Abstract
Objective: This study is to investigate the effects of mesenchymal stem cell (MSC) transplantation in burn treatment. Methods: Wharton’s Jelly was stripped from neonatal umbilical cord, and human umbilical cord MSCs were then cultured. Burn models were constructed in male SD rats weighted at 200 ± 5 g, and the rats were randomly divided into control and MSCs transplantation groups. The rats in transplantation group were injected subcutaneously with MSCs (2×106) at 24 h after burning. Blood samples were collected at 0 d, 1 d, 2 d, 3 d, 5 d and 7 d after burning and the contents of white blood cells (WBC), C-reactive protein (CRP), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10 ) were detected. The wound healing rate at 7 d, 14 d, 21 d and 28 d together with the wound healing time were compared and analyzed statistically by analysis of variance (ANOVA). Results: WBC and CRP in control group increased significantly at 1 d and 2 d, 2 d and 3 d, respectively. IFN-γ, IL-6 and IL-10 levels in serum showed increasing till 5th day and TNF-α arrived its peak value at 7th day. By contrast, WBC, CRP, TNF-α, IL-6 and IL-10 in the MSCs transplantation group showed slight increase after burning and the differences were verified by statistically analysis. IFN-γ showed no significant difference between the two groups. MSCs transplantation group showed significantly higher wound healing rate at 14 d, 21 d, 28 d and showed shorter wound healing time than control. Conclusions: MSCs transplantation could suppress secondary inflammatory reaction by lowering inflammatory cytokines after burning, thus promoting wound healing and scald repair in burn animal model.
Keywords: MSCs, transplantation, burn, cytokines, immunoregulation, immunosuppression
Introduction
Burn trauma can cause local and systemic inflammatory response, and induce the production of a variety of inflammatory mediators such as interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin-10 (IL-10) and interleukin-6 (IL-6) [1]. Proinflammatory cytokines of IFN-γ, TNF-α and IL-6 not only can induce the release of other cytokines, but also can promote the migration of neutrophiles cells and the adhesion between neutrophiles and endothelial cells. This can increase the release of inflammatory mediators [2]. Excessive release of the inflammatory mediators will further aggravate tissue organ damages and cause localized depletion of inflammatory cytokines, thus leading to local infection and deterioration [3]. Therefore, inflammatory cytokine regulation and excessive inflammatory response inhibition are significant to the treatment of severe burns.
Mesenchymal stem cells (MSCs), with the capacities of self-renewal and multipotent differentiation, are originated from mesoderm mesenchymal tissues. They can differentiate into epidermal cells, sebaceous, sweat glands and other skin appendages cells, as a result, they have the potential for treatment of burns. Wu et al. indicated that MSCs transplantation could promote skin healing, differentiation of endothelial cells and formation of blood vessels [4]. Gao et al. reported that the MSCs those systemically or locally transplanted could not only migrate to the damaged skin tissues and promote epithelial tissue repair, but also could transdifferentiate into sebaceous glands, epidermal keratinocytes, follicular epithelial cells and other skin appendages [5]. In addition, MSCs have immunomodulatory capacity. Under different conditions, they can play anti-inflammatory roles or immune-enhancing roles [6-8]. However, there are no related reports focusing on the regulation effects of MSCs transplantation on inflammatory responses in burn models.
In this study, MSCs were transplanted into burn rat models by subcutaneous injection. The inflammatory indices such as WBC, CRP, IFN-γ, TNF-α, IL-6 and IL-10 were tested and compared between MSCs-transplanted and non-transplanted groups. Meanwhile, the tissue pathological changes of the rat skins were also compared. The regulation of MSCs transplantation on inflammatory responses in burn models was further discussed.
Materials and methods
Isolation and culture of MSCs
MSCs were obtained from umbilical cords of newborns. The umbilical cords were obtained from the normal newborns in the General Hospital of Urumqi. The puerperas signed the written consents after informed. Briefly, the umbilical cord was cut into 1-1.5 cm small segments, then, Wharton’s jelly was separated and cut into 1 mm2 pieces The samples were suspend with a medium containing 89% DMEM-F12, 10% fetal bovine serum, 1% glutamine (GIBCO, USA), 100 U/mL penicillin and 100 mg/mL streptomycin (Solarbio, Beijing) and cultured in the incubator at 37°C with 5% CO2. The medium was changed every 3 days. When the cells reached 70~80% confluence, they were passaged at a ratio of 1:2 or 1:3 for several passages. The P2 (the second passage) -P4 (the fourth passage) cells were used for the subsequent animal experiments.
