Abstract
MicroRNAs (miRNAs) have emerged as important regulators that potentially play critical roles in cancer cell biological processes. Previous studies have shown that miR-204 plays an important role in various human cancers. However, the underlying mechanisms of this microRNA in breast cancer remain largely unknown. In the present study, we investigated that miR-204 expression level was markedly reduced in both the human breast cancer tissue and cultured breast cancer cell lines (MCF-7, MDA-MB-231). Overexpression of miR-204 inhibited the proliferation and promoted the apoptosis in breast cancer cells, which were reversed by co-transfection of miR-204 inhibitor. We validated that Janus kinase 2 (JAK2), as a direct target of miR-204, is overexpressed in breast cancer. Knockdown of JAK2 suppressed cell viability and induced apoptosis in breast cancer cells. Moreover, the level of miR-204 is negatively correlated with p-STAT3 and anti-apoptotic genes BCl-2 and surviving in breast cancer. In conclusions, miR-204 targets JAK2 and suppressed JAK2 and p-JAK2 expression in breast cancer, which further inhibit the activation of STAT3, BCl-2 and survivin. These findings indicate that manipulation of miR-204 expression may represent a novel therapeutic strategy in the treatment of breast cancer.
Keywords: Breast cancer, miR-204, JAK2, proliferation, apoptosis
Introduction
Breast cancer is a malignant neoplasm originating from breast tissue, accounting for 29% of all female cancers [1]. Although the advances in diagnosis and appropriately systemic therapy, including surgery, radiation and chemotherapy, contribute to the prognosis of breast cancer, breast cancer is the most common cause of cancer death in females worldwide. So it is urgent to investigate the underlying mechanism mechanisms of breast cancer metastasis.
Janus kinase (JAK, or “Just another kinase”) is a family of intracellular, non-receptor protein tyrosine kinases (PTKs), including JAK1, JAK2, JAK3, and TYK2, which selectively associate with the cytoplasmic domains of various cytokine receptors [2,3]. JAK2 is a crucial intracellular mediator of cytokine and hormone signaling. When cytokines bind to a cell-surface receptor, the receptor dimerizes and leads to phosphorylation and activation of JAK tyrosine kinases, which in turn phosphorylate the receptor to allow binding and phosphorylation of cytoplasmic STAT proteins, which dimerize and translocate to the nucleus to regulate transcription of various target genes [4,5]. Accumulating evidences showed that aberrant JAK2 signaling is linked to a wide variety of tumors and plays an important role in proliferation, differentiation and apoptosis [6,7]. STAT3 is the preferred downstream targets of phosphorylated JAK2 and is constitutively activated in a variety of human tumors and possesses oncogenic potential and anti-apoptotic activities [8,9]. Recent results showed that JAK2 acts as an oncogene in breast cancer and the alterations to the JAK/STAT pathway are particularly identified in breast cancer [10,11].
MicroRNAs (miRNAs), an abundant class of ~22 nucleotide small noncoding RNAs, post-transcriptionally regulate gene expression through binding to multiple target mRNAs (mRNAs) [12-14]. miRNAs, as important regulators, are significantly involved in the development of many types of human diseases. The dysregulated expression of miRNAs is closely associated with carcinogenesis and cancer progression [15]. For example, miR-21 and miR-657, as oncogenic miRNA, are up-regulated in breast cancer and reported to promote cancer progression [16,17], whereas miR-124, miR-101, miR-29 and miR-145, as tumor-suppressor, are downregulated in breast cancer [13,14]. Recent studies showed that downregulated expression of miR-204 was significantly associated with metastasis of breast cancer [18]. However, the underlying mechanism remains unclear. This study was designed to investigate the potential molecular mechanisms of miR-204 mediated anti-tumor effects in breast cancer cells.
Materials and methods
Human tissue samples
Human breast cancer tissues were provided by Affiliated Hospital of Binzhou Medical College after approval was obtained from the Univer-sity’s Ethics Committee on the Use of Human Samples. The tissues were obtained from 20 individuals undergoing surgery due to invasive ductal carcinoma or invasive lobular carcinoma. The sampled tissues were immediately snap-frozen in liquid nitrogen and stored at -80°C.
