Abstract
Ovarian cancer, the most lethal gynecological cancer, related closely to tumor stage. High-grade ovarian cancer always results in a late diagnose and high recurrence, which reduce survival within five years. Until recently, curable therapy is still under research and anti-angiogenesis proves a promising way. Tumor-derived exosomes are essential in tumor migration and metastases such as angiogenesis is enhanced by exosomes. In our study, we have made comparison between high-grade and unlikely high-grade serous ovarian cancer cells on exosomal function of endothelial cells proliferation, migration and tube formation. Exosomes derived from high-grade ovarian cancer have a profound impact on angiogenesis with comparison to unlikely high-grade ovarian cancer. Proteomic profiles revealed some potential proteins involved in exosomal function of angiogenesis such as ATF2, MTA1, ROCK1/2 and so on. Therefore, exosomes plays an influential role in angiogenesis in ovarian serous cancer and also function more effectively in high-grade ovarian cancer cells.
Keywords: Ovarian cancerexosome, angiogenesis, proteomic profiles
Introduction
Ovarian cancer remains the worst prognosis in gynecological cancer, with 140,000 women dying of ovarian cancer every year in the world [1]. Majority of women suffering from ovarian caner will relapse and developed resistance to further treatment despite advanced technology in chemotherapy and surgery [2]. Heterogeneity of ovarian cancer may be responsible for high recurrence, which adds great difficulty to improve the prognosis of it [3]. Introducing new ways such as targeting on tumor angiogenesis may be urgent and effective way in ovarian cancer treatment.
It is well known that solid tumor development depends largely on angiogenesis. Angiogenesis is a significant feature of cancer, which refers to aberrant formation of new blood vessels to support tumor growth, migration and metastasis. In previous studies, a number of regulators of pathogenic angiogenesis have been identified, but our understanding of this field is still incomplete [4]. Endothelial cells are the most important components of vessel barrier, also are the main anti-angiogenesis target. Endothelial cells ability of proliferation, migration and tube formation are all involved in angiogenesis. Unknown factors such as exosomes targeted on endothelial cells in angiogenesis are essential to be figured out.
Exosomes ranging in size from 40-100 nm in diameter, secreted by cells are proposed to be mechanism through which secreted cells pass signals to targeted cells. A great deal of information reveals the complexity of exosomes from different cell types, containing 194 lipids, 4,563 proteins, 1639 mRNA and 764 microRNAs and demonstrating their intimate relationship related to tumor development [5,6]. Research on treatment of prostate cancer has achieved significant progress that targeted antigen localization to exosomes might be a viable way [7]. However, exosomes applied in ovarian cancer therapy still remains deeper researches. Based on heterogeneity of ovarian cancer and characters of exosomes derived from tumor cells, we have focused on the role of exosomes in angiogenesis to explore the effectiveness and feasibility of exosomes in ovarian cancer treatment.
Materials and methods
Cell lines and cell culture
Serous ovarian epithelial cancer is the most common pathologic type among multiple ovarian cancers. SKOV3 cell lines represented unlikely high-grade of serous ovarian cancer and CAOV3 cell lines represented high-grade serous ovarian cancer [8]. Cell lines were all purchased from Shanghai Institute of cell library, China. All ovarian cancer cell lines were cultured in RPMI medium 1640 (Hyclone) added with 10% (vol/ vol) fetal bovine serum (Hyclone). Primary human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC) and cultured in M199 medium (GBICO) supplemented with 10% (vol/vol) FBS, 1 µg/ml hydrocortisone and 10 ng/ml epidermal growth factor (EGF). 2 mM L-glutamine, 100 U/ml streptomycin and 100 U/ml penicillin were supplemented to all media. All cells were cultured at 37°C with 5% CO2 and 95% air in an humidified incubator.
Exosome extraction
Exosomes were extracted from cell conditioned media by differential centrifugation. SKOV3 and SKOV3 cells were starved for 72 h when the cells reached 80% confluence. Condition media were collected from starved cells and centrifuged at 3,000 g, 15 min at 4°C to eliminate cell debris. Supernatant were then centrifuged at 20,000 g for 30 min at 4°C. Exosome pellets were obtained by ultracentrifugation at 100,000 g for 80 min at 4°C. Pellets were suspended by large volume of PBS and ultracentrifugation at 100,000 g for 80 min at 4°C. The wash step was repeated once and protein concentration of exosomes was determined by BCA Protein Assay Kit (Thermo).
