the activity of MMP-2/9, and contributed to the migration of cervical cells.
Keywords: HPV-16E6/E7, matrix metalloproteinases, migration, cervical cancer
Introduction
Cervical cancer (CC) is one of main cause of cancer-related death in women [1] and the second most common cancer among women worldwide [2], and the persistent infection with high-risk human papillomaviruses (HPVs) accounts for more than 50% of cervical cancer cases worldwide [3]. The viral oncoprotein, E6 or E7 of HPV16 interacts with such cellular proteins as the cell cycle protein pRb [4] and Forkhead box M1b (FoxM1b) [5], regulates the cell cycle and causes cell transformation and immortalization [6]. The HPV16E7 binds to pRb and promotes the degradation of pRb via a proteasome-mediated process [4], or promotes cellular proliferation by interacting with the DREAM (DP, RB-like, E2F and MuvB) complex at two distinct phases of the cell cycle [7]. HPV16E6 oncoprotein promotes the Cyclin-dependent kinase (Cdk1 and Cdk 2), degrades the tumor suppressor p53 and abrogates cell cycle checkpoints [8,9]. It also up-regulates FOXM1 through the myeloid zinc finger 1/NK2 homeobox 1 (MZF1/NKX2-1) axis which is required for HPV-associated tumorigenesis [10].
Matrix metalloproteinases (MMPs) belong to a family of zinc-dependent proteases responsible for degradation of extracellular matrix that is required for migration and metastasis of cancer cells [11]. The presence of MMPs has indicated to be associated with the infection of HPV in cervical cancers [12,13]. Quite a few of MMPs, such as MMP-2, MMP-7, MMP-9, and MMP-10 have been reported in invasive cervical carcinomas [14,15]. However, only MMP-2 [16] and MMP-9 [17] are shown to correlate with poor prognosis in patients. And the correlation between HPV oncoproteins and MMPs in cervical cancers remains to be further identified. Far more recently, both MMP-2 and membrane type 1-MMP (MT1-MMP) were found active in squamous cell carcinomas of cervix [15]. And what’s more, MT1-MMP activated pro-MMP-2 in melanoma cells [18] and other tumor cells [19].
In the present study, we investigated the regulation of E6/E7 oncoproteins from high risk HPV16 on the expression of MMPs (MT1-MMP, MMP-2 and MMP-9) and the migration of cervical cancer cells. And then we examined the role of the upregulated MT1-MMP, MMP-2 and MMP-9 in the migration promotion by the E6/E7 oncoproteins of HPV16 in cervical cancer cell lines.
Materials and methods
Cells, reagents, cell culture and treatment
Cervical cancer Caski and SiHa cells, both of which were E6-, E7 and E6E7-positive, and cervical cancer C33A cells were purchased from the American type culture collection (ATCC, Rockville, MD, USA). The three kinds of cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% Fetal bovine serum (FBS, GIBCO, Rockville, MD, USA) and 1% penicillin-streptomycin cocktail (Sigma Aldrich, St. Louis, MO, USA), at 37°C in a humidified atmosphere of 5% CO2. To overexpress the E6 or E7 of HPV16 in C33A cells, the E6 or E7 cDNA sequences, which were amplified from the SiHa cells and were cloned into the eukaryotic expression vector, pcDNA3.1 (+). And the recombinant E6-pcDNA3.1 (+), E7-pcDNA3.1 (+) or pcDNA3.1 (+) control plasmid was transfected into C33A cells with lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). 12, 24 or 36 hours post transfection, cells were respectively subject to cellular mRNA analysis, western blot analysis or migration assay. To knockdown the E6 or E7 in Caski or SiHa cells, we performed the lentivirus-mediated shRNA knockdown of E6 or E7 expression. The shRNAs targeting E6 or E7 were designed [20] by the ambion online program (http://www.ambion.com), with a nonsense sequence as control. The shRNA-E6, shRNA-E7, or shRNA-Ctrl sequences were confirmed by BLAST to targeted sequence. Lentiviruses were generated in HEK293T cells by co-transfection of each recombinant E6- or E7-pRNAT-U6.1 plasmid with pHelper plasmid. Viral titer was determined by quantitatively counting the numbers of GFP-positive cells post virus infection, under fluorescence microscope (Olympus, Tokyo, Japan). Caski or SiHa cells with 85% confluence were transducted with shRNA-harboring lentivirus at a multiplicity of infection (MOI) of 10, and 12 or 24 hours later, cells were propagated for mRNA analysis or western blot analysis. MMP-2 Inhibitor I (sc-204092, 5 μM) and MMP-9 Inhibitor I (sc-311437, 5 μM) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were utilized to inhibit the activity of MMP2, MMP3 and MMP9, respectively in Caski or SiHa cells, 12 hours before mRNA analysis, or 24 hours before western blot analysis or MMP-2/9 activity assay.
