Abstract
Objective: In esophageal cancer, depth of wall penetration, reflected by T classification, represents the most important prognostic variable. Our study aimed to investigate the impact of tumor length, measured as the longitudinal length, on the outcome of esophageal squamous cell carcinoma (ESCC) patients. Methods: The survival data of 362 ESCC patients who underwent surgical resection as the primary treatment between 1999 and 2007 were collected retrospectively. Receiver-operator characteristic analysis was applied to identify the optimal cut-off values. Results: 4.0 cm was identified as the optimal cut-off value within the whole group. Tumor length greater than 4.0 cm was associated with increasing T stage (P = 0.001), N stage (P = 0.046), and tumor differentiation (P = 0.033). Univariate analysis and multivariate analysis both found that tumor length greater than 4.0 cm was associated with worse overall survival compared with shorter tumors (P < 0.001). It appeared to have a greater impact on N0-N1 (P < 0.001, P = 0.026, respectively) than N2-N3 and appeared to have a higher impact on the lower-stage patients than the higher-stage patients. Conclusions: Tumor length proved to be an independent prognostic parameter for ESCC patients, especially for node-negative and lower-stage patients. More attention should be paid to its role in the management of ESCC.
Keywords: Esophageal cancer, tumor length, cancer-specific survival, multivariate analysis
Introduction
The determination of the prognostic factors in patients with esophageal cancer is essential for predicting the clinical prognosis and identifying appropriate treatment strategies. Many previous studies detected a number of independent prognostic factors of esophageal cancer with great predictive value, such as the pathological status of lymph nodes in patients with esophageal cancer [1-3], depth of tumor invasion [4,5], and several biological molecular markers [6,7]. The most important clinicopathological characteristic identifying patients with esophageal cancer at high risk for therapeutic failure is T classification, which is based on depth of wall penetration across the different layers of the esophageal wall. Recent studies reported that the prognostic value of horizontal tumor extent were relatively limited and contradictory. Moreover, these prognostic factors are often obtained postoperatively and are not available for surgeon at the time of surgical resection to confirm the safe margin and the extent of radical resection. It is because they mainly depend on a postoperative histologic examination of the resected specimen. In contrast, tumor length, given as the longitudinal length of tumor, can be observed and measured easily before or during operation without any special measurement tools. Tumor length attracts more attention from surgeons than commonly believed and might have a direct impact on patients’ surgical management and treatment outcome. However, the prognostic value of tumor length in patients with esophageal cancer is still under debate [8-17]. Some studies have uncovered that tumor length functions as an independent prognostic indicator [9,10,12,15], whereas Khan and colleagues [18] identified tumor length as an unreliable indicator and reported that tumor length did not affect the survival of patients with esophageal cancer independently. Therefore, it is necessary for the present study to re-evaluate the prognostic value of tumor length in esophageal carcinoma.
Recently, a comprehensive study clearly indicated that tumor size, in particular the maximum horizontal tumor diameter, represented a valuable prognosticator in gastric cancer [19]. The optimal cut-off value was determined by receiver-operator characteristic (ROC) analysis and was found to be 3.5 cm. The present study aims to explore the prognostic clinical significance of tumor length in the subgroups of es-ophageal cancer patients. As inconsistency in previous reports caused by inappropriate cut-off values, we assessed the optimal cut-off values by ROC analysis and systematically examined these factors.
Patients and methods
Patients
The retrospective study described herein consisted of 362 consecutive patients who underwent curative resection (R0) for histologically confirmed esophageal squamous cell carcinoma (ESCC) at the Department of Esophageal Cancer Surgery, Cancer Hospital of Tianjin Medical University, between January 1999 and December 2007. Ages ranged between 24 and 83 years with an average age of 54.5 years. Of these, 267 patients were male and 95 patients were female. Twenty eight patients (7.7%) showed upper 1/3 esophageal cancer, 234 patients (64.6%) middle 1/3 esophageal cancer, and 100 patients (27.7%) lower 1/3 esophageal cancer. No death events occurred during the initial hospitalization and one month after operation. No patients received neoadjuvant chemotherapy or radiotherapy because of presumptive treatment-related changes in T classification. All 362 patients received complete evaluations before surgery, including physical examination, biochemistry tests, upper endoscopy, barium esophagography, chest and ab-dominal computed tomography scan, and neck and abdominal ultrasonography. Most patients received a trans-thoracic esophagectomy with a two-field lymphadenectomy either by a left thoracic or thoraco-abdominal approach or using the Ivor-Lewis procedure. Others received three-field lymph node dissection. Esophageal reconstruction was performed using the stomach via the esophageal bed. The study protocol was approved by the local ethics committee of Cancer Hospital of Tianjin Medical University and all patients provided written consent.