Cell culture
Human embryonic kidney cells (HEK293; ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS; Hyclone, USA). The human breast cancer cell lines MCF-7 and MD-MB-231 were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI 1640 (Hyclone, USA) supplemented with 10% FBS. MCF-10A cell line, a non-tumorigenic epithelial cell line, was cultured in DMEM/F12 (1:1) (Hyclone, USA) supplemented with 5% horse serum, 10 µg/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin and 0.5 μg/ml hydrocortisone. MCF-10A was purchased from Cell Resource Center (Beijing, China).
miRNAs and siRNA transfection
Negative control (NC) , miR-204 mimics (mimics) and miR-204 inhibitors (inhibitors) were synthesized by GenePharma (Shanghai, China). JAK2 siRNA was purchased from Cell Signaling Technology. The cells were transfected with NC, mimics, inhibitors, or siRNA using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer’s protocol.
Real-time PCR
The total RNA from the human tissues and cell lines was extracted using Trizol (Invitrogen, USA) according to the manufacturer’s instructions. The levels of miR-204 and JAK2 mRNA were quantified using the qRT-PCR (quantitative real-time PCR) miRNA Detection Kit (Ambion, USA) combined with SYBR Green qPCR Master Mix (Tiangen) on ABI 7300 (Applied Biosystems, Foster, CA) [19]. The following JAK2 primers were used for PCR detection: 5’-GGGAGGTGGTCGCTGTAAAA-3’ (forward); 5’-ACCAGCACTGTAGCACACTC-3’ (reverse). The relative expression levels of miR-204 and JAK2 were calculated through normalization to their internal controls, U6 and GAPDH, respectively. The relative expression intensity values were calculated as 2-ΔΔCt.
MTT assay
The 3-(4,5-dimethylthiazal-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was used to estimate cell viability [20]. Briefly, cells were plated at a density of 1×104 cells per well in 96-well plates. After exposure to specific treatment, the cells were incubated with MTT at a final concentration of 0.5 mg/ml for 4 h at 37°C. After the removal of the medium, 150 mM DMSO solutions were added to dissolve the formazan crystals. The absorbance was read at 570 nm using a multi-well scanning spectrophotometer reader. Cells in the control group were considered 100% viable.
Flow cytometry
Annexin V and PI fluorescein staining kit (Bender MedSystems, Austria) were utilized to measure H9c2 cell apoptosis by following the manufacturer’s instruction. Briefly, 5×105 cells were suspended in 500 μl 1× binding buffer (10 mM HEPES pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Cells were then incubated with Annexin V (1:20) for 5 min followed by incubation with propidium iodide (PI, 1 mg/ml) for 15 min. Apoptosis rate was evaluated by Flow Cytometry.
Western blot
Protein lysate were separated by 10% SDS-polyacrylamide gel electrophoresis and then electroblotted onto PVDF membranes. The primary antibodies against JAK2, p-JAK2, anti-p-STAT3 (tyr705), Bcl-2 and survivin were purchased from Cell Signaling Technology, with GADPH antibody (Cell Signaling Technology) as an internal control. Densitometric analysis was done with Labworks Image Acquisition and Analysis Software (UVP, Upland, CA).
Luciferase reporter assays
Twenty-four hours before transfection HEK-293 cells were seeded into a 24-well plate (~3×104 /well), then the cells were cotransfected with Renilla luciferase and luciferase reporter plasmids containing miR-204 or vector control and wild-type or mutated target gene 3’UTR with Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, luciferase activities were measured using dual-luciferase reporter assay system (Promega). Firefly luciferase activities were normalized to Renilla luciferase.
Statistical analysis
Data are expressed as mean ± SD and analyzed by Student’s t-test. Compared with respective controls, P values of <0.05 were considered statistically significant.
Results
MiR-204 was significantly down-regulated in human breast cancer tissues and cell lines
Previous study has been revealed that miR-204 was dysregulated in breast cancer issues, so we examined miR-204 expression level in breast cancer. RT-PCR was used to quantify its expression in 24 couples of breast cancer tissues (BCTs) and normal adjacent tissues (NATs). miR-204 was significantly decreased in BCTs compared with NATs (Figure 1A), which was in accordance with the previous study [18]. Furthermore, the expression of miR-204 in breast cancer cell lines (MCF-7 and MDA-MB-231) was compared with breast epithelial cell line MCF-10A. As shown in Figure 1B, miR-204 expression level was significantly decreased in MCF-7 and MDA-MB-231 cells, compared with the MCF-10A cell. These results suggested that miR-204 might be involved in breast cancer progression.
Figure 1.