RNA extraction and quantitative real-time polymerase chain reaction
Total cellular mRNA from the cervical cancer cell lines was extracted using Trizzol agents (Thermo Fisher Scientific, Waltham, MA, USA) according to the manual, and was supplemented with Rnase inhibitor (TaKaRa Bio Inc., Tokyo, Japan). Real-time quantitative PCR (RT-qPCR) with the SYBR PrimeScript RT-qPCR Kit (TaKaRa Bio Inc., Tokyo, Japan) was performed to analyze the mRNA of MT1-MMP, MMP-2/9 or HPV16E6/E7, with β-actin as an internal control. Primers were synthesized by Yuanshen Bio-tech (Wuhan, China). The RT-qPCR was performed with a Lightcycler 480 II (Roche, Mannheim, Germany). All data were normalized to β-actin and expressed as the fold change over control and calculated with the ∆∆Ct method [21].
Western blot analysis
The cultured cervical cancer cells post various treatment were collected and lysed with an ice-cold lysis buffer (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s guidance, and were supplemented with a protease inhibitor cocktail (Pierce, Rockford, IL, USA). Protein samples were separated by 10 or 12% SDS-PAGE gel, and were transferred to a nitrocellulose membrane. The target protein was detected, via successive inoculation with anti-MT1-MMP, MMP-2, MMP-9 (all three antibodies were from Thermo Fisher Scientific, Rockford, IL, USA), anti-HPV16E6, anti-HPV16E7 (both were from Abbiotec, San Diego, CA, USA) or anti-β-actin antibody (Sino Biological, Beijing, China), a peroxidase-conjugated secondary antibody (Pierce, Rockford, IL, USA) and the electro-chemo-luminescence (ECL) detection system (Amersham, Uppsala, Sweden).
Migration assay
The wound healing assay was performed to investigate the migration of cervical cancer cells. C33A, Caski or SiHa cells with 85% confluence on the 12-well plates post the above-mentioned treatment were scratched with a scraper. 36 hours post scratching, the cellular growth was observed, and numbers of invading cells across the baseline were counted.
MMP-2/9 activity assay, ELISA assay for supernatant MMP-2/9
The MMP-2/9 activity was assayed with a QuickZyme Human MMP-2 activity assay (QuickZyme BioSciences, CE Leiden, Netherlands). The cervical cancer lyzed cellular samples were pipetted into the pre-coated plate, and then human MMP-2 or MMP-9 presenting in the sample was captured by the MMP-2- or MMP-9-specific antibody. After washing, the pro-detection enzyme which was activated by the active MMP-2 into an active detection enzyme was added each well to cleave the chromogenic substrate, resulting in generation of a yellow color that can be measured at 405 nm using a plate reader. The MMP-2 or MMP-9 in the supernatant of cervical cancer cells were assayed with MMP-2 or MMP-9 ELISA kits (Amersham Pharmacia Biotech, Orsay, France) according to the manufacturer’s instructions. And the value for MMP-2 or MMP-9 was read by a spectrophotometer at 405 nm.