Pathological examination
The tumor length was measured according to the following procedure. First, the resected esophagus was cut open along the longitudinal axis so the whole mucosa could be observed clearly. Then, the esophageal specimen was naturally placed on a flat table without any stretching assistance. The longitudinal length of each ESCC specimen was examined with a handheld ruler and recorded in the operation notes as accurate as possible. The distance between the tumor border and both the proximal and distal cut ends was also recorded. The specimens were sent for pathology examination after preservation in 10% neutral buffered formalin. The depth of tumor invasion, grade of differentiation, and lymph node involving status were recorded according to the results of pathologic reports. All patients were staged using the seventh edition of the AJCC TNM staging system [20].
Follow-up
Follow-up ranged from six to 144 months with a median of 84 months. All patients received the laboratory checks every three months (including blood count, liver enzymes and tumor markers CEA and CA 19-9); after three years the interval was extended to six months. Chest computed tomography scans and ultrasonography of the abdomen were obtained at six-month intervals; after three years the interval was extended to 12 months.
Statistical analysis
Survival time was defined as the time from the date of surgery until death or until the most recent follow-up appointment. Survival curves were calculated according to the Kaplan-Meier method and compared by the log-rank test.
Univariate analysis was used to estimate the prognostic significance of potential parameters. Variables that predicted survival in a statistically significant manner (P < 0.05) were used to build a multivariate model. Multivariate analysis was performed using the Cox proportional hazards model. Backward stepwise elimination of variables was used to construct the final model. The optimal cut-off values for tumor size as a prognostic variable were chosen from an ROC analysis with the criterion variable “tumor length” and “progress” as the condition variable. In addition, a log linear model was adopted to evaluate the association between tumor length and other clinicopathologic features. For all analyses, P-values were two-sided and only P < 0.05 was considered significant.
All statistical calculations were performed using SPSS Software for Windows (version 19.0; SPSS Inc, Chicago, Ill).
Results
Tumor length
Tumor length ranged from 0.5 to 11.0 cm (mean, 4.4 cm; median, 4.0 cm). ROC analysis indicated that the median tumor length of 4.1 cm was selected as threshold value owing to sensitivity and specificity (Table 1). As shown in Figure 1, there are no ESCC patients with tumor lengths between 4.0-4.2 cm. In addition, the median tumor length was found to be 4.0 cm and thus, a tumor length of 4.0 cm was defined as the cut-off value for further analysis. A significant survival difference was observed among the subgroups of patients with esophageal cancer using the cut-off value of 4.0 cm (Figure 2). Patients with a tumor length of 4.0 cm or less (n = 188) had a better median survival time (26 versus 13 months) with a higher threeyear (41.5% versus 25.9%) and five-year survival rate (36.7% versus 17.8%) than those with a tumor length greater than 4.0 cm (P < 0.001). Using the Cox regression model, tumor length still displayed a significantly predictive factor for overall survival [hazard ratio (HR): 1.537; 95% confidence interval (CI): 1.182-1.997; P = 0.001.
Table 1.