Flow cytometry
The MSCs were identified with flow cytometry (Beckman-Coulter, California, USA). The tested cell surface markers included CD29, CD34, CD44, CD45 and CD105. All the antibodies were purchased from eBioscience, Inc. (San Diego, CA, USA).
Alizarin red staining and oil red staining
Adipogenic differentiation was induced in expanded UC-MSCs by adding 0.5 mM 1-methyl-3-isobutylxanthine, 1 µM dexamethasone, 10 µM insulin and 200 µM indomethacin in the medium. After induced for 2 weeks, the accumulation of neutral lipid vacuoles in induced MSCs was detected by Oil Red O and examined under light microscope. The general steps were described as follows. The cells were fixed with 75% alcohol for 1 h and were washed with distilled water three times. Then, the cells were stained with 0.3% Oil red O for 30 min at room temperature and washed with 70 % alcohol for 30 min.
Osteogenic differentiation was induced in expanded UC-MSCs by adding 10 mM beta -glycerol phosphate, 50 µM ascorbate, 0.1 µM dexamethasone in the medium. After induced for 2 weeks, the calcium compounds induced MSCs were fixed with 95% ethanol for 10 min and were washed with distilled water three times. Then, the cells were stained with 0.1% alizarin red-Tris-Hcl (pH 8.0) for 30 min at 37°C and were washed with distilled water three times. At last, the cells were observed under light microscope.
HE staining
The animals were sacrificed with an overdose of anesthetics and the burn areas were removed and transferred into 10% neutral-buffered formalin until tissue processing for histological evaluation. The specimens were embedded in paraffin, and sections of 5 µm in thickness were provided and stained using hematoxylin and eosin (H&E) and studied by a routine light microscope (Olympus, Tokyo, Japan).
Preparation of burn model
A total of 84 male Sprague-Dawley rats (supplied by the Experimental Animal Center of Xinjiang Medical University), with a mean weight of 200 ± 5 g, were randomly divided into two groups: the transplantation group (n = 42) and the control group (n = 42). The rats were housed separately in a SPF lab, with free access to water and adaptively fed for a week before establishment of burn model. After anesthetized with 3% pentobarbital at a concentration of 1.0 mL/kg, the dorsal hair of the rats was removed. An iron mold was burned to red and put on the rat back for 25 s to prepare burn model when the measured bottom (diameter of 1.5 cm) center temperature was 200-210°C (MT4 portable visible infrared thermometer, FLUKE, USA).
Cell transplantation
At 24 hours after burn, P3 MSCs were suspended with 0.5 mL normal saline and transplanted to the rats in the transplantation group with a concentration of 2×106 cells/rat. The MSCs were subcutaneously injected to multi-points at about 0.5 cm from the burn parts.
Detection of inflammatory markers
The WBC was counted with cell counting board and CRP was detected by nephelometric immunoassay method (Beckman Coulter Immage 800, California, USA). The contents of IFN-γ, TNF-α, IL-6 and IL-10 were measured by ELISA (Solarbio, Beijing, China).
Wound healing rate and healing time observation
At the 0 d, 1 d, 2 d, 3 d, 5 d and 7 d after burn, 6 rats in each group were taken and anesthetized with 3% pentobarbital at a concentration of 1.0 mL/kg. Blood was collected for inflammatory indices detection via inferior vena cava after abdomen opening. The rats were then sacrificed, and the burn parts of the skin tissues were obtained for HE staining [9]. At 7 d, 14 d, 21 d and 28 d after burn, 6 rats in each group were used for wound healing rates measurement and complete wound healing time calculation. The wound diameters of the rats in the two groups were measured, and the wound healing rates were calculated. The calculation formula was: Healing rate = (Original wound area-Non-healing wound area)/Original wound area. According to the healing condition of the burn wound, the healing time was recorded as the required time of rats in each group for complete epithelialization of the wound.
Statistical analysis
All the statistical analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA). Comparison between the two groups was performed using analysis of variance (ANOVA). A P value < 0.05 was considered as statistically significant.
Results
Identification of MSCs
To make sure the cells separated were MSCs but not other cell types, a series of experiments were carried out. After 3 days of culture, the P3 adherent cells mainly exhibited fusiform shapes, grew evenly and arranged regularly (Figure 1A). Flow cytometry results showed that the positive rates of CD29, CD44, CD90 and CDl05 surface markers were all over 95%, while CD34 and CD45 were not expressed (Figure 1B). After adipogenic and osteogenic differentiation, positive staining of Oil Red O and Alizarin Red were observed (Figure 1C and 1D). These results together indicated that the cells separated were MSCs.