Results
Overexpression of HPV16 E6/E7 oncoproteins promoted MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells
To investigate the association of MT1-MMP, MMP-2 or MMP-9 upregulation with the HPV16 E6/E7 in cervical cancers, we examined the level of MT1-MMP, MMP-2 and MMP-9 in the HPV16 E6/E7-positive cervical cancer cell lines, Caski and SiHa cells, with C33A cells as control. As shown in Figure 1A, the mRNA level of MT1-MMP, MMP-2 or MMP-9 was significantly higher in both Caski and SiHa cells than in the HPV16 E6/E7-negative C33A cells (P<0.01 or P<0.001). And the western blot results confirmed the upregulation of MT1-MMP, MMP-2 and MMP-9 at protein level in both Caski and SiHa cells, than in C33A cells (Figure 1B; P<0.05, P<0.01 or P<0.001). To reconfirm such association of MMPs upregulation with the HPV16 E6/E7 in cervical cancers, we overexpressed the E6, E7 or E6E7 of HPV16 with an eukaryotic expression vector, pcDNA3.1 (+) in C33A cells, and examined the promotion to MMPs at both mRNA and protein levels. It was indicated that the E6-HPV16-pcDNA3.1 (+) (abbreviated to E6-HPV16), E7-HPV16-pcDNA3.1 (+) (abbreviated to E7-HPV16), or the E6E7-HPV16-pcDNA3.1 (+) (abbreviated to E6E7-HPV16) significantly promoted the E6, E7 or E6E7 at both mRNA (either P<0.01; Figure 1C) and protein (either P<0.001; Figure 1D and 1E) levels in C33A cells. And what’s more, the western blot analysis demonstrated that the promotion to E6, E7 or E6E7 significantly upregulated MT1-MMP, MMP-2 and MMP-9 at protein level (P<0.05 or P<0.01; Figure 1D and 1F). Thus, we confirmed that the E6, E7 or E6E7 oncoprotein of HPV16 promoted the MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells.
Figure 1.
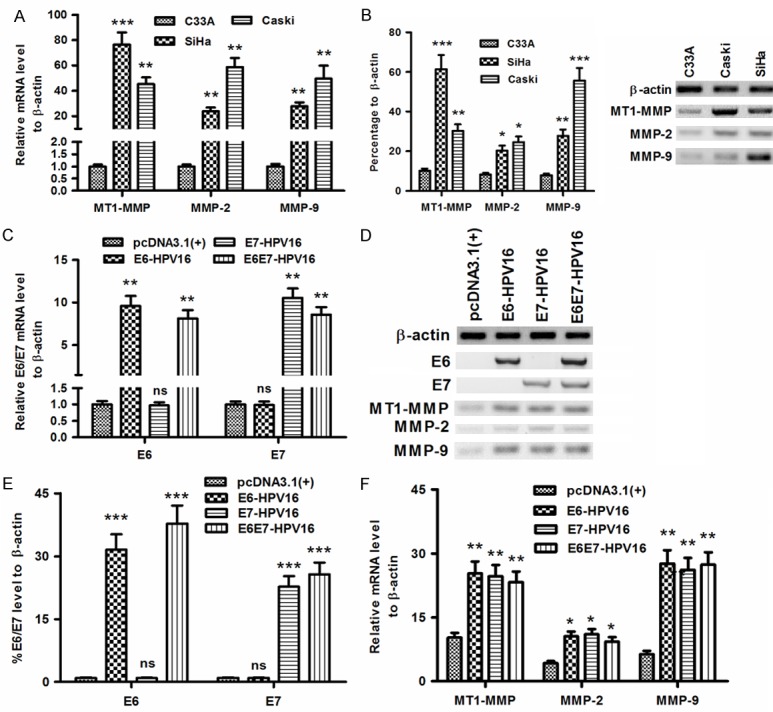
The level of MT1-MMP, MMP-2 and MMP-9 in HPV16E6/E7-positive or HPV16E6/E7-overexpressed cervical cancer cells. (A and B) MT1-MMP, MMP-2 and MMP-9 in C33A, Caski or SiHa cells at mRNA (A) or protein (B) level; (C-E) The mRNA (C) or the protein (D and E) level of E6, E7, or E6E7 in the C33A cells which were transfected with E6-HPV16-pcDNA3.1 (+), E7-HPV16-pcDNA3.1 (+), or E6E7-HPV16-pcDNA3.1 (+); (D) and F: MT1-MMP, MMP-2 and MMP-9 at protein (D) or mRNA (F) level in the C33a cells which were transfected with E6-HPV16-pcDNA3.1 (+), E7-HPV16-pcDNA3.1 (+), or E6E7-HPV16-pcDNA3.1 (+). All results were representative of three independent experiments, *P<0.05, **P<0.01, ***P<0.001, ns no significance.