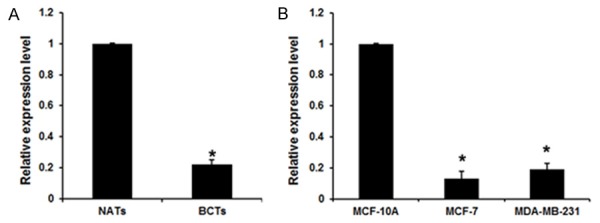
miR-204 was down-regulated in human breast cancer tissues and cell lines. A. The level of miR-204 was decreased in breast cancer tissues compared with NATs; *P<0.01 vs. NATs. B. miR-204 was absent in MCF-7 and MDA-MB-231 cells compared with MCF-10A; n=4 *P<0.01 vs. MCF-10A.
Effects of miR-204 on cell proliferation and apoptosis of breast cancer
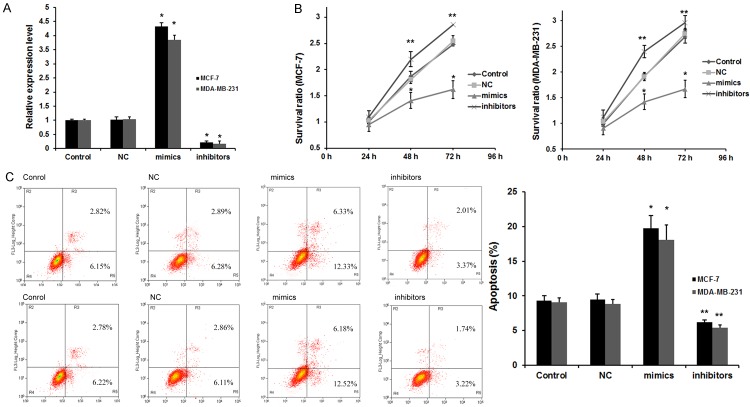
To further reveal the role of miR-204 in breast cancer development, we transfected miR-204 mimics or inhibitors into MCF-7 and MDA-MB-231 cells to overexpress or silence miR-204 expression. As showed in Figure 2A (right), after tranfected with miR-204 mimics, miR-204 expression was effectively upregulated, and miR-204 expression was greatly downregulated in MCF-7 and MDA-MB-231 cells after tranfected with miR-204 inhibitors.
Figure 2.
miR-204 regulates the viability and apoptosis of breast cancer cells. A. miR-204 level after transfection. *P<0.01 vs. Control and NC group. B. miR-204 decreased the viability of breast cancer cells by MTT assay. The cell viability was significantly decreased after transfection with miR-204 mimics for 48 h; n=6 *P<0.01 and **P<0.05 vs. Control (negative control) group. C. Apoptosis of breast cancer cells after miR-204 transfection for 48 h by flow cytometry. Data are presented as means ± SEM of three separate experiments; *P<0.01 and **P<0.05 vs. Control group.
An MTT assay was performed to detect the viability of MCF-7 and MDA-MB-231 cells after transfection with 50 nM miR-204 for 24, 48, and 72 h. MTT assays showed that overexpression of miR-204 significantly decreased cell viability and inhibition of miR-204 increased viability of MCF-7 and MDA-MB-231 cells (Figure 2B) with a higher inhibitory effect observed 48 h after miR-204 treatment, which indicates that miR-204 contributes to breast cancer tumorigenesis. We next assessed the requirement of mir-204 for cell apoptosis. As shown in Figure 2C, transfecting both MCF-7 and MDA-MB-231 cells with mir-204 mimics increased cell apoptosis to a large extent. On the contrary, treating MCF-7 and MDA-MB-231 cells with miR-204 inhibitors resulted in a significantly lower rate of apoptosis.
Together, the above results indicate that miR-204 performs tumor suppressor role by suppressing cell proliferation and inducing apoptosis in breast cancer.