Transmission electron microscopy
Exosomes were fixed in 2.5% glutaraldehyde incacodylatd buffer, stained with 0.75% uranyl formate. Exosomes were observed by transmission electron microscope (NanoPort) at 6 kV acceleration voltage and images were recorded.
Western blotting
Exosomes were lysed with IP lysis buffer (Beyotime) supplemented with Protease Inhibitor Mixture (Roche). Cell lysates were separated used electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gel, transferred onto nitrocellulose membranes, blocked in 5% BSA supplemented 0.1% Tween. After blocking, membranes incubated with primary antibodies: anti-CD63 (rabbit anti-human polyclonal antibody, Abcam). Odyssey Infrared Imaging System (Li-COR) was used to visualized after incubated by goat anti-rabbit secondary antibody with IRDye 800CW-labeled (Li-COR Biosciences, Lincoln, NE). The densitometry was quantified by ImageJ software (NIH, Bethesda, MD).
Cell viability assay
Cell viability was measured using a Cell Counting Kit-8 (CCK8, Dojindo). HUVECs were seeded in 96-well plates at 5,000 cells per well overnight, then treated with exosomes, which respectively derived from SKOV3 and CAOV3 cell lines at concentrations of 10 µg/ml and 50 µg/ml and PBS as vector. Absorbance was measured at 450 nm after 0, 24, 48, 72 h after incubation with exosomes. Absorbance was determined at 450 nm after 2 hours of incubation.
Cell migration assay
This assay was performed as described before in our lab [9]. HUVECs were harvested after being starved overnight, Cells were seeded to the top chambers of 8 um pore cell culture (Millipore) at 20,000 cells with serum-free medium, then co-incubated with 10 µg/ml exosomes purified from CAOV3, 50 µg/ml exosomes derived from SKOV3 as described above as respectively effective concentration and PBS as vector for 8 hours. Fixed the migrated cells affixed to bottom membranes, stained with crystal violet, and calculated every five stochastic fields under the microscope (Olympus).
Endothelial cell tube formation assays in vitro
50 µl of growth factor reduced matrigel (BD Bioscience) were bedded into each well of a 96-well plate and polymerized at 37°C for 30 minutes. HUVECs were seeded at 10,000 cells per 96-well and suspended in conditioned medium (PBS contrasted to exosomes derived from CAOV3 and SKOV3 cell lines at respectively concentrations of 10 µg/ml and 50 µg/ml) for 4 h. The angiogenic property was determined by calculating the number of tubes per 400× microscopic field.
Chorioallantoic membranes (CAMs) angiogenesis assay
CAMs angiogenesis assay was carried out to confirm the effect of exosomes on blood vessel formation in developing fertilized chicken eggs in vivo-like conditions. Eggs after fertilized for 9 days were incubated at 37°C in atmosphere in humidified conditions for two days. On the third day, a small hole was performed at the end of the egg which with air hole. Mixed cellulose esters of 5 mm diameter carried PBS in contrast to exosomes respectively at concentration of 50 µg/ml from SKOV3 and 10 µg/ml from CAOV3 were kept in the hole. The windows were closed by transparent tape. Another 72 h of incubation, CAMs were photographed using a camera (Nikon, Japan). Nascent vessel formation around membranes within 1 mm and 5 mm were respectively quantified, and the data are represented as mean ± SE of total number of vessels in 3 independent CAMs for each treatment. This experiment was repeated with similar data.
Cell morphology monitor
HUVECs were co-cultivated with exosomes derived from CAOV3 and SKOV3 cell lines at respective concentrations of 10 µg/ml and 50 µg/ml for 24 h, also setting PBS as vector. The morphology of cells was monitored under microscope and photograph by camera at 4 h (Olympus).
Proteomic mass spectrometry analysis
We collected exosomes, which were derived from high-grade serous ovarian cancer (CAOV3) and unlikely high-grade serous ovarian cancer (SKOV3), respectively analyzed proteins of them by proteomic mass spectonmetry analysis according to manual. Identified proteins were analyzed by David (http://david.abcc.ncifcrf.gov/). This website allows classifying proteins to facilitate high-throughput analysis. Differentially expressed proteins participate on angiogenesis were also referred to PubMed search results. We figured out different proteins which might influence angiogenesis that can be detected in exosomes derived from CAOV3 but not SKOV3.