Knockdown of HPV16 E6/E7 oncoproteins reduced MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells
To reconfirm the promotion by the E6, E7 or E6E7 oncoprotein of HPV16 to the MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells, we abrogated the expression of E6-HPV16 or E7-HPV16 in SiHa cells with lentivirus-mediated shRNA. The recombinant lentivirus (Lenti-shRNA-E6 or Lenti-shRNA-E7) was packed by tranfecting the recombinant plasmid containing E6- or E7-specific shRNA sequences in HEK293T cells. Then the SiHa cells were infected with the lentivirus (Lenti-shRNA-E6, Lenti-shRNA-E7 or Lenti-shRNA-Ctrl). Compared to the control shRNA group, the E6- or E7-specific shRNA (shRNA E6 or shRNA E7) significantly downregulated the mRNA level of E6 or E7 for 47 or 56 %, downregulated the mRNA level of E6E7 for 64% (either P<0.05; Figure 2A). And the expression of E6 or E7 at protein was also downregulated from 30.23 ± 3.63% to 13.44 ± 1.35% (for E6), or from 14.16 ± 1.58% to 6.47 ± 0.71% (for E7) (P<0.05 or P<0.01; Figure 2B). Moreover, the expression of MT1-MMP, MMP-2 and MMP-9 was also regulated by the shRNA E6 or shRNA E7. Figure 2C indicated that all the three MMPs were downregulated at mRNA levels post the E6- or E7-knockdown. Surprisingly, only MT1-MMP and MMP-9 were downregulated at protein level within the Lenti-shRNA-E6- or Lenti-shRNA-E7-infected SiHa cells (P<0.05 or P<0.01; Figure 2D), the downregulation of MMP-2 was not significant (Figure 2D). In addition, the MMP-2 or MMP-9 level in the supernatant of the Lenti-shRNA-E6- or Lenti-shRNA-E7-infected SiHa cells was significantly downregulated (either P<0.05; Figure 2E). Thus, we confirmed that the knockdown of HPV16 E6/E7 oncoproteins reduced MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells.
Figure 2.
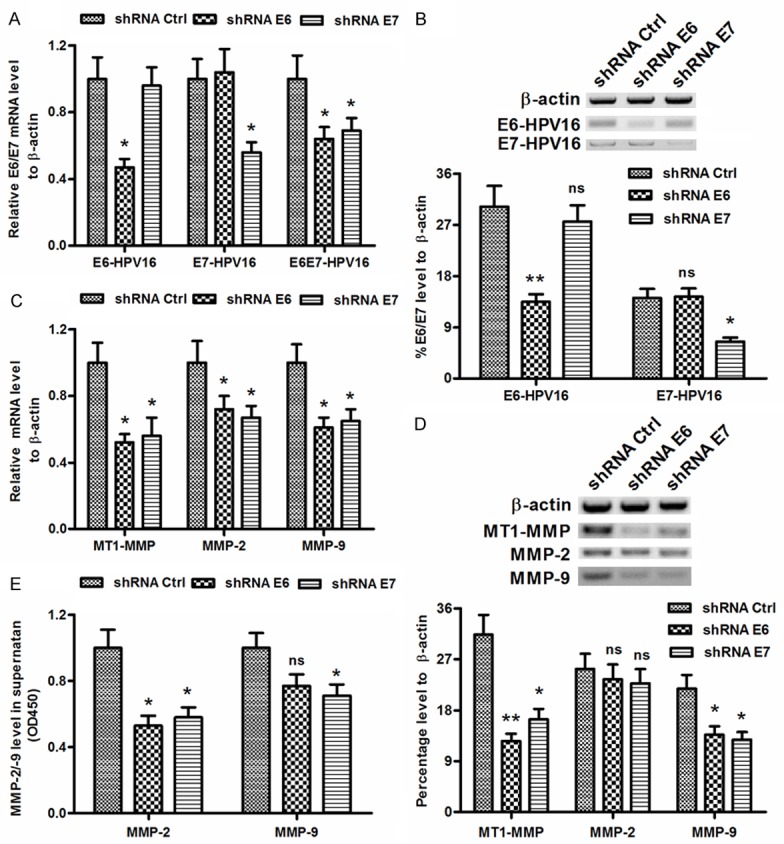
HPV16E6/E7 knockdown by shRNAs reduced MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells. (A and B) The mRNA (A) or the protein (B) level of E6 and E7 in the SiHa cells which were infected with Lenti-shRNA-E6, Lenti-shRNA-E7 or Lenti-shRNA-Ctrl. (C and D) The mRNA (C) or the protein (D) level of MT1-MMP, MMP-2 and MMP-9 in the SiHa cells which were infected with Lenti-shRNA-E6, Lenti-shRNA-E7 or Lenti-shRNA-Ctrl. (E) The MMP-2 and MMP-9 level in the supernatant of SiHa cells post the infection with Lenti-shRNA-E6, Lenti-shRNA-E7 or Lenti-shRNA-Ctrl. Each value was averaged for three independent experiments, *P<0.05, **P<0.01, ns no significance.