JAK2 is a direct target of miR-204
In order to elucidate the underlying molecular mechanism, we performed a bioinformatic analysis using mirco-RNA.org (http://www.microrna.org/microrna/home.do) to predict the possible target gene of miR-204. We found that JAK2 contained theoretical miR-204 binding sites in its 3’ UTR (Figure 3A). To demonstrate whether JAK2 was directly targeted by miR-204, we performed luciferase reporter gene assay in HEK 293 cells. As shown in Figure 3A, co-transfection of miR-204 suppressed the luciferase activity of the reporter containing wild-type JAK2 3’ UTR sequence, but failed to inhibit that of mutated JAK2 by dual-luciferase reporter assay. These data suggested that miR-204 could directly target the 3’UTR sequences of JAK2. Additionally, we found overexpression of miR-204 significantly inhibited both the mRNA (Figure 3B) and protein (Figure 3C) expression of JAK2, which was abolished by co-transfection of miR-204 inhibitor. Moreover, the relative level of the phosphorylated form of JAK2 (p-JAK2) was consistent with the total JAK2 protein (Figure 3C). These results demonstrated that endogenous JAK2 expression is directly targeted and regulated by miR-204 and suggested that miR-204 downregulation might result in overexpression of JAK2 in breast cancer.
Figure 3.
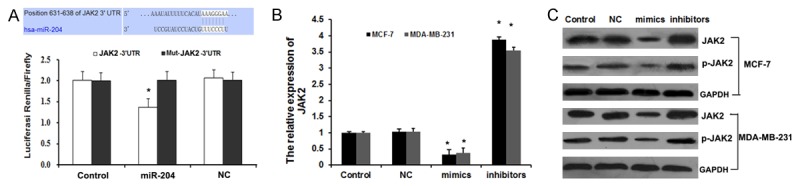
JAK2 is a direct target of miR-204. A. The potential interaction between miR-204 and two putative binding sites in the JAK2 3’-UTR predicted by Target Scan. The mutant sequences are equivalent to the wild-type ones with the exception of mutations at the 3’ end of the target site. The luciferase activities were analyzed in HEK293 cells 48 h after transfection. The data are presented as the means ± SEM of three separate experiments, *P<0.05. B. qRT-PCR analyses were performed to examine the effects of miR-204 on expression of JAK2. Error bars represent ±S.E. and *, P<0.01 versus control. C. Western blotting was performed to determine JAK2 and p-JAK2 protein levels in MCF-7 and MDA-MB-231 cells.
miR-204 regulates cell apoptosis by targeting JAK2 in breast cancer
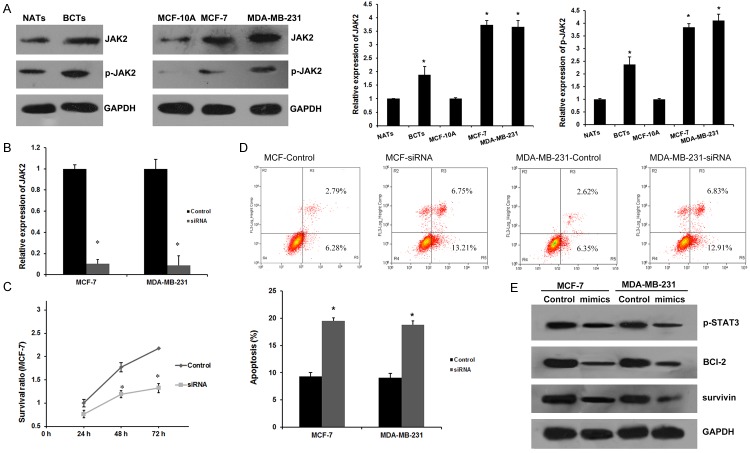
We examined the expression of JAK2 in breast cancer tissues and cell lines. We found that JAK2 is overexpressed in breast cancer tissues and breast cancer cell lines (MCF-7 and MDA-MB-231) than in NATs and MCF-10A cell line, respectively. The same qualitative alteration was observed in terms of the expression of phosphorylated JAK2 (Figure 4A). To further confirm the role of JAK2 in the anti-neoplastic effects of mir-204, we silenced JAK2 by RNAi in both MCF-7 and MDA-MB-231 cells. The effect of JAK2 siRNA on JAK2 mRNA was determined by qRT-PCR (Figure 4B). As shown in Figure 4C and 4D, specific JAK2 siRNAs markedly suppressed cell proliferation and apoptosis. Therefore, we concluded that JAK2 serves as a direct mediator of the antitumor effects of miR-204 in breast cancer carcinogenesis.