Statistical analysis
All data have been analyzed as the mean ± standard deviation (SD). Student’s t-test has been performed for calculating statistical significance between groups with SPSS version 11.5 (SPSS Inc.). Differences with P values (*) < 0.05 are referred to significant level.
Results
Exosomes extracted from conditioned media were typical
We chose the SKOV3 cell lines as models for unlikely high-grade serous ovarian cancer, and CAOV3 as models for high-grade serous ovarian cancer for studying cancer-secreted exosomes. Exosomes extracted from conditioned media manifests unique cup-shaped result under electron microscopy and ranging from 40-100 nm (Figure 1A). Exosomes purified by ultracentrifugation have been illustrated by highly CD63-positive nanovesicles which are used as exosome marker (Figure 1B).
Figure 1.
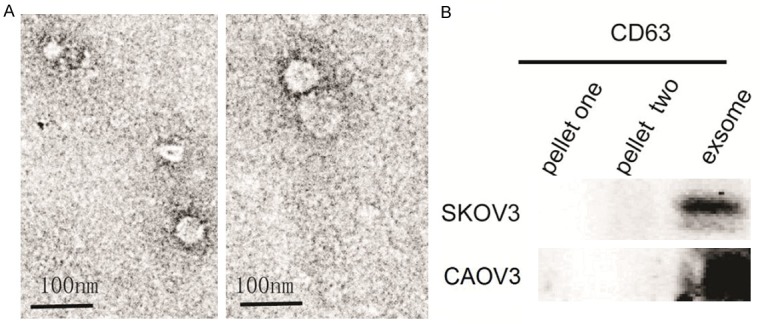
Characterization of exosomes derived from ovarian cancer. A. Electron microscopy photographs indicating intact exosomes range from 40-100 nm (scale bar: 100 nm). B. Analysis showed pellets of ultracentrifugation highly expressed of exosome markers CD63 compared with pellets of centrifugation.
Exosomes derived from ovarian cancer promote the viability and migration of HUVECs
We have focused on exosomal function on endothelial cells in this research for their principal barrier function while growth and metastasis. We identified that exosomes purified from CAOV3 could significantly increase the viability (Figure 2A) and migration of HUVECs (Figure 2C) at the concentration of 10 µg/ml. Exosomes purified from SKOV3 significantly prompted the viability and migration of HUVECs only when reaching the concentration of 50 µg/ml (Figure 2B, 2C).
Figure 2.
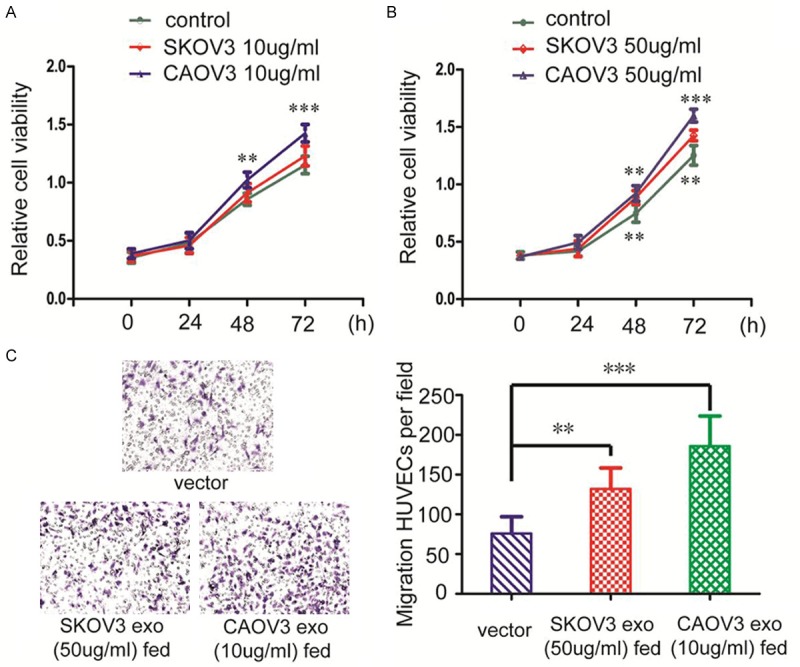
Exosomes derived from ovarian cancer have enhanced cell viability of HUVECs. (A) 10 µg/ml of exosomes purified from CAOV3 significantly enhanced cell viability of HUVECs after 48 h, but corresponding concentration of exosomes purified from SKOV3 prove of failed to significantly enhance cell viability of HUVECs in 72 h. (B) When the concentration of exosomes derived from CAOV3 and SKOV3 reached 50 µg/ml, cell viability of HUVECs all significantly enhanced after 48 h. (C) 10 µg/ml of exosomes purified from CAOV3 and 50 µg/ml of exosomes purified from SKOV3 enhanced HUVECs migration. The enhancement were more obviously in exosomes derived from CAOV3 than that from SKOV3. Quantification analysis data signified the means ± standard deviation (SD), n = 5; **P < 0.01, ***P < 0.001.