Sensitivity and specificity value of tumor length calculated by ROC analysis
| Threshold | sensitivity | specificity | sensitivity + specificity |
|---|---|---|---|
| -0.5000 | 1.000 | 0.000 | 1.000 |
| 0.7000 | 1.000 | 0.012 | 1.012 |
| 0.9500 | 1.000 | 0.023 | 1.023 |
| 1.2500 | 0.993 | 0.035 | 1.028 |
| 1.6500 | 0.986 | 0.081 | 1.067 |
| 1.9000 | 0.986 | 0.105 | 1.090 |
| 2.1000 | 0.935 | 0.174 | 1.109 |
| 2.3500 | 0.931 | 0.174 | 1.106 |
| 2.6000 | 0.866 | 0.267 | 1.133 |
| 2.7500 | 0.862 | 0.267 | 1.130 |
| 2.9000 | 0.859 | 0.267 | 1.126 |
| 3.2500 | 0.721 | 0.430 | 1.151 |
| 3.6000 | 0.645 | 0.547 | 1.191 |
| 3.8500 | 0.641 | 0.558 | 1.199 |
| 4.1000 | 0.533 | 0.686 | 1.219 |
| 4.3500 | 0.529 | 0.686 | 1.215 |
| 4.7500 | 0.464 | 0.733 | 1.196 |
| 5.2500 | 0.315 | 0.872 | 1.187 |
| 5.7500 | 0.268 | 0.895 | 1.163 |
| 6.1000 | 0.181 | 0.930 | 1.111 |
| 6.3500 | 0.178 | 0.930 | 1.108 |
| 6.7500 | 0.156 | 0.930 | 1.086 |
| 7.2500 | 0.105 | 0.965 | 1.070 |
| 7.7500 | 0.076 | 0.965 | 1.041 |
| 8.2500 | 0.029 | 0.977 | 1.006 |
| 8.7500 | 0.018 | 0.988 | 1.006 |
| 9.2500 | 0.011 | 0.988 | 0.999 |
| 9.7500 | 0.007 | 0.988 | 0.996 |
| 10.5000 | 0.000 | 0.988 | 0.988 |
| 12.0000 | 0.000 | 1.000 | 1.000 |
Figure 1.
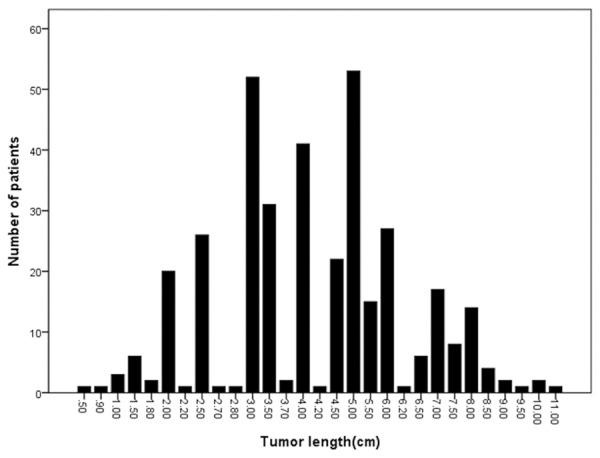
Histogram of the number of patients as a function of longitudinal tumor length.
Figure 2.
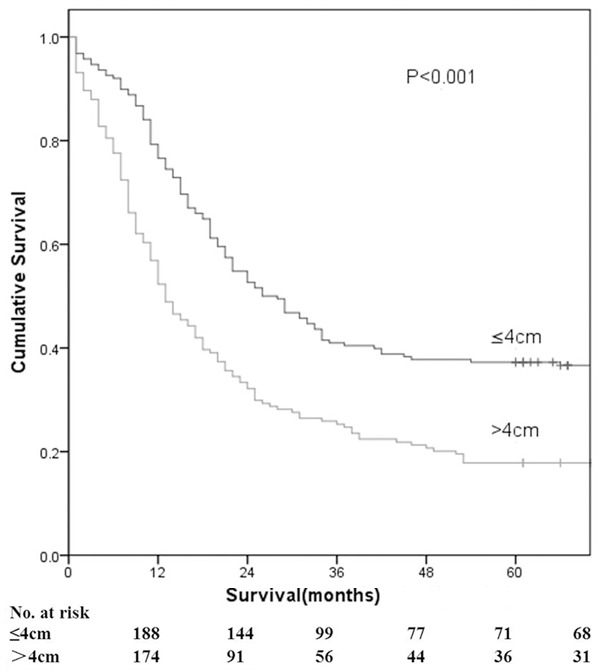
Kaplan-Meier survival curves for 362 patients as a function of tumor length (4.0 cm).
Univariate analysis
Univariate analyses were performed using the Kaplan-Meier method to assess the predictive capability of each variable assessed. These data are summarized in Table 2. Age, blood type, venous invasion and adjuvant therapy were not found to be statistically associated with survival. As expected, variables constituting the clinical and pathologic staging systems were predictive of survival. The strongest prognostic factors on univariate analysis were tumor length (P < 0.001), T stage (P < 0.001), and N stage (P < 0.001). The grade of the tumor (p < 0.001) and sex (P = 0.026) was also found to be statistically associated with survival.