Manipulated HPV16 E6/E7 levels correlated with the migration of cervical cancer cells
To further identify the pro-oncogenic role of E6-, E7- or E6E7-HPV16 in cervical cancers, we then investigated the influence of the above-mentioned oncoproteins on the migration of cervical C33A cells, by a wound healing assay. As shown in Figure 3A, there were more cells invading the baseline in the E6-, E7- or E6E7-overexpressed cells, compared to the pcDNA3.1 (+) control cells, post an inoculation of 36 h (P<0.01; Figure 3B). On the other side, we examined the migration of Caski or SiHa cells, post the knockdown of E6-HPV16 or E7-HPV16 with the lentivirus-mediated shRNA. The E6 or E7 knockdown with shRNA E6 or shRNA E7 significantly inhibited the migration of Caski cells, compared to the control shRNA (P<0.05; Figure 3C). We also repeated the migration inhibiting experiment in SiHa cells. As shown in Figure 3C, there were less migratory cells in the E6- or E7-abrogated SiHa cells, compared to the SiHa cells post the transduction with control shRNA (P<0.05). All these results revealed that the knockdown of E6-HPV16 or E7-HPV16 by shRNA reduced the migration of cervical cancer cells in vitro.
Figure 3.
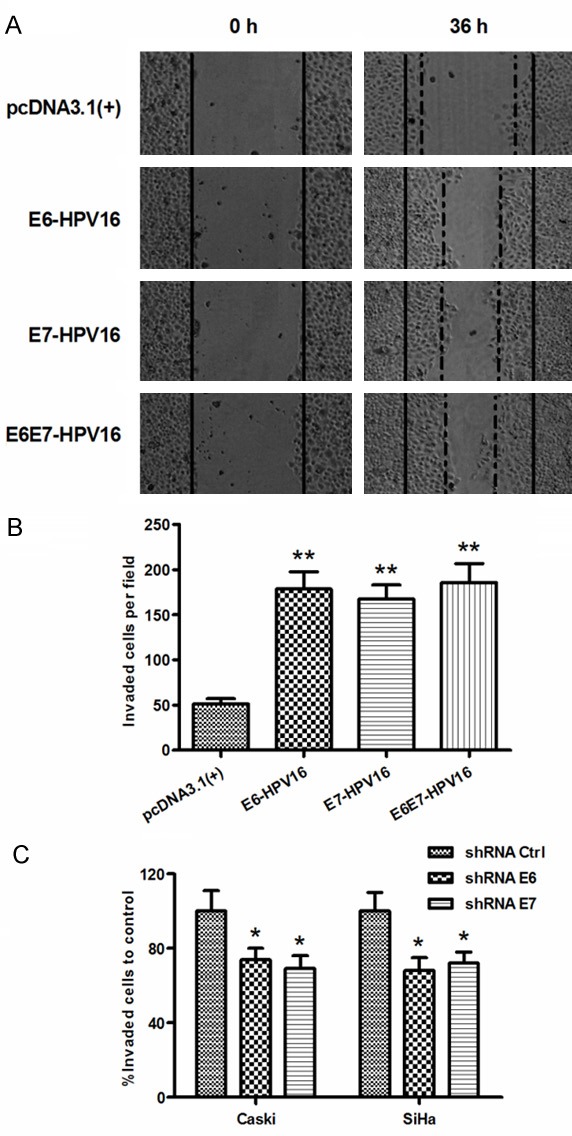
HPV16E6/E7 knockdown by shRNAs reduced MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells. A: Migration of C33A cells post the transfection with E6-HPV16-pcDNA3.1 (+), E7-HPV16-pcDNA3.1 (+), or E6E7-HPV16-pcDNA3.1 (+) for 36 hours by scratch assay. Solid lines were shown as a baseline. B: The migratory cells were counted respectively in each group; C: Migration of Caski or SiHa cells post the knockdown of E6 or E7 by scratch assay. Each experiment was performed separately in triplicate. Statistically significant was showed as ns, no significance, *P<0.05 or **P<0.01.