Figure 1.
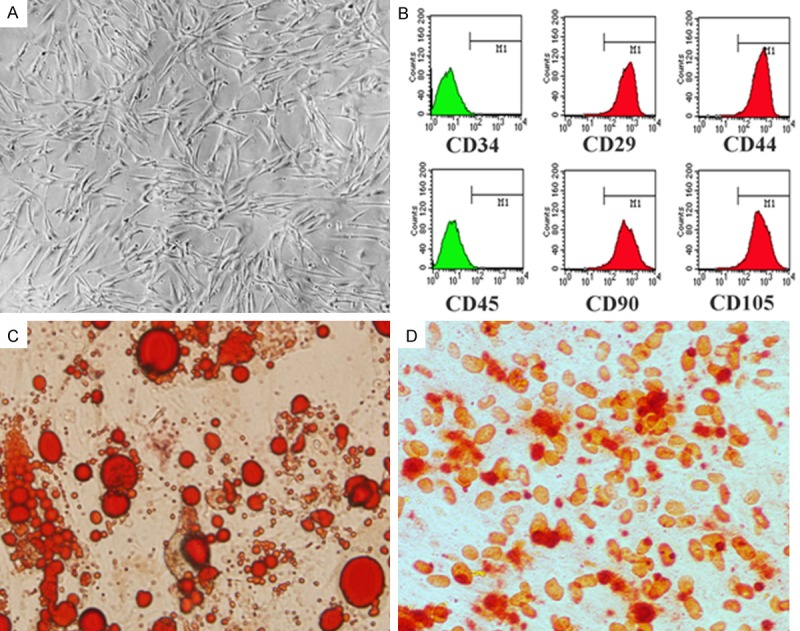
Culture and identification of MSCs. A. The stable proliferation of MSCs. B. Cell surface markers tested by FCM. C. Oil Red O staining after adipogenic differentiation (40×). D. Alizarin Red staining after osteogenic differentiation (40×).
Time-dependent changes of the inflammatory markers
To investigate the function of MSCs on burn wounds, the amounts of the inflammatory markers were detected at different times. At 24 hours after burn, all the inflammatory markers in the control group showed increasing trends to varied degrees. The WBC (Table 1; Figure 2A) and CRP (Table 2; Figure 2B) levels reached peaks at the 2nd and 3rd day, respectively. IFN-γ (Table 3; Figure 2C), IL-6 (Table 3; Figure 2E) and IL-10 (Table 3; Figure 2F) reached peaks at the 5th day after burn. TNF-α (Table 3; Figure 2D) reached the peak at the 7th day. The contents of WBC, CRP, IFN-γ, TNF-α, IL-6 and IL-10 in the rats blood samples of the transplantation group were all reduced compared to the control group. WBC decreased most significantly at the 2nd day (P < 0.05), CRP decreased significantly at both the 2nd and 3rd days (P < 0.01), TNF-α and IL-10 both decreased from the 2nd day to the 7th day (P < 0.05), and IL-6 reduced significantly from the 3rd day to the 7th day (P < 0.05). There was no significant difference in IFN-γ between the two groups (Table 3; Figure 2C). In conclusion, the forementioned results showed that MSCs could reduce release of inflammatory markers.
Table 1.
WBC counts of the rats in different groups at varied time points (x ± s, ×109, n = 6)
| Group | 0 d | 1 d | 2 d | 3 d | 5 d | 7 d |
|---|---|---|---|---|---|---|
| Transplantation | 9.50 ± 0.94 | 10.42 ± 1.40 | 9.81 ± 1.76Δ | 9.38 ± 1.23 | 10.13 ± 1.33 | 9.99 ± 1.04 |
| Control | 9.52 ± 1.15 | 10.56 ± 1.54 | 14.23 ± 1.25 | 10.88 ± 1.62 | 9.55 ± 1.23 | 8.66 ± 1.24 |
Note: Compared with control,
P < 0.05.
Figure 2.