Metastatic ovarian cancer derived exosomes promote tube formation both in vitro and in vivo
Angiogenic tube formation assays in vitro have shown 10 µg/ml exosomes purified from CAOV3 induce tube formation of endothelial cells to a greater extent in contrast to those at the concentration of 50 µg/ml derived from SKOV3 (Figure 3A, 3B). The vivo vessel formation also performed the similar results at the same concentration of two kinds of exosomes as in vitro (Figure 3C, 3D).
Figure 3.
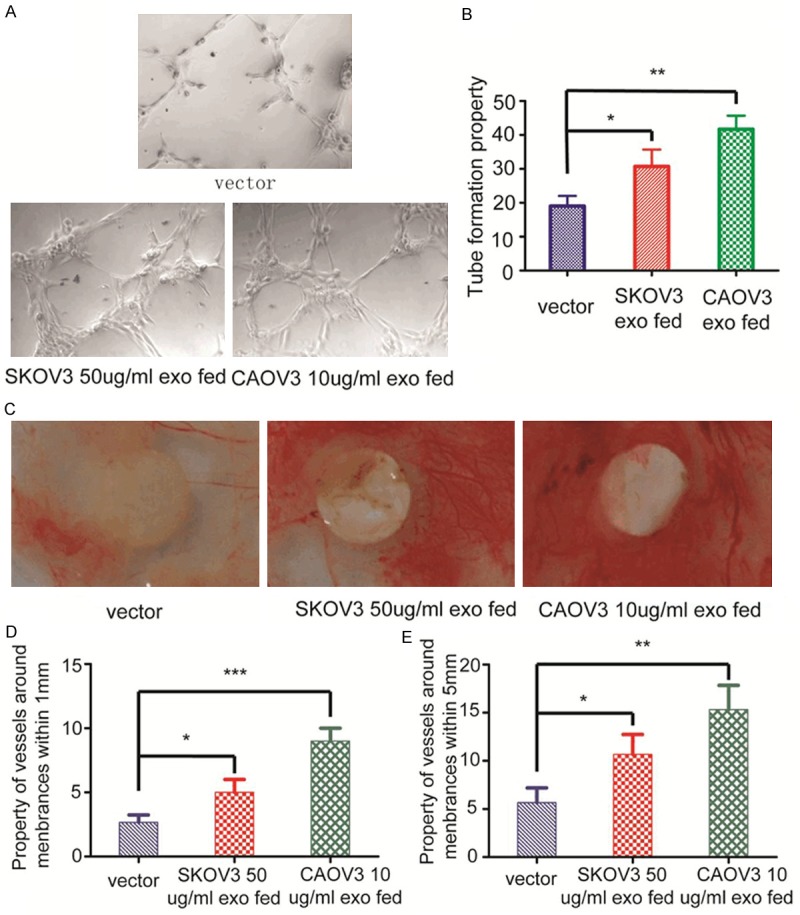
Exosomes derived from ovarian cancer have enhanced tube formation ability both in vitro and in vivo. A. 10 µg/ml exosomes purified from CAOV3 induce tube formation of endothelial cells to a greater extent in contrast to those at the concentration of 50 µg/ml derived from SKOV3. B. Property of tube analysis indicated the means ± standard deviation (SD), n = 3; *P < 0.05, **P < 0.01. C. Just as in vitro study, 10 µg/ml exosomes derived from CAOV3 induce CAMs vessel formation to a greater extent in contrast to those at the concentration of 50 µg/ml purified from SKOV3. D. Property of vessels analysis around membranes within 1 mm diameter signified the means ± standard deviation (SD), n = 3; *P < 0.05, ***P < 0.001. E. Property of vessels analysis around membranes within 5 mm diameter indicated the means ± standard deviation (SD), n = 3; *P < 0.05, **P < 0.01.
