Abstract
Aim: The aim of this study was to explore the role of hydrogen sulfide on wound healing in diabetic rats. Methods: Experimental diabetes in rats was induced by intraperitoneal injection of streptozotocin (STZ) (in 0.1 mol/L citrate buffer, Ph 4.5) at dose of 70 mg/kg. Diabetic and age-matched non-diabetic rats were randomly assigned to three groups: untreated diabetic controls (UDC), treated diabetic administrations (TDA), and non-diabetic controls (NDC). Wound Healing Model was prepared by making a round incision (2.0 cm in diameter) in full thickness. Rats from TDA receive 2% sodium bisulfide ointment on wound, and animals from UDC and NDC receive control cream. After treatment of 21 days with sodium bisulfide, blood samples were collected for determination of vascular endothelial growth factor (VEGF), intercellular cell adhesion molecule-1 (ICAM-1), antioxidant effects. Granulation tissues from the wound were processed for histological examination and analysis of western blot. Results: The study indicated a significant increase in levels of VEGF and ICAM-1 and a decline in activity of coagulation in diabetic rats treated with sodium bisulfide. Sodium bisulfide treatment raised the activity of superoxide dismutase (SOD) and heme oxygenase-1 (HO-1) protein expression, and decreased tumor necrosis factor α (TNF-α) protein expression in diabetic rats. Conclusions: The findings in present study suggested that hydrogen sulfide accelerates the wound healing in rats with diabetes. The beneficial effect of H2S may be associated with formation of granulation, anti-inflammation, antioxidant, and the increased level of vascular endothelial growth factor (VEGF).
Keywords: Hydrogen sulfide, wound healing, diabetes
Introduction
Diabetes mellitus, a serious chronic metabolic disorder, results from the absolute or/and relative insufficiency of insulin. Long-term hyperglycemia produces excessive reactive oxygen species which can impair vessel and lead to vasculopathy called as diabetic vascular complications [1-3]. Diabetic vascular complications can injure some important tissues such as kidney, heart, and arteries [4-6]. Diabetic foot ulcer (DFU) is one of the most serious, and fearing complications in diabetic patients due to its particular difficulty to heal. Twenty-five of the patients with diabetes are in risk of developing the foot ulcer [7]. It is estimated that over 1% of the patients with diabetes leads to amputation as result of DFU per year [8], and amputation results in a high rate of mortality. Rate of 5-year mortality is as high as 70% in diabetic patients with amputation [9]. The pathogenesis of DFU is complex and still far from being fully understood, thus methods of effective treatment to DFU are lacking. The injury of vessels resulting from oxidative stress leads to vasoconstriction, coagulation and the peripheral circulation failure, which are important factors in the development of diabetic ulcer [10-12]. Therefore, angiogenesis and vasculogenesis play pivotal roles in the repair of wound tissues.
Hydrogen sulfide (H2S) was known as a colorless, foul and poisonous gas, but massive researches show that it exerts diverse vital roles in physiology and pathophysiology, and regarded as the third gaseous signaling molecule following the nitric oxide (NO) and carbon monoxide (CO). Endogenous H2S is produced from L-cysteine as the main substrate by catalysis of cystathionine beta-synthase (CBS) and cystathionine-gamma-lyase (CSE) [13]. Increasing studies indicate that H2S executes various biological functions such as reducing oxidative stress [14,15], regulating inflammation [16]. Chronic inflammation and oxidative stress are involved in pathogenesis of some human diseases, for example, diabetes and its complications, cardiovascular disease, and tumor etc [17-20]. Accumulated studies show that H2S can regulate proliferation and migration of endothelial cells to augment angiogenesis [21,22], exert anti-hypertensive effect through vasorelaxation [23], inhibit activation and aggregation of platelet [24], and protect myocardium against ischemia/reperfusion (I/R) injury [25].
Therefore, the present study aimed to evaluate the effects of H2S on wound healing in rats with diabetes, and the related mechanisms involving vascular endothelial growth factor expression, and change of inflammatory cytokines.
Materials and methods
Preparation of STZ-induced diabetes
Healthy male SD rats (weighing 250-300 g) were obtained from Animal Center at Wannan Medical College and housed in a standard facility at 22°C room temperature and a twelve-hour alternate between light and dark. After a week of environmental adaptation, experimental diabetes in healthy male SD rats (weighing 250-300 g) was induced by intraperitoneal injection of streptozotocin (STZ; in 0.1 mol/L ice-cold citric acid-sodium citrate buffer, pH 4.5) at dose of 70 mg/kg. After 72 hours of STZ administration, blood samples from the tail vein were collected for measure of glucose levels. Rats were diagnosed as diabetic while blood glucose concentration >16.7 mmol/L.