Table 2.
Univariate analysis of patients using Kaplan Meier method
| Factor | Cases | 5-Year survival | 95% CI | P-value |
|---|---|---|---|---|
| ALL | 362 | 27.6 | 20.0 (16.893-23.107) | ---- |
| Age | 0.834 | |||
| < 60 | 150 | 27.3 | 21.0 (16.336-25.664) | |
| ≥ 60 | 212 | 27.8 | 19.0 (15.159-22.841) | |
| Sex | 0.026 | |||
| Male | 267 | 24.6 | 18.0 (14.681-21.319) | |
| Female | 95 | 36.2 | 26.0 (18.876-33.124) | |
| Blood type | 0.855 | |||
| O | 103 | 28.2 | 19.0 (11.767-26.233) | |
| A | 107 | 26.2 | 20.0 (14.544-25.456) | |
| B | 109 | 26.1 | 20.0 (16.182-23.818) | |
| AB | 33 | 36.4 | 21.0 (6.370-35.630) | |
| Tumor site | 0.042 | |||
| Upper | 28 | 10.7 | 11.0 (1.925-20.075) | |
| Middle | 234 | 29.9 | 20.0 (15.964-24.036) | |
| Lower | 100 | 27.0 | 19.0 (13.383-24.717) | |
| Surgical approach | 0.011 | |||
| Left thoracic | 239 | 30.1 | 22.0 (17.131-26.869) | |
| Ivor-Lewis | 55 | 27.8 | 21.0 (16.896-25.104) | |
| Tri-incisional | 68 | 17.6 | 15.0 (8.942-21.058) | |
| Tumor Length | < 0.001 | |||
| ≤ 4.0 cm | 188 | 36.7 | 26.0 (19.841-32.159) | |
| > 4.0 cm | 174 | 17.8 | 13.0 (10.063-15.937) | |
| T stage | < 0.001 | |||
| T1 | 7 | 85.7 | N/A | |
| T2 | 57 | 45.6 | 42.0 (N/A) | |
| T3 | 273 | 23.8 | 19.0 (15.964-22.036) | |
| T4 | 25 | 12.0 | 10.0 (7.552-12.448) | |
| N stage | < 0.001 | |||
| N0 (0) | 285 | 32.6 | 24.0 (18.988-29.012) | |
| N1 (1-2) | 51 | 7.8 | 12.0 (8.119-15.881) | |
| N2 (3-6) | 18 | 16.7 | 15.0 (10.842-19.158) | |
| N3 (≥ 7) | 8 | 0 | 4.0 (0.0-8.158) | |
| Grade | < 0.001 | |||
| Well differentiated | 42 | 38.1 | 18.0 (0.0-38.112) | |
| Moderately differentiated | 268 | 29.1 | 22.0 (18.794-25.206) | |
| Poorly differentiated | 52 | 11.5 | 11.0 (7.974-14.026) | |
| Venous invasion | 0.590 | |||
| No | 360 | 27.8 | 20.0 (16.747-23.253) | |
| Yes | 2 | 0 | 17.0 (N/A) | |
| Adjuvant therapy | 0.932 | |||
| No | 249 | 30.1 | 19.0 (15.512-22.488) | |
| Yes | 113 | 22.1 | 21.0 (17.094-24.906) |
Multivariate analysis
For multivariate survival analysis, all factors associated with a significant prognostic prediction for overall survival using univariate analysis were included to determine the independent prognostic factors for patients with resectable ESCC. As shown in Table 3, tumor length, T stage, N stage, tumor grading, and tumor site plays a potential role as independent prognostic factors (P < 0.05). Sex and surgical approach were not found to be independent prognostic factors (P > 0.05).
Table 3.