Metastatic ovarian cancer derived exosomes changes HUVECs morphology
Due to the property of exosomes promoting HUVECs migration and tube formation, the morphology change of HUVECs after exosome being co-cultivated have been monitored. After HUVECs cocultivated with exosomes purified respectively from CAOV3 (10 µg/ml) and SKOV3 (50 µg/ml) after 4 h, cells have turned from cobble-stone morphology to round which lasted for at least 24 h (Figure 4).
Figure 4.
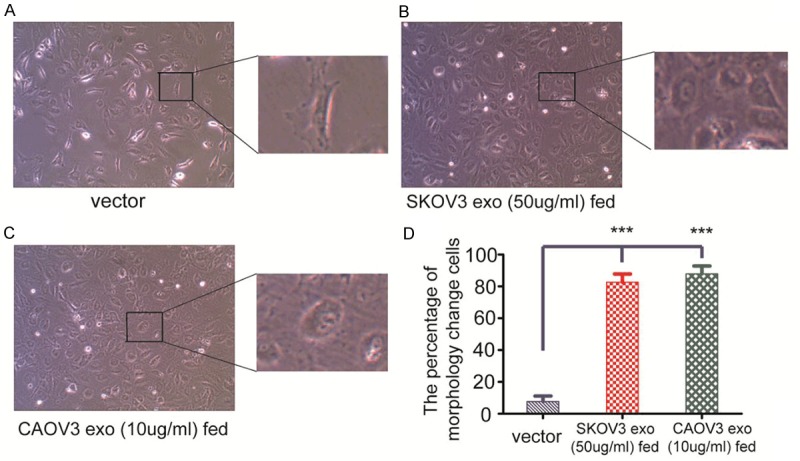
HUVECs morphology changes. A. After HUVECs being co-cultivated with PBS for 4 h, the morphology of HUVECs remain cobble-stone like. B. After HUVECs being co-cultivated with 10 µg/ml exosomes derived from CAOV3, the morphology of HUVECs have changed morphology from cobble-stone to round. C. When 50 µg/ml exosomes derived from SKOV3 co-cultivated with HUVECs for 4 h, the majority of HUVECs have also changed morphology from cobble-stone to round. D. Quantification analysis data signified the means ± standard deviation (SD), n = 3; ***P < 0.001.
Differential proteomic analysis of exosomes from CAOV3 and SKOV3
In order to discover the mechanism of different behaviors of different grade of ovarian cancer on angiogenesis, we used mass spectrometry analysis to compare protein components in above two cell lines. 10 exosomal proteins related to angiogenesis were identified only in CAOV3 but not in SKOV3 (Table 1). These proteins might influence angiogenesis between high-grade serous ovarian cancer and unlikely high-grade cell lines identified by mass spectrometry analysis.
Table 1.
Differential proteomic analysis of exosomes from CAOV3 and SKOV3 related to angiogenesis
| Symbol | Entrez Gene Name | Location | Type(s) |
|---|---|---|---|
| ATF2 | Activating transcription factor 2 | Nucleus | Protein coding |
| MTA1 | Metastasis-associated protein 1 | Nucleus | Protein coding |
| ABCB1 | ATP-Binding Cassette, Sub-Family B, Member 1 | Plasma membrane | Protein coding |
| SARS | Seryl-TRNA Synthetase | Cytosol | Protein coding |
| HNRNPA | Heterogeneous nuclear ribonucleoprotein A | Nucleus | Protein coding |
| ROCK1 | Rho-Associated, Coiled-Coil Containing Protein Kinase 1 | Golgi apparatus, cytosol | Protein coding |
| ROCK2 | Rho-Associated, Coiled-Coil Containing Protein Kinase 2 | Cytoskeleton, nucleus | Protein coding |
| HMGB1 | High Mobility Group Box 1 | Nucleus, excellular | Protein coding |
| HMGB2 | High Mobility Group Box 2 | Nucleus, excellular | Protein coding |
| HDAC2 | Histone Deacetylase 2 | Nucleus | Protein coding |
Discussion
Ovarian cancer is the most deadly gynecological tumor due to a late diagnose, high recurrence and lack of effective medicine [10]. The prognosis of ovarian cancer in advanced stage has only 20% survivals rate in 5 years. However, if detected at an early stage (stage I, FIGO 2000), the survival can reach over 90% [11]. Reliable early diagnose and treatment especially anti-high-grade ovarian cancer. Exploring new treatment such as anti-angiogenesis is essential to ovarian cancer therapy. Exosomes have been found in biological fluids and have a series of typical proteins, lipids and RNAs composition that varies of depending on the cells from which they originate. Exosomal constituents plays an influential role in tumor migration and metastases, including damaging the intact barrier of endothelial cells. Zhou W et al. has figured out mir-105 delivered by exosomes secreted exclusively by breast cancer but not benign tumor destroyed the barrier of endothelial cells, which contribute to tumor development [12]. Umezu T et al. found hypoxic multiple myeloma cells shed exosomal miR-135b enhanced angiogenesis by factor-inhibiting HIF-1 [13]. Ekstrom et al. pointed out that WNT5A signaling induces melanoma cells release of exosomes containing pro-angiogenic proteins IL-6, VEGF and MMP2 [14]. However, seldom surveys have covered exosomes from ovarian caner and angiogenesis [15]. We initially discover ovarian cancer can secreted exosomes, which are loaded diverse components derived from ovarian cancer cells of different grade malignancy, have played unlikely functions on angiogenesis.