Wound healing model in rats
Diabetic animals and age-matched non-diabetic rats were randomly assigned to three groups: untreated diabetic controls (UDC), treated diabetic administrations (TDA), and non-diabetic controls (NDC). Animals were fed with standard pellet diet and water ad libitum. Wound healing model in rats was prepared as previously described method [26]. In brief, animals were anaesthetized by intraperitoneally injecting 10% chloral hydrate at a dose of 300 mg/kg. After hind dorsum of each rat was shaved, and disinfected with 75% ethanol, a round incision (2.0 cm in diameter) was made in full thickness. Rats from UDC and NDC receive control cream, and rats from TDA receive 2% sodium bisulfide ointment on wound. The wound healing was evaluated by measuring the size of wounded area on days 5, 10, 15 and 21. After 21 days of ointment dosing, animals from all groups were anaesthetized with chloral hydrate (300 mg/kg i.p.) and sacrificed, and fasting blood samples and granulation tissues from wound were collected for biochemical analyses.
Analyses of coagulation
Plasma were separated from fasting blood samples by centrifugation at 1300 × g. Coagulation was analyzed with appropriate kits on semi auto analyzer by the enzymatic colorimetric methods (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Change of antioxidant effects
SOD activity and MDA level were determined in serum by commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Measure of VEGF and ICAM-1
Levels of VEGF and ICAM-1 in serum were measured with rat VEGF and ICAM-1 specific ELISA kit (Hefei Bomei Biotechnology CO., LTD, China) according to manufacturer’s protocol. The levels of VEGF and ICAM were expressed ng/L pg/ml, respectively.
Estimation of wound closure rate
The wound was snapped with digital camera, and its closure rate was analyzed with Image. Pro Plus 5 software. The rate was calculated by the formula: the closure rate of wound (%) = (initial area - detected area)/initial area × 100%.
Histological examinations
At the end of experiment, granulation tissues from wound were harvested and fixed in 4% neutral formalin for pathological analysis. Fixed tissue was embedded in paraffin, 5-μm sections were stained with hematoxylin-eosin (HE) and Masson, respectively. Stained sections were used to observe the formation granulation tissues under a light microscope. The small blood vessels in wounds were counted in high power field.
Western blotting analysis
Granulation tissues (0.2 g) were collected, lysed and homogenized in 2 ml of lysis buffer containing 10 mmol/L Na3VO4, 2 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L EDTA, 100 mmol/L sodium pyrophosphate, 100 mmol/L NaF, 50 mmol/L HEPES, 2 μg/mL leupeptin, 2 μg/mL aprotinin and 1% Triton X-100 (v/v) for 20 min. Lysate was centrifuged at 12,000 g for 10 min at 4°C. Proteins from supernatants were denatured. Equal amounts of protein were separated by 12% sodium dodecyl sulfate polyacrylamide gel and then transferred to 0.2-μm nitrocellulose membranes. The membranes were blocked in Tris buffer (Ph 7.5) containing 10 mmol/L Tris, 150 mmol/L sodium chloride, 0.1% Tween -20, and 5% nonfat dry milk for 2 h. Then, the membranes were incubated with a rabbit polyclonal antibodies β-actin, HO-I, TNF-α (Wuhan Boster Bioengineering Limited Company, China) overnight at 4°C. After extensively washing, the membranes were incubated with a horseradish peroxidase-conjugated secondary anti-rabbit antibody for 1 h. The membranes were rinsed three times, and then visualized by DAB (Bio Basic Inc. Canada).
Statistics
All values were presented as mean ± standard deviation (SD). Statistical evaluation was made via one-way analysis of variance using SPSS16.0. Difference between groups was performed by Student’s t-test. Values of P<0.05 were considered as significant.
Results
General characteristic
Blood glucose levels from rats injected with STZ were increased over experimental time, and reduced weight, increased urinary volume and water consumption were observed in STZ-treated animals.