Prognostic factors retained in multivariate analysis by Cox model
| Factor | χ2 | P-value | RR | 95% CI |
|---|---|---|---|---|
| Sex (Male/Female) | 2.659 | 0.103 | 0.770 | 0.562-1.054 |
| Surgical approach (left thoracic/Ivor-Lewis/Tri-incisional) | 1.052 | 0.591 | 1.173 | 0.811-1.695 |
| Tumor length (≤ 4.0 cm/> 4.0 cm) | 10.322 | 0.001 | 1.537 | 1.182-1.997 |
| T stage (T1/T2/T3/T4 ) | 11.797 | 0.008 | 3.874 | 2.526-28.529 |
| N stage (N0/N1/N2/N3) | 27.239 | < 0.001 | 1.929 | 1.369-2.718 |
| Grade (Well/Moderately/Poorly) | 7.121 | 0.028 | 1.175 | 1.376-1.778 |
| Tumor site | 5.897 | 0.052 | 0.538 | 0.326-0.888 |
Correlation analysis
To explore the possibility of tumor length in patients with ESCC to be an independent prognosticator, the correlation of tumor length with the clinicopathologic variables of ESCC patients was assessed by both the Pearson Chi-square test and the log linear model. As shown in Table 4, tumor length was associated with several clinicopathologic factors such as sex, depth of invasion, status of lymph nodes and histologic grading. However, the results showed that the status of lymph nodes (βtn = 0.143, P = 0.003) and the depth of invasion (βti = 0.159, P = 0.006) were the only two variables with significant linear by linear association with the tumor length independent of other clinicopathologic factors. The longer diameter of tumor at the longitudinal axis was associated with the deeper tumor invasion and more lymph nodes were involved.
Table 4.
Clinicopathologic features of patients according to tumor length
| Variables | Tumor Length | χ2 | P | |
|---|---|---|---|---|
|
| ||||
| ≤ 4.0 cm (%) | > 4.0 cm (%) | |||
| Age | 1.588 | 0.208 | ||
| < 60 | 72 (48.0) | 78 (52.0) | ||
| ≥ 60 | 116 (54.7) | 96 (45.3) | ||
| Sex | 21.182 | 0.001 | ||
| Male | 120 (44.8) | 148 (55.2) | ||
| Female | 68 (51.9) | 26 (27.7) | ||
| Blood type | 0.134 | 0.987 | ||
| O | 54 (52.4) | 49 (47.6) | ||
| A | 55 (51.4) | 52 (48.6) | ||
| B | 61 (51.3) | 58 (48.7) | ||
| AB | 18 (54.5) | 15 (45.5) | ||
| Tumor site | 0.686 | 0.709 | ||
| Upper | 16 (57.1) | 12 (42.9) | ||
| Middle | 123 (52.6) | 111 (47.4) | ||
| Lower | 49 (49.0) | 51 (51.0) | ||
| Surgical approach | 2.084 | 0.353 | ||
| left thoracic | 127 (53.1) | 112 (46.9) | ||
| Ivor-Lewis | 31 (55.6) | 24 (44.4) | ||
| Tri-incisional | 30 (44.1) | 38 (55.9) | ||
| T stage | 16.359 | 0.001 | ||
| T1 | 7 (100.0) | 0 (0) | ||
| T2 | 39 (68.4) | 18 (31.6) | ||
| T3 | 133 (48.7) | 140 (51.3) | ||
| T4 | 9 (36.0) | 16 (64.0) | ||
| N stage | 7.555 | 0.046 | ||
| N0 (0) | 157 (55.1) | 128 (44.9) | ||
| N1 (1-2) | 18 (35.3) | 33 (64.7) | ||
| N2 (3-6) | 10 (55.6) | 8 (44.4) | ||
| N3 (≥ 7) | 3 (37.5) | 5 (62.5) | ||
| Pathological TNM | 5.372 | 0.029 | ||
| IB | 11 (78.6) | 3 (21.4) | ||
| IIA | 49 (52.1) | 45 (47.9) | ||
| IIB | 89 (54.6) | 74 (45.4) | ||
| IIIA | 25 (41.7) | 35 (58.3) | ||
| IIIB | 9 (60.0) | 6 (40.0) | ||
| IIIC | 5 (31.3) | 11 (68.7) | ||
| Differentiation | 4.479 | 0.033 | ||
| Well | 22 (52.4) | 20 (47.6) | ||
| Moderately | 146 (54.5) | 122 (45.5) | ||
| Poorly | 20 (38.5) | 32 (61.5) | ||
| Venous invasion | 0.003 | 0.956 | ||
| No | 187 (51.9) | 173 (48.1) | ||
| Yes | 1 (50.0) | 1 (50.0) | ||
Survival comparison according to pathological stage and lymph node metastasis in two groups (tumor length of ≤ 4.0 cm versus > 4.0 cm)
According to the AJCC/TNM staging system, ESCC was divided into six stages: stage IB, stage IIA, stage IIB, stage IIIA, stage IIIB, and stage IIIC. On the basis of tumor length, each stage was divided into two groups, tumor length of ≤ 4.0 cm versus > 4.0 cm. There were significant differences in overall 5-year survival rates between the two groups according to stages IIA, IIB, and IIIA (P = 0.034, 0.001, and 0.022, respectively; Table 4; Figure 3). In addition, further analysis was performed to assess the correlation between overall survival and lymph node metastasis in patients with ESCC. The tumor length of ESCC presented a significant impact on ESCC patients with N0 and N1 (P < 0.001, Figure 4) rather than the cohort with N2 or N3.