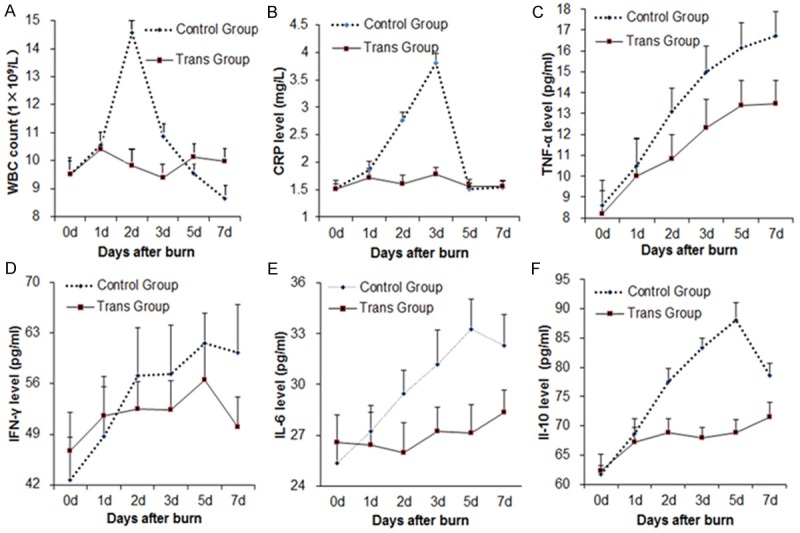
The longitudinal change trends of inflammatory markers in the rats. A-F. showed the levels of WBC, CRP, IFN-γ, TNF-α, IL-6 and IL-10 respectively. Blue line indicated the Control Group while red line did the Transplantation Group.
Table 2.
CRP contents of the rats in different groups at varied time points (x ± s, mg/l, n = 6)
| Group | 0 d | 1 d | 2 d | 3 d | 5 d | 7 d |
|---|---|---|---|---|---|---|
| Transplantation | 1.51 ± 0.10 | 1.71 ± 0.18 | 1.61 ± 0.16ΔΔ | 1.77 ± 0.14ΔΔ | 1.56 ± 0.12 | 1.56 ± 0.10 |
| Control | 1.52 ± 0.14 | 1.85 ± 0.17 | 2.77 ± 0.13 | 3.81 ± 0.18 | 1.51 ± 0.11 | 1.54 ± 0.13 |
Note: Compared with control,
P < 0.01.
Table 3.
The contents of INF-γ, TNF-α, IL-6 and IL-10 of the rats in different groups at varied time points (x ± s, pg/ml, n = 6)
| Group | 0 d | 1 d | 2 d | 3 d | 5 d | 7d | |
|---|---|---|---|---|---|---|---|
| INF-γ | Transplantation | 46.67 ± 5.39 | 51.50 ± 9.54 | 52.50 ± 13.80 | 52.33 ± 14.05 | 56.50 ± 11.00 | 60.33 ± 6.56 |
| Control | 42.67 ± 5.89 | 48.67 ± 9.91 | 57.17 ± 13.56 | 57.33 ± 15.73 | 61.67 ± 15.15 | 50.00 ± 6.16 | |
| TNF-α | Transplantation | 8.18 ± 1.15 | 9.99 ± 1.78 | 10.85 ± 1.14Δ | 12.30 ± 1.36Δ | 13.38 ± 1.22Δ | 13.48 ± 1.11ΔΔ |
| Control | 8.60 ± 1.20 | 10.49 ± 1.35 | 13.10 ± 1.10 | 14.99 ± 1.23 | 16.17 ± 1.16 | 16.75 ± 1.13 | |
| IL-6 | Transplantation | 26.59 ± 1.59 | 26.42 ± 1.94 | 25.95 ± 1.81 | 27.25 ± 1.40Δ | 27.15 ± 1.65ΔΔ | 28.37 ± 2.30Δ |
| Control | 25.37 ± 1.25 | 27.24 ± 1.52 | 29.47 ± 2.35 | 31.19 ± 2.41 | 33.28 ± 1.74 | 32.30 ± 1.80 | |
| IL-10 | Transplantation | 62.37 ± 2.74 | 67.22 ± 2.42 | 68.84 ± 2.35ΔΔ | 67.86 ± 1.90ΔΔ | 68.77 ± 2.34ΔΔ | 71.44 ± 2.52ΔΔ |
| Control | 61.37 ± 1.47 | 68.63 ± 2.52 | 77.55 ± 2.26 | 83.42 ± 1.49 | 88.15 ± 2.95 | 78.68 ± 2.02 | |
Note: Compared with control,
P < 0.05;
P < 0.01.
Comparison of the healing rate and time
To investigate the function of MSCs on healing rate and time, healing rate and time in both groups were recorded and compared. The healing rate of the transplantation group was significantly higher than that of the control group at varied time points (Table 4; Figure 3). The healing time of the transplantation group (29 ± 2.8 days) was significantly shortened compared to that of the control group (35 ± 2.4 days). To sum up, these results suggest that MSCs greatly increase the healing of burn wounds.