Effects of H2S on wound healing
To evaluate the wound closure, the wound area was measured, and macroscopic differences of the wounds were observed. There was no significant difference in closure rate of wound, and the wounds were swelling and purulent in the next day. After 5 days, the closure rate of the wound from TDA rats was significantly increased when compared to the DNC rats (P<0.01) (Table 1). Treatment with H2S accelerated reepithelization of the wounds in diabetic rats (Figure 4). At 21 d, the wounds from TDA rats were almost healed.
Table 1.
Effect of H2S on closure rate of wound (mean ± SD)
| NDC | UDC | TDA | |
|---|---|---|---|
| Day 5 | 21.8±4.9 | 12.7±4.7** | 18.5±5.3## |
| Day 10 | 53.4±6.3 | 35.9±6.4** | 55.7±7.2## |
| Day 15 | 78.0±5.8 | 51.4±9.2** | 83.4±7.6## |
| Day 20 | 92.9±5.2 | 76.3±8.2** | 93.8±4.7## |
Significantly different: P<0.01 vs. NDC;
Significantly different: P<0.01 vs. UDC;
n=10 per group.
Figure 4.
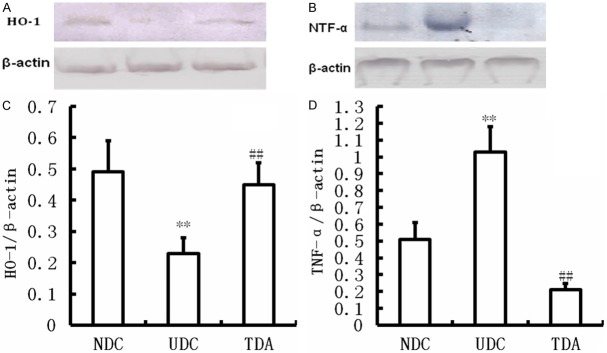
Effects of H2S on HO-1 and TNF-α protein expression. Western blot showing levels of HO-1 (A) and TNF-α (B) in the wound of Non-diabetic Controls, Untreated diabetic Controls, Treated diabetic administrations. **Significantly different: P<0.01 vs. NDC; ##Significantly different: P<0.01 vs. UDC; n=6 per group.
Effects of H2S on coagulation activity
The change of coagulation activity was observed in this study. The results showed that prothrombin time (PT) and thrombin time (TT) were shortened in diabetic rats compared with NDC rats (P<0.01) (Table 2), and hydrogen sulfide treatment significantly improved PT and TT (P<0.01) (Table 2). While level of fibrinogen in serum was elevated in UDC. Treatment with H2S reduced level of fibrinogen in serum (P<0.01) (Table 2).
Table 2.
Effect of H2S on coagulation activity (mean ± SD)
| NDC | UDC | TDA | |
|---|---|---|---|
| Prothrombin time (s) | 12.2±0.6 | 10.2±0.3** | 10.9±0.3## |
| Thrombin time (s) | 28.3±1.2 | 20.5±2.8** | 26.0±1.5## |
| Fibrinogen (mg/ml) | 1.73±0.1 | 2.91±0.2** | 1.79±0.1## |
Significantly different: P<0.01 vs. NDC;
Significantly different: P<0.01 vs. UDC;
n=10 per group.
Antioxidant effects of H2S
MDA, a production of lipid peroxidation reactions, was significantly increased in serum from UDC rats compared with that of NDC (P<0.01) (Figure 1), and reduced SOD activity was observed (P<0.01) (Figure 1). Treatment with H2S raised SOD activity, and reduced MDA production.
Figure 1.
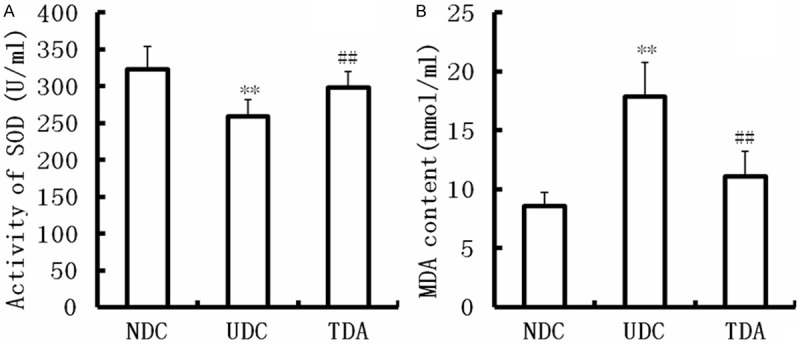
Effect of H2S on SOD activity and MDA level. SOD activity was increased and MDA level was decreased by H2S. Data are means ± SD from 10 rats. **Significantly different: P<0.01 vs. NDC; ##Significantly different: P<0.01 vs. UDC.