Figure 3.
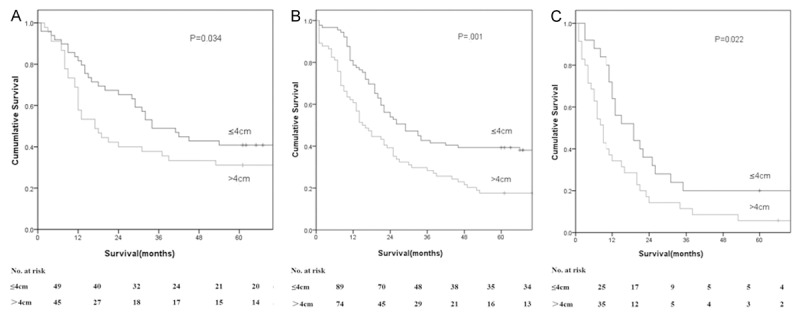
Survival as a function of tumor length and pathologic stage. A. Stage IIA. B. Stage IIB. C. Stage IIIA.
Figure 4.
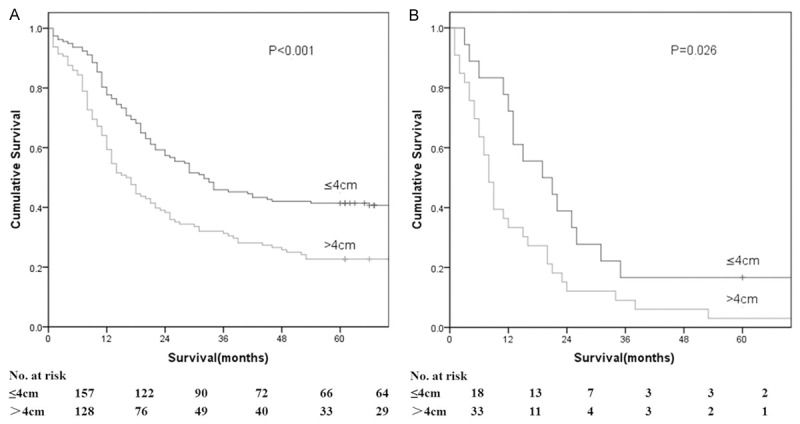
Survival as a function of tumor length and N stage. A. Stage N0. B. Stage N1.
Discussion
Risk stratification of ESCC patients for prognostic purposes is mainly based on the TNM staging system [21]. It depends on the anatomic extent of disease and is assessed using a combination of primary tumor extent, local lymph node spread, and presence or absence of distant metastasis [22]. According to the TNM classification, the T subcategory reflects depth of tumor penetration within or beyond the esophageal wall, whereas data regarding the biologic significance of longitudinal tumor extent, as assessed by measuring the longitudinal length, are scarce and contradictory. Some researchers have demonstrated that tumor length independently influences prognosis, while others stressed that the tumor longitudinal length of esophageal carcinoma measured by gross or histological examination is a rough indicator of actual length and it should not be considered as an independent prognostic factor. Gaur et al. [16] divided tumor length into two subgroups according endoscopic esophageal tumor length (≤ 2 and > 2 cm) and found that tumor length independently influenced the survival of patients. Griffiths et al. [10] categorized tumor length by cut-points of 3.5 cm. In their study, the independent prognostic role of tumor length was also evidenced. Recently, Wang et al. [9] defined a threshold of 3 cm for dividing patients into two groups and their results indicated that tumor length was an independent prognostic factor. However, in another similar study, Khan et al. [18] found that tumor length was not a clinical predictor of ESCC patient survival (P = 0.861). In view of the facts that tumor length is the primary tumor-related information for surgeons during operation, it is necessary to clarify the prognostic value of tumor length in ESCC.