Figure 4.
miR-204 regulates apoptosis by targeting JAK2 in breast cancer cells. A. JAK2 and p-JAK2 expression levels in human breast cancer tissues and cell lines. *P<0.01 vs. NATs or MCF-10A group. B. miRNA levels of JAK2 in MCF-7 and MDA-MB-231 cells after treated with JAK2 for 48 h. *P<0.01 vs. Control group. C. JAK2 siRNA decreased the viability of breast cancer cells by MTT assay. The cell viability was significantly decreased after transfection with JAK2 siRNA for 48 h; n=6 *P<0.01 vs. Control group. D. Apoptosis of breast cancer cells after JAK2 siRNA treatment for 48 h by flow cytometry. Data are presented as means ± SEM of three separate experiments; *P<0.01 vs. Control group. E. p-STAT3, Bcl-2, survivin proteins were detected after transfection of miR-204 mimics.
It is widely accepted that the activation of JAK2-STAT3 cascade is involved in a variety of pathologies, including cancer transformation. STAT3 is a downstream target of JAK2, which is activated by JAK2 to p-STAT3. As shown in Figure 4E, miR-204 mimics significantly reduced the expression of p-STAT3 in MCF-7 and MDA-MB-231 cells. As a significant transcription factor, STAT3 can contribute to carcinogenesis by up-regulating target oncogenes, such as BCl-2 and survivin [21]. As expected, western blotting analysis revealed that a pretreatment with miR-204 mimics could inhibit the expression of BCl-2 and survivin in breast cancer.
All together, these results suggested that miR-204 participates cell proliferation and apoptosis of breast cancer by regulating JAK2-STAT3 signaling and the downstream target genes BCl-2 and survivin.
Discussion
Breast cancer morbidity ranks the first accounting for 29% in all the women suffering cancer [1]. Although the advances in diagnosis and appropriately systemic therapy contribute to the prognosis of breast cancer, breast cancer is the most common cause of cancer death in females worldwide. In this study, we showed that miR-204 is down-regulated in breast cancer tissues and cells, which is consistent with that of a previous study [18]. Furthermore, we discovered an important downstream target of miR-204, JAK2, a crucial intracellular mediator of cytokine and hormone signaling, which accounts for the pro-apoptotic effects of miR-204 in breast cancer cells.
It has been well demonstrated that microRNAs (miRNAs), as post-transcriptional gene regulators, potentially play essential roles in multiple biological processes, including cell proliferation, cycle, differentiation, angiogenesis, invasion, and migration. Recently, accumulating deregulated miRNAs were observed in various cancer cells. These miRNAs involves in the regulation of tumor proliferation, metastasis, and invasion and offer potential therapeutic targets for cancer intervention and treatment [15]. miR-204, as a tumor suppressor microRNA, was shown to be down-regulated in human intrahepatic cholangiocarcinoma, head and neck squamous cell carcinoma, gastric cancer, non-small-cell lung carcinoma and endometrial cancer [22-26]. A functional analysis has demonstrated that miR-204 acts as a tumor suppressor by inhibiting NUAK1 expression in non-small-cell lung carcinoma [25]. Moreover, miR-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favorable clinical outcome [27]. Recent study showed that decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer [18]. However, the underlying mechanism is still unclear. In this study, we explored the role of miR-204 in breast cancer. Consistently, we observed the down-regulation of miR-204 in human breast cancer samples and cell lines, and its pro-apoptotic activity in breast cancer.
JAK2 is a non-receptor tyrosine kinase and is involved in the proliferation, angiogenesis, immune evasion, and anti-apoptosis of cancer cells [5]. Constitutive activation of the JAK signaling pathway is observed in a variety of cancers [28], including human breast cancer [10,29], so inhibition of JAK2 was consider as a novel strategy in developing anticancer agents [30-32]. Consistent with these results, our study showed that JAK2 is overexpressed in both breast cancer tissue and cell lines. Furthermore, dual-luciferase reporter assay showed that JAK2 is the direct target of miR-204 and JAK2 expression was suppressed by miR-204 in breast cancer, which was alleviated by co-transfection of miR-204 inhibitors. We further confirm the role of JAK2 in the anti-neoplastic effects of mir-204 by silencing JAK2 with siRNA. The specific JAK2 siRNAs markedly suppressed cell proliferation and apoptosis. Therefore, we concluded that JAK2 serves as a direct mediator of the antitumor effects of miR-204 involved in breast cancer carcinogenesis.