Besides elemental studies on exosomes, clinical applications of exosomes have proved a big improvement. In diagnosis, laboratory and clinical research have already revealed exosomes to be a reliable and accessible biological specimen of biomarkers and early discovery of illness, with many unknown to be explored [5,16]. In therapy, exosomes derived from tumor have been applied to present tumor antigens and carried them to T cells, further induce the anti-tumor immune response, resulting in tumor cell death [17]. These findings manifested that tumor-secreted exosomes may be promising in developing a vaccine of cell-free and anti-cancer to fix the issue of exosome-mediated drug resistance [16,18].
Considering clinical application of exosomes, in current studies, we contrasted exosomes from different grades of serous ovarian cancer and discovered their different effects on angiogenesis. Serous ovarian caner is most common among ovarian tumor. Based on re-evaluating cell lines of ovarian cancer, we have set two cell lines as our survey models: CAOV3 as possibly high-grade serous ovarian caner and SKOV3 as unlikely high-grade serous ovarian cancer. To our interest, whatever in proliferation, migration or tube formation of HUVECs, exosomes from CAOV3 manifested more aggressive behavior than that from SKOV3. For instance, 10 µg/ml exosomes from CAOV3 could more effectively enhanced tube formation ability in vitro and in vivo than 50 µg/ml exosomes from SKOV3. This exclusively discovery suggested that high-grade of serous ovarian cancer facilitate metastasis through shedding tumor-derived exosomes to induce angiogenesis.
Exosomes are full of common constituents which are fusion of multivesicular bodies (MVB), attachment to target cells and so on, such as CD9, CD63, CD81, HSP70 and HSP 90 that could be markers for discrimination of exosomes [16]. Tumor-derived exosomes had packaged other special composition from original cells which play roles directly or indirectly in angiogenesis. Thus we searched in PubMed for proteins that were reported to be related to angiogenesis and analyzed their expression profiles between exosomes derived from SKOV3 and CAOV3. First of all, many cytosol proteins were packaged by exosomes and performed paracrine function to targeted cells [19]. Secondly, many proteins lies in high-grade serous ovarian cancer functioned directly or indirectly in angiogenesis. For instance, ATF2, MTA1, SARS, ROCK1/2 can be detected in exosomes of high-grade cell lines of ovarian cancer but not in those of unlikely high-grade cell lines. In present studies, EBNA1 binding to ATF2 activates AP-1 transcription factor pathway and elevated expression of AP-1 targets VEGF which prompt angiogenesis in vitro [20]. HIF-1 alpha (hypoxia-inducible factor-1 alpha) can be stabilized by MTA1, resulting in enhancement of angiogenesis [21]. SARS is attributed to the malformation of vessel through canonical activity in zebra fish [22]. ROCK1 & 2 have revealed to exhibit overlapping and unique regulator in angiogenesis and angiosarcoma tumor progression [23].