Furthermore, in present study we determined HO-1, an antioxidase, expression. As shown in Figure 4, HO-1 protein expression was decreased in the wounds from UDC rats compared with that of NDC (P<0.01) (Figure 4). Hydrogen sulfide increased HO-1 protein expression in the wounds from TDA rats (P<0.01) (Figure 4).
Effects of H2S on the inflammatory responses
The protein level of TNF-α showed a significant increase in the granulation tissues from UDC compared with NDC (P<0.05) (Figure 4). The change was corrected by treatment with H2S (P<0.05) (Figure 4). The present study showed the increases in leucocyte and leukomonocyte counts (P<0.05), and leukocyte infiltration was present in the wounds from UDC. Leucocyte and leukomonocyte counts were near to normal (P<0.05) (Table 3), and leukocyte infiltration was hardly observed in the granulation tissues from rats treated with H2S (Figure 3).
Table 3.
Effect of H2S on leukocyte and leukomonocyte (mean ± SD)
| NDC | UDC | TDA | |
|---|---|---|---|
| Leukocyte (109/L) | 3.91±0.53 | 22.88±2.00** | 12.21±0.88## |
| Leukomonocyte (109/L) | 2.78±0.56 | 12.66±2.82** | 7.33±0.76## |
| The number of vessel | 18±3.1 | 12±2.5** | 20±3.7## |
Significantly different: P<0.01 vs. NDC;
Significantly different: P<0.01 vs. UDC;
n=10 per group.
Figure 3.
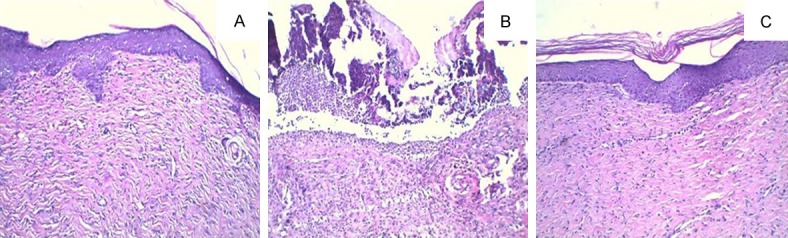
Photomicrographs of H-E staining in the wound tissue of each group. A. Non-diabetic control; B. Untreated diabetic controls; C. Treated diabetic administrations.
Effects of H2S on angiogenesis
The number of the small vessels in the granulation tissues was counted in present study under microscope. Our results showed the increasing number of the minute vessels in the granulation tissues from TDA compared with UDC (P<0.05) (Table 3). Furthermore, we determined cytokines which effect angiogenesis. Levels of VEGF and ICAM-1 were decreased in UDC compared with NDC (P<0.05) (Figure 2). Treatment with H2S improved Levels VEGF and ICAM-1 (P<0.05) (Figure 2).
Figure 2.
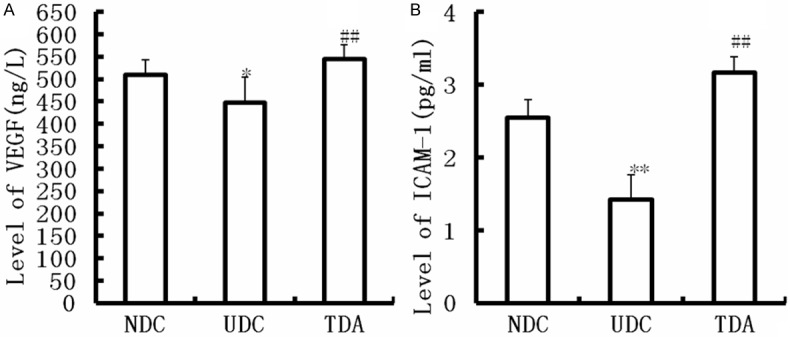
Effect of H2S on levels of VEGF and ICAM-1. Data are means ± SD from 10 rats. **Significantly different: P<0.01 vs. NDC; ##Significantly different: P<0.01 vs. UDC.
Discussion
Impaired wound healing is one of the most serious and costly diabetic complications. Wound healing is a complex pathophysiological process in diabetes, and effective treatment remains unknown. Numerous studies have shown that it is beneficial to wound healing by improving angiogenesis, anti-inflammation, and antioxidant in wound tissues. In present study, our results showed that diabetic rats with wound showed inflammatory characteristics such as purulence and increased expression of TNF-α, reduced antioxidant and angiogenesis. Treatment with H2S to diabetic rats accelerated wound closure, increased level of VEGF, reduced TNF-α protein expression, and improved antioxidant and angiogenesis.