Table 4.
Healing rates at different time points after burn (%)
| Group | 7 d | 14 d | 21 d | 28 d |
|---|---|---|---|---|
| Transplantation | 14.3 ± 1.28 | 47.2 ± 1.72Δ | 76.2 ± 2.06Δ | 97.2 ± 2.74Δ |
| Control | 12.5 ± 1.39 | 33.8 ± 1.56 | 50.4 ± 1.78 | 79.4 ± 2.09 |
Note: Compared with control,
P < 0.05.
Figure 3.
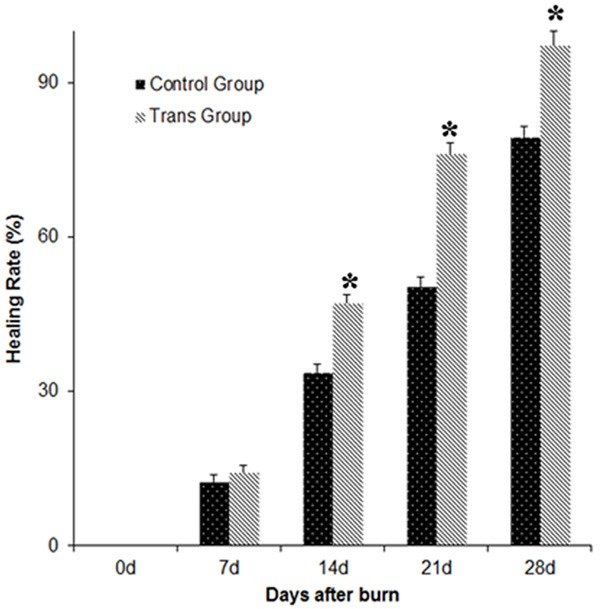
The longitudinal change trends of the healing rates of the rats wounds. Compared with the control group, there was a significant difference (P < 0.05).
Histopathological observation
To identify the role of MSCs on granulation tissues growth, Histopathological examination was carried out. Histopathological examination confirmed that the models were Grade III burns (Figure 4A and 4D). Granulation tissues appeared in both groups 14 days after burn (Figure 4B and 4E), however, there were less new granulation tissues in the control group (Figure 4E). The numbers of capillaries and fibroblasts in the transplantation group were significantly increased compared to the control group, and there were more abundant granulation tissues formed (Figure 4B). At 28 days after burn, the transplantation group had a wealth of new capillaries and clear structures for each skin layer (Figure 4C), while in the control group, the skin layers were obscure and there was more congestion in the neovascularization (Figure 4F). The Histopathological examination results illustrated that MSCs could promote granulation tissues growth after burn wounds.
Figure 4.
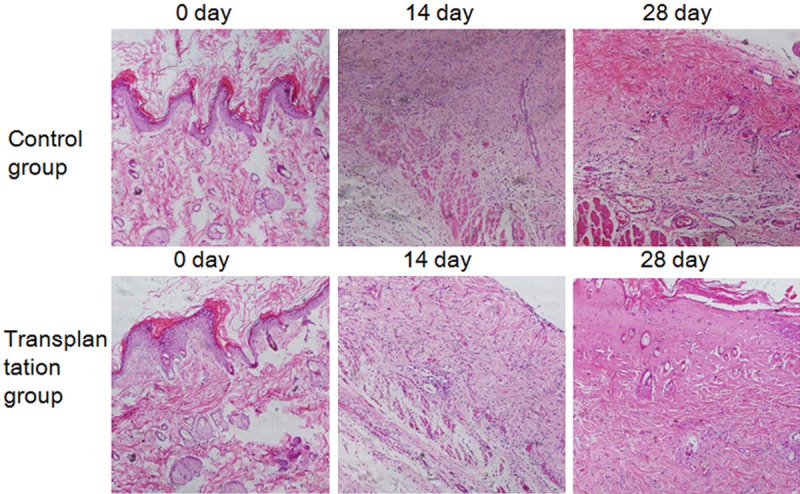
Histological HE staining of rats burnt tissues. (A-F) Showed the skin specimens at 0 d, 14 d and 28 d after burning respectively. The Control Group: (A-C); the Transplantation Group: (D-F).