Chemical inhibition of MMP-2/9 inhibited the migration of cervical cancer cells
To further confirm the association of cervical cancer migration with MMP-2/9 activity, we utilized the MMP-2 inhibitor I and MMP-9 inhibitor I to decrease the activity of MMP-2 and MMP-9 in Caski or SiHa cells, and then evaluated the migration of two cervical cancer lines. It was shown in Figure 4A that both inhibitors did not regulate the expression of MMP-2 or MMP-9 at either mRNA (Figure 4A) or protein (Figure 4B) level. However, the activity of MMP-2 or MMP-9 was inhibited by the MMP-2 inhibitor I or MMP-9 inhibitor I respectively in Caski cells (P<0.05; Figure 4C) or in SiHa cells (P<0.01; Figure 4D). Compared to the 0.84 ± 0.095 (OD405 value) or 1.37 ± 0.150 of MMP-2 activity in control Caski cell group, the activity value MMP-2 or MMP-9 decreased to 0.46 ± 0.040 (OD405 value) or 0.73 ± 0.086 respectively by the MMP-2 inhibitor I or MMP-9 inhibitor I. And the MMP-2 activity in SiHa cells also decreased from 0.68 ± 0.082 to 0.35 ± 0.040 by the MMP-2 inhibitor I; and the MMP-9 activity in SiHa cells also decreased from 0.84 ± 0.093 to 0.32 ± 0.037 by the MMP-9 inhibitor I. And what’s more, the MMP-2 inhibitor I or the MMP-9 inhibitor I significantly inhibited the migration of Caski or SiHa cells, with a reduction of 31.82% or 32.76% (P<0.05; Figure 4E). Taking together, these results demonstrated that the MMP-2/9 contributed to the migration of cervical Caski or SiHa cells.
Figure 4.
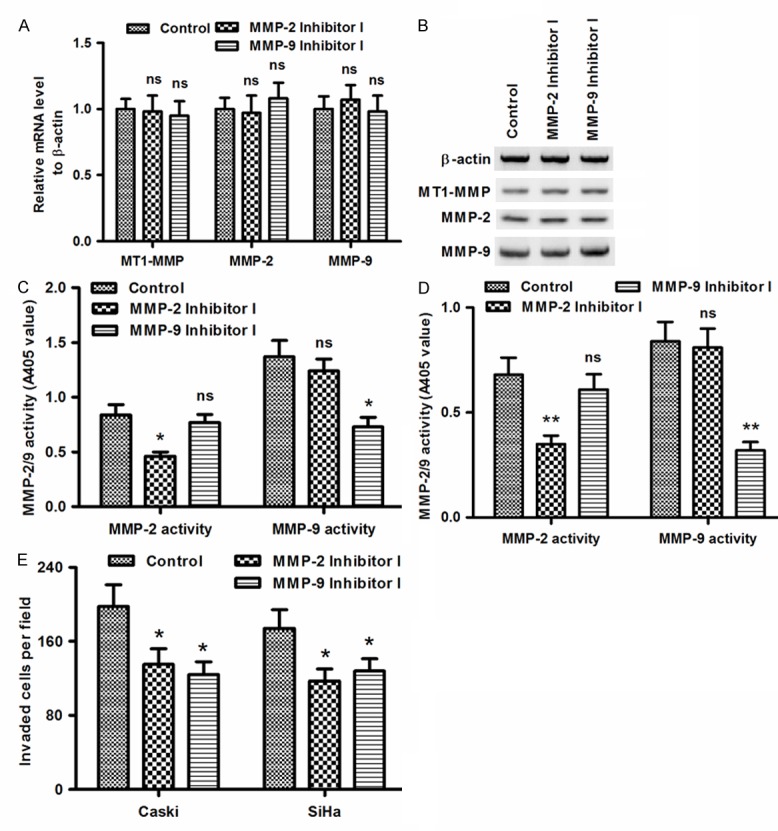
Chemical inhibition of MMP-2 or MMP-9 downregulated the migration of Caski or SiHa cells. (A and B) MT1-MMP, MMP-2 and MMP-9 in Caski cells at mRNA (A) or protein (B) level, post the treatment with 5 μM MMP-2 inhibitor I or 5 μM MMP-9 inhibitor I; (C and D) Reduced activity of MMP-2 or MMP-9 in Caski (C) or SiHa (D) cells post the treatment with 5 μM MMP-2 inhibitor I or 5 μM MMP-9 inhibitor I; (E) Migration of Caski or SiHa cells post the treatment with 5 μM MMP-2 inhibitor I or MMP-9 inhibitor I. Each value was averaged for triplicate independent results. Statistically significant was showed as *P<0.05, **P<0.01 or ns, no significance.