Increasing studies from both clinic and experiment show that sustained hyperglycemia results in reduced antioxidant and elevated oxidative stress involved in development of diabetes and its complications via impairing vascular functions [27,28]. Reactive oxygen species (ROS) causing oxidative stress aggravates wound in diabetes, therefore, antioxidant productions may improve wound healing and healing of foot ulcers in diabetes [29]. H2S, a gasotransmitter, plays various roles in physiology and pathophysiology. Some studies indicated that H2S itself is not a potent antioxidant compared with other antioxidants [30-32], but H2S enhances the antioxidant effect via elevating endogenous antioxidase such as SOD [25,33]. H2S can inhibit cytochrome C oxidase and regulates mitochondria function [34]. Our results showed that H2S treatment increased the activity of SOD, decreased MDA content. The findings suggest that H2S improves the wound healing in diabetic rats by enhancing antioxidant.
Inflammatory response is an important factor in cutaneous wound healing in diabetes, and failure of diabetic wound to heal was associated with proinflammatory cytokines such as TNF-α, and IL-6 which were increased in diabetes [35-37]. Metabolic disturbance resulting from diabetes led to the decrease in migration of inflammatory cells and chemotaxis of leukocytes [38], which results in delayed wound healing in diabetes. It has been found that H2S decreased leukocyte adhesion to the vascular endothelium, and downregulated proinflammatory cytokine expression [39-41]. In this study, the significantly increased expression of TNF-α protein was observed in the granulation tissues from untreated STZ-diabetic rats. H2S decreased expression of TNF-α protein and leukocyte adhesion.
Oxidative stress and inflammatory response in diabetes damaged vascular endothelium, which resulted in disorder of coagulation and anticoagulation, peripheral vessel disease, and peripheral circulation failure [11,12]. Therefore, improved homeostasis of coagulation and anticoagulation, and angiogenesis may play an important role in diabetic wound healing. In this study, we found the increased fibrinogen in serum from diabetic rats, which are in agreement with previous finding from the literature [42]. H2S reduced level of fibrinogen. Angiogenesis plays an important role in wound healing via accelerating the formation of granulation tissue. In the study, we demonstrated that the number of capillary in H2S-treated wounds was increased, indicating that H2S promote angiogenesis. Furthermore, we found the rise in levels of VEGF and ICAM-1 in diabetic rats treated with H2S compared with untreated diabetic rats. VEGF is one of the key cytokine which stimulate angiogenesis via cell migration and proliferation [43]. ICAM-1, an inducible transmembrane protein, mediates leukocyte adhesion to endothelial cells, and activates endothelial cells, which promotes angiogenesis [45,46].
In present study, our results demonstrate that H2S can accelerate diabetic wound healing by promoting angiogenesis in granulation tissues, which is associated with the increased level of vascular endothelial growth factor. Elevated anti-inflammation and antioxidant are involved in beneficial effect of H2S to wound healing in diabetes. We suggest that it is important to further explore the precise mechanism of H2S to heal diabetic wound for clinical application.
Acknowledgements
This work was supported by The Army Key Project (No. BJN14C001), The National Natural Science Foundation of China (No. 81172790), and initial funding of Wannan Medical College (No. 06020204).
Disclosure of conflict of interest
None.