Exosomal proteins that may involve in high-grade ovarian cancer metastasis that still needs to explore deeper and more specified. Previous studies have established that besides proteins, many exosomal RNAs, micro RNAs also target angiogenesis [13]. Genomic profiles are also essential for exploring pathogenesis of ovarian cancer. In our study, we exclusively show exsomes derived from different grade of ovarian cancer cell lines have diverse functions on angiogenesis. These results implicated exosomes involved in a wide range of migration and metastasis in ovarian cancer. Targeted on exosomes in ovarian cancer treatment proved to be a promising treatment in our study. On the other hand, we performed this study in vitro which needs in vivo study for further support. Genome research focus on mRNAs and micro RNAs in exosomes from different cell lines, can effectively discover those who are engaged in angiogenesis. The relationship between proteins, mRNAs and micro RNAs are essential to research differences lies in exosomes from different grades of ovarian cancer, which may figure out the underlying reason for diverse outcome of ovarian cancer.
In conclusion, exosomal constituents derived from high-grade ovarian cancer have a profound impact on angiogenesis with comparison to unlikely high-grade ovarian cancer. ATF2, MTA1 and other proteins may play a key role in exosomal enhancement of tumor development.
Acknowledgements
Thank you for Fudan University of Medicine for proteomic mass spectrometry analysis.
Disclosure of conflict of interest
None.
References
- 1.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 2.Grzankowski KS, Carney M. Quality of life in ovarian cancer. Cancer Control. 2011;18:52–8. doi: 10.1177/107327481101800107. [DOI] [PubMed] [Google Scholar]
- 3.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Abraham S, McKenzie JA, Jeffs N, Swire M, Tripathi VB, Luhmann UF, Lange CA, Zhai Z, Arthur HM, Bainbridge JW, Moss SE, Greenwood J. LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature. 2013;499:306–11. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach A, Zhang HG, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci. 2015;72:1–10. doi: 10.1007/s00018-014-1710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Brinkmann K, Dirmeier U, Laus R, Delcayre A. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011;71:5235–44. doi: 10.1158/0008-5472.CAN-10-4076. [DOI] [PubMed] [Google Scholar]
- 8.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu ZY, Yang XM, Cheng MJ, Zhang R, Ye J, Yi H, Ao JP, Zhang ZG, Xu CJ. Dysregulated cell mechanical properties of endometrial stromal cells from endometriosis patients. Int J Clin Exp Pathol. 2014;7:648–55. [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Li J, Yang X, Wang Y, Yu Y, Ye J, Xu C, Qin W, Zhang Z. MCAM is a novel metastasis marker and regulates spreading, apoptosis and invasion of ovarian cancer cells. Tumour Biol. 2012;33:1619–28. doi: 10.1007/s13277-012-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–15. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–57. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstrom EJ, Bergenfelz C, von Bülow V, Serifler F, Carlemalm E, Jönsson G, Andersson T, Leandersson K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer. 2014;13:88. doi: 10.1186/1476-4598-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappellesso R, Tinazzi A, Giurici T, Simonato F, Guzzardo V, Ventura L, Crescenzi M, Chiarelli S, Fassina A. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122:685–93. doi: 10.1002/cncy.21442. [DOI] [PubMed] [Google Scholar]
- 16.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 17.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–81. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–42. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas SN, Liao Z, Clark D, Chen Y, Samadani R, Mao L, Ann DK, Baulch JE, Shapiro P, Yang AJ. Exosomal proteome profiling: a potential multi-marker cellular phenotyping tool to characterize hypoxia-induced radiation resistance in breast cancer. Proteomes. 2013;1:87–108. doi: 10.3390/proteomes1020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neil JD, Owen TJ, Wood VH, Date KL, Valentine R, Chukwuma MB, Arrand JR, Dawson CW, Young LS. Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitro. J Gen Virol. 2008;89:2833–42. doi: 10.1099/vir.0.2008/003392-0. [DOI] [PubMed] [Google Scholar]
- 21.Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis. 2009;26:215–27. doi: 10.1007/s10585-008-9233-8. [DOI] [PubMed] [Google Scholar]
- 22.Fukui H, Hanaoka Rand Kawahara A. Noncanonical activity of seryl-tRNA synthetase is involved in vascular development. Circ Res. 2009;104:1253–9. doi: 10.1161/CIRCRESAHA.108.191189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montalvo J, Spencer C, Hackathorn A, Masterjohn K, Perkins A, Doty C, Arumugam A, Ongusaha PP, Lakshmanaswamy R, Liao JK, Mitchell DC, Bryan BA. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr Mol Med. 2013;13:205–19. doi: 10.2174/1566524011307010205. [DOI] [PMC free article] [PubMed] [Google Scholar]