Signal transducer and activator of transcription (STAT) proteins are family of transcription factors whose activity is associated with wide variety of biological processes, including cell proliferation, apoptosis and tumor progression [33,34]. Among other STATs, the constitutive activation of STAT3 is frequently reported in ~60% of breast tumors [35]. Because STAT3 is a downstream molecule of JAK2, JAK2 phosphorylates STAT3 protein, which dimerizes and translocate to the nucleus to regulate transcription of various target oncogenes, such as BCl-2 and survivin [21,36,37]. BCl-2 and survivin functions as anti-apoptotic genes and pro-metastatic genes in various human cancers [21,38,39]. Here we further investigated the effect of miR-204 on the expression of STAT3, BCl-2 and survivin in breast cancer. In this study, western blotting analysis revealed that a pretreatment with miR-204 mimics could inhibit the expression of BCl-2 and survivin in breast cancer, suggesting that miR-204 regulates cell apoptosis of breast cancer via BCl-2/survivin pathway.
In conclusion, the present study demonstrated that miR-204 directly targets JAK2 and induces breast cancer cell apoptosis through the inhibition of STAT3/BCl-2/survivin pathway. Our findings highlight the potential of miR-204 as a prognosis marker and therapeutic target for breast cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 4.Qian C, Wang J, Yao J, Wang L, Xue M, Liu W, Si J. Involvement of nuclear JAK2 signaling in AG490-induced apoptosis of gastric cancer cells. Anat Rec (Hoboken) 2013;296:1865–1873. doi: 10.1002/ar.22820. [DOI] [PubMed] [Google Scholar]
- 5.Qian CJ, Yao J, Si JM. Nuclear JAK2: form and function in cancer. Anat Rec (Hoboken) 2011;294:1446–1459. doi: 10.1002/ar.21443. [DOI] [PubMed] [Google Scholar]
- 6.Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Updat. 2010;13:67–78. doi: 10.1016/j.drup.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vera J, Rateitschak K, Lange F, Kossow C, Wolkenhauer O, Jaster R. Systems biology of JAK-STAT signalling in human malignancies. Prog Biophys Mol Biol. 2011;106:426–434. doi: 10.1016/j.pbiomolbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Valentino L, Pierre J. JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol. 2006;71:713–721. doi: 10.1016/j.bcp.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Behera R, Kumar V, Lohite K, Karnik S, Kundu GC. Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis. 2010;31:192–200. doi: 10.1093/carcin/bgp289. [DOI] [PubMed] [Google Scholar]
- 11.Harry BL, Eckhardt SG, Jimeno A. JAK2 inhibition for the treatment of hematologic and solid malignancies. Expert Opin Investig Drugs. 2012;21:637–655. doi: 10.1517/13543784.2012.677432. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 14.Tutar L, Tutar E, Tutar Y. MicroRNAs and Cancer; an Overview. Curr Pharm Biotechnol. 2014;15:430–7. doi: 10.2174/1389201015666140519095304. [DOI] [PubMed] [Google Scholar]
- 15.Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N. Regulation of breast cancer and bone metastasis by microRNAs. Dis Markers. 2013;35:369–387. doi: 10.1155/2013/451248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang HH, Liu X, Liang DS, Lu YJ, Shan HL, Jiang HC. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem. 2012;30:631–641. doi: 10.1159/000341444. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Yang L, Liu X, Chen W, Chang L, Chen L, Loera S, Chu P, Huang WC, Liu YR, Yen Y. MicroRNA-657 promotes tumorigenesis in hepatocellular carcinoma by targeting transducin- like enhancer protein 1 through nuclear factor kappa B pathways. Hepatology. 2013;57:1919–1930. doi: 10.1002/hep.26162. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7:3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 19.Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, Yang B. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Che W, Xue J, Zheng C, Tang K, Zhang J, Wen J, Xu Y. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 2013;32:655–662. doi: 10.1159/000354469. [DOI] [PubMed] [Google Scholar]
- 21.Huang K, Li LA, Meng YG, You YQ, Fu XY, Song L. Arctigenin promotes apoptosis in ovarian cancer cells via the iNOS/NO/STAT3/survivin signalling. Basic Clin Pharmacol Toxicol. 2014;115:507–511. doi: 10.1111/bcpt.12270. [DOI] [PubMed] [Google Scholar]