According to our data, tumor length was found to be significantly correlated with prognosis in univariate survival analysis and it still kept its prognostic value in multivariate survival analysis similar to earlier studies reported. In addition, positive associations between tumor length and other clinicopathologic features, such as status of lymph nodes and depth of tumor invasion, were indicated by log linear model results. All mentioned findings supported a notion that the tumor length of ESCC was ascribed to a powerful clinical marker for the prognosis and the aggressiveness of ESCC. As previously reported [18], tumor size has important effects on the prognosis of patients with esophageal cancer and is always used as a fundamental characteristic to assess esophageal cancer. In light of our results, tumor length is found to be a reliable predictor of survival for patients with ESCC. Moreover, tumor length can help surgeons evaluate the depth of tumor invasion efficiently and the degree of lymph nodes metastasis preoperatively or intraoperatively. All of these advantages can help surgeons identify appropriate treatment strategies such as determining appropriate extent of resection and level of lymphadenectomy in the management of ESCC. From novel viewpoint, the great correlations of tumor length with lymph node status and depth of tumor invasion may account for its independent clinical value.
We determined the clinical value of the clinical variable “tumor length of ESCC” in the subpopulation of patients. Esophageal tumor length yielded a greater impact on patients with N0 or N1 rather than patients with N2 or N3. Our data demonstrated that tumor length contributes to the survival of ESCC patients with stage IIA, IIB and IIIA, indicating that patients with a tumor length of ≤ 4 cm displayed a survival advantage relative to those with a tumor length of < 4 cm. Our subgroup analysis confirmed the observations of Yendamuri et al. [15] and Wang et al. [9] in that tumor length may be correlated to locoregional lymph node metastasis in patients with ESCC and not to distant metastasis of ESCC. Nevertheless, Wang et al. [9] found that tumor length had no significant impact on the survival of N1 ESCC patients (P = 0.187), which is controversial to our results. The reason for the discrepancy remains elusive, but may be partially attributed to the relatively small sample size under the retrospective analysis. Wang and associates analyzed 160 ESCC patients who underwent curative esophagectomy for N1 disease. In conclusion, tumor length may serve as a good prognosis predictor in patients with ESCC.
It was noticed that the cut-points for categorizing tumor length varied in different studies. Since the differences in cut-points might be considered as the reason for the varied results among different studies, we examined our data with every reported grouping method described in the literature. The results showed that tumor length remained an independent prognostic indicator in our study regardless of the categorization method. The question may be raised of which method of categorizing tumor length is optimal. However, our data did not allow us to directly address this issue; larger-scale, multi-centric, prospective studies are needed to answer this question in near future. In conclusion, tumor length, measured as the longitudinal length, is an important prognostic parameter for patients with ESCC, especially for nodenegative and lower-stage patients. Therefore, it appears that the addition of tumor length to ordinary classification factors such as depth of invasion, lymph node metastasis, and distant metastasis may be a useful strategy in the stage classification in ESCC. Further prospective studies, however, are warranted to validate the proposed cut-off values that were determined by ROC analysis in our dataset.
Disclosure of conflict of interest
None.