Discussion
MMPs have been found to be upregulated in almost every type of human cancer and to correlate the invasiveness and metastasis and even poor prognosis [22,23]. Early expression of MMPs helps to remodel the extracellular matrix (ECM) and to release ECM- and/or membrane-bound growth factors, which provides a favorable microenvironment for the establishment of the primary tumor [24]. MMPs are also involved in cell migration by removing sites of adhesion, exposing new binding sites, cleaving cell-cell or cell-matrix receptors, and releasing chemoattractants from the ECM [25]. Moreover, the increased expression and activation has been indicated to be associated with the progression and recurrence of human cervical cancers [26]. The oncogenesis of HPV-16 early proteins E6 and E7 has been well recognized in the cervical cancers. Either HPVE6 or HPVE7 has confirmed to be implicated in the regulation of cervical cancer cell cycling [4,5,7-9]. And multiple cellular proteins such as pRb [4], Forkhead box M1b (FoxM1b) [5], DREAM (DP, RB-like, E2F and MuvB) complex [7], Cyclin-dependent kinase (Cdk1 and Cdk 2) [8,9] have be confirmed to be regulated by the HPVE6 or HPVE7 and to influence the cycle of cervical cancer cells. More recently, it was reported that HPV16E6 induced cervical cancer cell migration directly via regulating p53 signaling [27].
And in the present study, we linked the oncogenicity of PVE6 or HPVE7 to the migration promotion of cervical cancer cells. The overexpression of HPV16 E6/E7 oncoproteins promoted MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells. MT1-MMP, MMP-2 and MMP-9 were upregulated in the HPV16 E6/E7-positive cervical cancer cell lines, Caski and SiHa cells at both mRNA and protein levels. And the overexpression of E6, E7 or E6E7 of HPV16 also significantly promoted MT1-MMP, MMP-2 and MMP-9. On the other side, the knockdown of HPV16 E6/E7 oncoproteins with shRNAs reduced MT1-MMP, MMP-2 and MMP-9 in cervical cancer Caski or SiHa cells. The E6- or E7-specific shRNA-harbored lentivirus significantly reduced the mRNA and protein levels of E6 or E7. Moreover, the expression of MT1-MMP, MMP-2 and MMP-9 was also downregulated by the shRNA E6 or shRNA E7. Thus, we confirmed that the promotion of HPV16 E6/E7 oncoproteins to MT1-MMP, MMP-2 and MMP-9 in cervical cancer cells.
And our further investigation indicated that the E6-, E7- or E6E7-HPV16 exerted a significant influence on the migration of cervical cancer cells. The overexpression of E6, E7 or E6E7 oncoprotein promoted the migration of cervical cancer C33A cells. On the other side, knockdown of E6-HPV16 or E7-HPV16 with the lentivirus-mediated shRNA significantly inhibited the migration of Caski or SiHa cells. All these results revealed that the knockdown of E6-HPV16 or E7-HPV16 by shRNA reduced the migration of cervical cancer cells in vitro. Moreover, the inhibition of MMP-2 or MMP-9 with the MMP-2 inhibitor I or MMP-9 inhibitor I significantly reduced the migration of cervical cancer Caski or SiHa cells. Taking together, our results demonstrated that the E6-HPV16 or E7-HPV16 promoted the activity of MMP-2/9, and contributed to the migration of cervical cells.
In summary, our study demonstrated that the E6 and E7 oncoproteins of high risk HPV16 promoted the activity of MT1-MMP, MMP-2 and MMP-9, and contributed to the migration of cervical cells.