References
- 1.Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013;9:7093–7114. doi: 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Zguira MS, Vincent S, Le Douairon Lahaye S, Malarde L, Tabka Z, Saiag B. Intense exercise training is not effective to restore the endothelial NO-dependent relaxation in STZ-diabetic rat aorta. Cardiovasc Diabetol. 2013;12:32. doi: 10.1186/1475-2840-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontbonne A, Eschwege E, Cambien F, Richard JL, Ducimetiere P, Thibult N, Warnet JM, Claude JR, Rosselin GE. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989;32:300–304. doi: 10.1007/BF00265546. [DOI] [PubMed] [Google Scholar]
- 5.Srikanth S, Deedwania P. Primary and secondary prevention strategy for cardiovascular disease in diabetes mellitus. Cardiol Clin. 2011;29:47–70. doi: 10.1016/j.ccl.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 6.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Bartus CL, Margolis DJ. Reducing the incidence of foot ulceration and amputation in diabetes. Curr Diab Rep. 2004;4:413–418. doi: 10.1007/s11892-004-0049-x. [DOI] [PubMed] [Google Scholar]
- 9.Faglia E, Clerici G, Clerissi J, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Lupattelli T, Morabito A. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J Vasc Endovasc Surg. 2006;32:484–490. doi: 10.1016/j.ejvs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Khanolkar MP, Bain SC, Stephens JW. The diabetic foot. QJM. 2008;101:685–695. doi: 10.1093/qjmed/hcn027. [DOI] [PubMed] [Google Scholar]
- 11.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 12.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 14.Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 2010;39:1161–1169. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 15.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyoshi N, Takabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–575. doi: 10.1093/carcin/bgh051. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Park Y, Zuidema MY, Hannink M, Zhang C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J Cardiol. 2011;3:18–24. doi: 10.4330/wjc.v3.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 21.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 22.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 24.Allison GL, Lowe GM, Rahman K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012;91:1275–1280. doi: 10.1016/j.lfs.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 26.Lau TW, Sahota DS, Lau CH, Chan CM, Lam FC, Ho YY, Fung KP, Lau CB, Leung PC. An in vivo investigation on the wound-healing effect of two medicinal herbs using an animal model with foot ulcer. Eur Surg Res. 2008;41:15–23. doi: 10.1159/000122834. [DOI] [PubMed] [Google Scholar]
- 27.Grieve DJ, Byrne JA, Cave AC, Shah AM. Role of oxidative stress in cardiac remodelling after myocardial infarction. Heart Lung Circ. 2004;13:132–138. doi: 10.1016/j.hlc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol. 2004;19:488–493. doi: 10.1097/01.hco.0000133657.77024.bd. [DOI] [PubMed] [Google Scholar]
- 29.Park NY, Lim Y. Short term supplementation of dietary antioxidants selectively regulates the inflammatory responses during early cutaneous wound healing in diabetic mice. Nutr Metab (Lond) 2011;8:80. doi: 10.1186/1743-7075-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 31.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamar J, Solymar M, Tanai E, Cseplo P, Springo Z, Berta G, Debreceni B, Koller A. Bioassay-comparison of the antioxidant efficacy of hydrogen sulfide and superoxide dismutase in isolated arteries and veins. Acta Physiol Hung. 2012;99:411–419. doi: 10.1556/APhysiol.99.2012.4.5. [DOI] [PubMed] [Google Scholar]
- 33.Searcy DG, Whitehead JP, Maroney MJ. Interaction of Cu, Zn superoxide dismutase with hydrogen sulfide. Arch Biochem Biophys. 1995;318:251–263. doi: 10.1006/abbi.1995.1228. [DOI] [PubMed] [Google Scholar]
- 34.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haidara MA, Mikhailidis DP, Rateb MA, Ahmed ZA, Yassin HZ, Ibrahim IM, Rashed LA. Evaluation of the effect of oxidative stress and vitamin E supplementation on renal function in rats with streptozotocin-induced Type 1 diabetes. J Diabetes Complications. 2009;23:130–136. doi: 10.1016/j.jdiacomp.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Guo Z, Xia Z, Jiang J, McNeill JH. Downregulation of NADPH oxidase, antioxidant enzymes, and inflammatory markers in the heart of streptozotocin-induced diabetic rats by N-acetyl-L-cysteine. Am J Physiol Heart Circ Physiol. 2007;292:H1728–1736. doi: 10.1152/ajpheart.01328.2005. [DOI] [PubMed] [Google Scholar]
- 38.Wysocki J, Wierusz-Wysocka B, Wykretowicz A, Wysocki H. The influence of thymus extracts on the chemotaxis of polymorphonuclear neutrophils (PMN) from patients with insulin-dependent diabetes mellitus (IDD) Thymus. 1992;20:63–67. [PubMed] [Google Scholar]
- 39.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 40.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 41.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 42.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Xie ZH, Guo YJ, Zhao CP, Jiang H, Song Y, Zhu ZY, Lai C, Xu SL, Bi JZ. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2011;411:620–626. doi: 10.1016/j.bbrc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Hou C, Shen L, Huang Q, Mi J, Wu Y, Yang M, Zeng W, Li L, Chen W, Zhu C. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials. 2013;34:112–120. doi: 10.1016/j.biomaterials.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 46.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]