Discussion
In this study, in order to verify that MSCs could inhibit inflammation and promote the subsequent repair in the burned rats, MSCs were subcutaneously injected to the burned rats, and then compared with the burned rats without transplantation. The results showed that the healing time of the MSCs transplantation group was 29 ± 2.8 days, which was significantly shortened while compared to that of the control group (35 ± 2.4 days). The healing rate of the transplantation group was significantly higher than that of the control group. We also detected the changes of WBC, CRP, and inflammatory cytokines IFN-γ, TNF-α, IL-6 and IL-10 in the burned rats. The results showed that 24 hours after burn, substantial rises appeared in WBC and CRP, while the serum inflammatory cytokines IFN-γ, TNF-α, IL-6 and IL-10 showed different degrees of increase in the control group. For the transplantation group, WBC, CRP and the inflammatory cytokines all exhibited varied degrees of reduction compared to the control group. The phenomenon of expression decrease of WBC, CRP, TNF-α, IL-6 and other inflammatory markers indicate that MSC transplantation reduces the levels of systemic inflammatory response and improves the repair process by inhibiting the post-burn inflammation in the burn rats. The known effect of IL-10 is to inhibit inflammation. Previous researches show that when exposed to the microenvironment with high proinflammatory cytokines, MSCs could reduce the proinflammatory cytokine TNF-α whereas up-regulate the anti-inflammatory cytokine IL-10 [10,11]. However, in this study, the level of IL-10 in transplantation group was lower than that of the control group. The seemingly contradictory phenomenon might be caused by two reasons. One is that, in the burned rat model, the body is in a state of compensated anti-inflammatory response syndrome (CARS) due to the production of a large number of proinflammatory cytokines. CARS could lead to excessive production of IL-10. While the proinflammatory cytokine level was not obviously increased after MSCs transplantation, the CARS response would be weaker, which in turn led to lower IL-10 level in transplantation groups [12]. Another is that MSCs might paracrine some active factors like indoleamine 2, 3-dioxygenase (IDO), nitric oxide (NO) and so on, to enhance the effects of IL-10, which accelerated its consumption, and thus made IL-10 level lower in the transplantation group than the control group. The IFN-γ showed obvious increasing trend after burn, and reached the peak at the 5th day. After MSCs transplantation, it showed no obvious difference from the control group, indicating that transplantation of MSCs has no significant effect on the production of IFN-γ.
MSCs have low immunogenicity and immunomodulatory properties, showing application promising in the treatment of transplantation rejection [13-15]. It is reported that the use of immunomodulatory properties of MSCs for the treatment of autoimmune disease, systemic inflammatory response syndrome (SIRS), graft versus host disease (GVHD) and other disease, could achieve satisfactory clinical effects [16]. In this study, after MSCs transplantation, we detected and compared the variance in the inflammatory markers levels between MSCs transplantation and non-transplantation groups. We found that MSCs transplantation could reduce inflammatory markers and shorten the wound healing time after burn, which means that MSCs could suppress the secondary inflammatory injury at the burned parts and help the subsequent skin repair and reconstruction.
In short, this study indicates that the immunomodulatory effects of MSCs have great significance in the burn healing process. However, in the course of burn treatment, how to grasp the best MSCs transplantation time and the specific mechanism of MSCs modulating immune response still needs in-depth research work.
Acknowledgements
This study was supported by the Science and Technology Foundation of Lanzhou Military Region (CLZ13JB15).
Disclosure of conflict of interest
None.
References
- 1.Dahiya P. Burns as a model of SIRS. Front Biosci (Landmark Ed) 2009;14:4962–4967. doi: 10.2741/3580. [DOI] [PubMed] [Google Scholar]
- 2.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Colotta F, Sciacca FL, Sironi M, Luini W, Rabiet MJ, Mantovani A. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am J Pathol. 1994;144:975–985. [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 5.Gao D, Gu C, Wu Y, Xie J, Yao B, Li J, Feng C, Wang J, Wu X, Huang S, Fu X. Mesenchymal stromal cells enhance wound healing by ameliorating impaired metabolism in diabetic mice. Cytotherapy. 2014;16:1467–1475. doi: 10.1016/j.jcyt.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 8.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramov Y, Golden B, Sullivan M, Botros SM, Miller JJ, Alshahrour A, Goldberg RP, Sand PK. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007;15:80–86. doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 10.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 11.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry PA, Antoniades CG, Hussain MJ, McPhail MJ, Bernal W, Vergani D, Wendon JA. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010;30:733–740. doi: 10.1111/j.1478-3231.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 13.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 15.Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, Yu Z, Li B, Xu C, Li Y, Wang J, Hu J, Lou X, Chen H. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]