References
- 1.Bollschweiler E, Baldus SE, Schröder W, Schneider PM, Hölscher AH. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–363. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 2.Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J Thorac Oncol. 2010;5:1467–1471. doi: 10.1097/jto.0b013e3181e8f6b1. [DOI] [PubMed] [Google Scholar]
- 3.Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–371. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 4.Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633–641. doi: 10.1097/SLA.0b013e3181656d07. [DOI] [PubMed] [Google Scholar]
- 5.de Manzoni G, Pedrazzani C, Verlato G, Roviello F, Pasini F, Pugliese R, Cordiano C. Comparison of old and new TNM systems for nodal staging in adenocarcinoma of the gastro-oesophageal junction. Br J Surg. 2004;91:296–303. doi: 10.1002/bjs.4431. [DOI] [PubMed] [Google Scholar]
- 6.Allameh A, Rasmi Y, Nasseri-Moghaddam S, Tavangar SM, Sharifi R, Sadreddini M. Immunohistochemical analysis of selected molecular markers in esophagus precancerous, adenocarcinoma and squamous cell carcinoma in Iranian subjects. Cancer Epidemiol. 2009;33:79–84. doi: 10.1016/j.canep.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Sarbia M, Ott N, Puhringer-Oppermann F, Brucher BL. The predictive value of molecular markers (p53, EGFR, ATM, CHK2) in multimodally treated squamous cell carcinoma of the oesophagus. Br J Cancer. 2007;97:1404–1408. doi: 10.1038/sj.bjc.6604037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roedl JB, Sahani DV, Colen RR, Fischman AJ, Mueller PR, Blake MA. Tumour length measured on PET-CT predicts the most appropriate stage-dependent therapeutic approach in oesophageal cancer. Eur Radiol. 2008;18:2833–2840. doi: 10.1007/s00330-008-1078-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang BY, Goan YG, Hsu PK, Hsu WH, Wu YC. Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:887–893. doi: 10.1016/j.athoracsur.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol. 2006;93:258–267. doi: 10.1002/jso.20449. [DOI] [PubMed] [Google Scholar]
- 11.Bollschweiler E, Baldus SE, Schröder W, Schneider PM, Hölscher AH. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–363. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 12.Twine CP, Roberts SA, Lewis WG, Dave BV, Rawlinson CE, Chan D, Robinson M, Crosby TD. Prognostic significance of endoluminal ultrasound- defined disease length and tumor volume (EDTV) for patients with the diagnosis of esophageal cancer. Surg Endosc. 2010;24:870–878. doi: 10.1007/s00464-009-0681-2. [DOI] [PubMed] [Google Scholar]
- 13.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U. S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 14.Bolton WD, Hofstetter WL, Francis AM, Correa AM, Ajani JA, Bhutani MS, Erasmus J, Komaki R, Maru DM, Mehran RJ, Rice DC, Roth JA, Vaporciyan AA, Walsh GL, Swisher SG. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2009;138:831–836. doi: 10.1016/j.jtcvs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Yendamuri S, Swisher SG, Correa AM, Hofstetter W, Ajani JA, Francis A, Maru D, Mehran RJ, Rice DC, Roth JA, Walsh GL, Vaporciyan AA. Esophageal tumor length is independently associated with long-term survival. Cancer. 2009;115:508–516. doi: 10.1002/cncr.24062. [DOI] [PubMed] [Google Scholar]
- 16.Gaur P, Sepesi B, Hofstetter WL, Correa AM, Bhutani MS, Watson TJ, Swisher SG. Endoscopic esophageal tumor length: a prognostic factor for patients with esophageal cancer. Cancer. 2011;117:63–69. doi: 10.1002/cncr.25373. [DOI] [PubMed] [Google Scholar]
- 17.Jeganathan R, McGuigan J, Campbell F, Lynch T. Does pre-operative estimation of oesophageal tumour metabolic length using 18F-fluorodeoxyglucose PET/CT images compare with surgical pathology length? Eur J Nucl Med Mol Imaging. 2011;38:656–662. doi: 10.1007/s00259-010-1670-3. [DOI] [PubMed] [Google Scholar]
- 18.Khan OA, Alexiou C, Soomro I, Duffy JP, Morgan WE, Beggs FD. Pathological determinants of survival in node-negative oesophageal cancer. Br J Surg. 2004;91:1586–1591. doi: 10.1002/bjs.4778. [DOI] [PubMed] [Google Scholar]
- 19.Jun KH, Jung H, Baek JM, Chin HM, Park WB. Does tumor size have an impact on gastric cancer? A single institute experience. Langenbecks Arch Surg. 2009;394:631–635. doi: 10.1007/s00423-008-0417-0. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th edition. Chicago: Springer; 2010. [Google Scholar]
- 21.Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633–641. doi: 10.1097/SLA.0b013e3181656d07. [DOI] [PubMed] [Google Scholar]
- 22.de Manzoni G, Pedrazzani C, Verlato G, Roviello F, Pasini F, Pugliese R, Cordiano C. Comparison of old and new TNM systems for nodal staging in adenocarcinoma of the gastro-oesophageal junction. Br J Surg. 2004;91:296–303. doi: 10.1002/bjs.4431. [DOI] [PubMed] [Google Scholar]