- 22.Qiu YH, Wei YP, Shen NJ, Wang ZC, Kan T, Yu WL, Yi B, Zhang YJ. miR-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell Physiol Biochem. 2013;32:1331–1341. doi: 10.1159/000354531. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Yang X, Huang Y, Fan H, Zhang Q, Wu Y, Li J, Hasina R, Cheng C, Lingen MW, Gerstein MB, Weichselbaum RR, Xing HR, Lussier YA. Network modeling identifies molecular functions targeted by miR-204 to suppress head and neck tumor metastasis. PLoS Comput Biol. 2010;6:e1000730. doi: 10.1371/journal.pcbi.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacconi A, Biagioni F, Canu V, Mori F, Di Benedetto A, Lorenzon L, Ercolani C, Di Agostino S, Cambria AM, Germoni S, Grasso G, Blandino R, Panebianco V, Ziparo V, Federici O, Muti P, Strano S, Carboni F, Mottolese M, Diodoro M, Pescarmona E, Garofalo A, Blandino G. miR-204 targets Bcl-2 expression and enhances responsiveness of gastric cancer. Cell Death Dis. 2012;3:e423. doi: 10.1038/cddis.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Zhang B, Sun X, Lu S, Liu Z, Liu Y, Li H, Wang L, Wang X, Zhao C. MiR-204 inhibits human NSCLC metastasis through suppression of NUAK1. Br J Cancer. 2014;111:2316–27. doi: 10.1038/bjc.2014.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung TK, Lau TS, Cheung TH, Yim SF, Lo KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, Barroilhet LM, Ng AS, Wong RR, Wang VW, Mok SC, Smith DI, Berkowitz RS, Wong YF. Dysregulation of microRNA-204 mediates migration and invasion of endometrial cancer by regulating FOXC1. Int J Cancer. 2012;130:1036–1045. doi: 10.1002/ijc.26060. [DOI] [PubMed] [Google Scholar]
- 27.Ryan J, Tivnan A, Fay J, Bryan K, Meehan M, Creevey L, Lynch J, Bray IM, O’Meara A, Tracey L, Davidoff AM, Stallings RL. MicroRNA-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favourable clinical outcome. Br J Cancer. 2012;107:967–976. doi: 10.1038/bjc.2012.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu LF, Zhu YB, Qiao MM, Zhong J, Tu SP, Wu YL. [Constitutive activation and clinical significance of Stat3 in human gastric cancer tissues and cell lines] . Zhonghua Yi Xue Za Zhi. 2004;84:2064–2069. [PubMed] [Google Scholar]
- 29.Balanis N, Wendt MK, Schiemann BJ, Wang Z, Schiemann WP, Carlin CR. Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. J Biol Chem. 2013;288:17954–17967. doi: 10.1074/jbc.M113.475277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma A, Kambhampati S, Parmar S, Platanias LC. Jak family of kinases in cancer. Cancer Metastasis Rev. 2003;22:423–434. doi: 10.1023/a:1023805715476. [DOI] [PubMed] [Google Scholar]
- 31.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, Armstrong B, Bebernitz G, Weng S, Wang L, Ye M, McEachern K, Chen H, Morosini D, Bell K, Alimzhanov M, Ioannidis S, McCoon P, Cao ZA, Yu H, Jove R, Zinda M. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 34.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 35.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butturini E, Carcereri de Prati A, Chiavegato G, Rigo A, Cavalieri E, Darra E, Mariotto S. Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoural cells to chemotherapeutic drugs. Free Radic Biol Med. 2013;65:1322–1330. doi: 10.1016/j.freeradbiomed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Hsu KW, Hsieh RH, Huang KH, Fen-Yau Li A, Chi CW, Wang TY, Tseng MJ, Wu KJ, Yeh TS. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis. 2012;33:1459–1467. doi: 10.1093/carcin/bgs165. [DOI] [PubMed] [Google Scholar]
- 38.Wen K, Fu Z, Wu X, Feng J, Chen W, Qian J. Oct-4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells: effects associated with STAT3/Survivin. Cancer Lett. 2013;333:56–65. doi: 10.1016/j.canlet.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Yeh CT, Huang WC, Rao YK, Ye M, Lee WH, Wang LS, Tzeng DT, Wu CH, Shieh YS, Huang CY, Chen YJ, Hsiao M, Wu AT, Yang Z, Tzeng YM. A sesquiterpene lactone antrocin from Antrodia camphorata negatively modulates JAK2/STAT3 signaling via microRNA let-7c and induces apoptosis in lung cancer cells. Carcinogenesis. 2013;34:2918–2928. doi: 10.1093/carcin/bgt255. [DOI] [PubMed] [Google Scholar]