Disclosure of conflict of interest
None.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Zur HH. Papillomavirus infections--a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 4.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 5.Jaiswal N, John R, Chand V, Nag A. Oncogenic Human Papillomavirus 16E7 modulates SUMOylation of FoxM1b. Int J Biochem Cell Biol. 2015;58C:28–36. doi: 10.1016/j.biocel.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCaprio JA. Human papillomavirus type 16 E7 perturbs DREAM to promote cellular proliferation and mitotic gene expression. Oncogene. 2014;33:4036–4038. doi: 10.1038/onc.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Liu Y, Zhao N, Chen H, Qiao L, Zhao W, Chen JJ. Role of Cdk1 in the p53-independent abrogation of the postmitotic checkpoint by human papillomavirus E6. J Virol. 2015;89:2553–62. doi: 10.1128/JVI.02269-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JE, Lee JI, Jin DH, Lee WJ, Park GB, Kim S, Kim YS, Wu TC, Hur DY, Kim D. Sequential treatment of HPV E6 and E7-expressing TC-1 cells with bortezomib and celecoxib promotes apoptosis through p-p38 MAPK-mediated downregulation of cyclin D1 and CDK2. Oncol Rep. 2014;31:2429–2437. doi: 10.3892/or.2014.3082. [DOI] [PubMed] [Google Scholar]
- 10.Chen PM, Cheng YW, Wang YC, Wu TC, Chen CY, Lee H. Up-regulation of FOXM1 by E6 oncoprotein through the MZF1/NKX2-1 axis is required for human papillomavirus-associated tumorigenesis. Neoplasia. 2014;16:961–971. doi: 10.1016/j.neo.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 12.Da SC, Brohem CA, Correa TC, Winnischofer SM, Nakano F, Boccardo E, Villa LL, Sogayar MC, Maria-Engler SS. Higher expression and activity of metalloproteinases in human cervical carcinoma cell lines is associated with HPV presence. Biochem Cell Biol. 2006;84:713–719. doi: 10.1139/o06-084. [DOI] [PubMed] [Google Scholar]
- 13.Smola-Hess S, Pahne J, Mauch C, Zigrino P, Smola H, Pfister HJ. Expression of membrane type 1 matrix metalloproteinase in papillomavirus-positive cells: role of the human papillomavirus (HPV) 16 and HPV8 E7 gene products. J Gen Virol. 2005;86:1291–1296. doi: 10.1099/vir.0.80551-0. [DOI] [PubMed] [Google Scholar]
- 14.Gilles C, Polette M, Piette J, Munaut C, Thompson EW, Birembaut P, Foidart JM. High level of MT-MMP expression is associated with invasiveness of cervical cancer cells. Int J Cancer. 1996;65:209–213. doi: 10.1002/(SICI)1097-0215(19960117)65:2<209::AID-IJC14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN, Huang SC, Hsu SM. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003;63:6537–6542. [PubMed] [Google Scholar]
- 16.Wang PH, Ko JL, Yang SF, Tsai HT, Tee YT, Han CP, Lin LY, Chen SC, Shih YT. Significant relation of tissue inhibitor of matrix metalloproteinase-2 and its combination with matrix metalloproteinase-2 to survival of patients with cancer of uterine cervix. Reprod Sci. 2011;18:798–808. doi: 10.1177/1933719111398143. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Wu T, Zhang B, Yao Y, Yin G. Matrix metalloproteinase-9 is a prognostic marker for patients with cervical cancer. Med Oncol. 2012;29:3394–3399. doi: 10.1007/s12032-012-0283-z. [DOI] [PubMed] [Google Scholar]
- 18.Shaverdashvili K, Wong P, Ma J, Zhang K, Osman I, Bedogni B. MT1-MMP modulates melanoma cell dissemination and metastasis through activation of MMP2 and RAC1. Pigment Cell Melanoma Res. 2014;27:287–296. doi: 10.1111/pcmr.12201. [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 20.Taxman DJ, Livingstone LR, Zhang J, Conti BJ, Iocca HA, Williams KL, Lich JD, Ting JP, Reed W. Criteria for effective design, construction, and gene knockdown by shRNA vectors. BMC Biotechnol. 2006;6:7. doi: 10.1186/1472-6750-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Woessner JJ. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 23.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 24.Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res. 2003;9:551–554. [PubMed] [Google Scholar]
- 25.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 26.Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN, Huang SC, Hsu SM. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003;63:6537–6542. [PubMed] [Google Scholar]
- 27.Au YC, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011;30:2401–2410. doi: 10.1038/onc.2010.613. [DOI] [PubMed] [Google Scholar]


