Abstract
Background: Autoimmune hepatitis (AIH) is a chronic, progressive, and immunologically mediated inflammatory liver disorder. The etiology of AIH still remains unknown. The aim of this study was to investigate the changes in intestinal permeability, bacterial translocation, and intestinal microbiome in patients with AIH and to evaluate the correlations of those changes with the stages of the disease. Methods: 24 patients with autoimmune hepatitis and 8 healthy volunteers were recruited for this study. We assessed (1) the integrity of tight junctions within the gut by immunohistochemical analysis of zona occludens-1 and occludin expression in duodenal biopsy specimens; (2) changes in the enteric microbiome by 16S rDNA quantitative PCR; and (3) the presence of bacterial translocation by the level of lipopolysaccharide (LPS) using ELISA. Results: Increased intestinal permeability, derangement of the microbiome and bacterial translocation occurred in AIH, which correlated with the severity of the disease. Conclusions: Autoimmune hepatitis is associated with leaky gut and intestinal microbiome dysbiosis. The impaired intestinal barrier may play an important role in the pathogenesis of AIH.
Keywords: Autoimmune hepatitis, bacterial, intestinal permeability, microbiota, translocation
Introduction
Autoimmune hepatitis (AIH) is a chronic, progressive, and immunologically mediated inflammatory liver disorder. It is characterized by female preponderance, elevated levels of serum transaminases, presence of autoantibodies and hypergammaglobulinemia, and interface hepatitis [1]. The etiology of AIH still remains unknown. Pathogenesis may be a result of the breakdown of immune tolerance; a genetic predisposition, and environmental factors triggering the autoimmune process, which in collaboration induce a T cell mediated response to liver antigens for the development of necroinflammation and tissue destruction [2].
A close relationship exists between the gut and liver is named “gut-liver axis” because of the anatomical construction and its unique vascular character [3]. The liver receives approximately 75% of its blood supply from the intestine through the portal vein [4] it represents the initial organ exposed to gut-derived products, including not only alimentary substances but also bacteria and their products [5]. The defensive mechanism maintaining the hepatic immune homeostasis mainly depends on the intestinal barrier functions and the detoxifying capacity of the liver in healthy condition [6,7]. However, disruption of the gut barrier by various pathological conditions can lead bacteria and their products such as LPS and unmethylated CpG containing DNA to the liver [8]. These gut-derived toxins may break liver homeostasis through aberrant activation of the innate immune system, triggering signaling pathways involved in liver inflammation [9].
Various studies suggested that the gut-liver axis plays an important role in the pathogenesis initiation and progression of many types of liver diseases [10-14]. However, there are currently no patient studies assessing gut barrier function and changes in the microbiome in patients with AIH. In this study, we examined AIH patients and found that the disease is associated with leaky gut and intestinal microbiome dysbiosis. Our data indicate that the impaired intestinal barrier may play an important role in the pathogenesis of AIH.
Methods
Patients
Twenty-four patients confirmed for AIH with different stages, were enrolled into this study from April 2011 to March 2013 at the Department of Digestive Diseases, General Hospital, Tianjin Medical University, Tianjin, China. The diagnosis of AIH was performed according to simplified criteria for the diagnosis of autoimmune hepatitis [15]. The AIH patients included 4 males and 20 females (57.3±20.4 years old). Based on the results of alanine aminotransferase (ALT) (normal ≤ 40 U/L) and ultrasound or CT of the abdomen, the patients were classified as AIH normal liver function group (ALT ≤ 40 U/L, no cirrhosis), AIH abnormal liver function group (ALT > 40 U/L, nocirrhosis) or AIH cirrhosis group (cirrhosis). None of the patients had received any treatment.
For comparison, we recruited 8 healthy volunteers matched for both sex and age. The group was composed of 2 males and 6 females (52.1±21.9 years old), with normal serological levels and hepatic ultrasound examination, and no family history of celiac disease.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and that it conforms to the provisions of the World Medical Association’s Declaration of Helsinki in 1995 (as revised in Tokyo 2004). The institutional Review Board and Ethical Committee of Tianjin Medical University General Hospital approved the study protocol. All patients provided written informed consent for their participation in this study.
Upper gastrointestinal endoscopy and duodenal biopsy
All participants took upper gastrointestinal endoscopy examination with a high-resolution, high-magnification videoendoscope (Olympus, model GIF-XQ240, Japan). The biopsy specimens were collected from the descending duodenum (two from the first and two from the second portion). The tissues were stained with hematoxylin and eosin and examined under blinded conditions by an experienced pathologist.
Immunohistochemistry for ZO-1 and occludin
Duodenal biopsy specimens were fixed in 4% buffered formalin and embedded in paraffin. Immunohistochemistry was performed on 5-micron sections. Anti-ZO-1 antibody (1:50, Santa Cruz, USA) and anti-occludin antibody (1:50, Santa Cruz, USA) and subsequently with avidin-biotin-peroxidase (Vector, CA). Reactions were detected with 3.3’-diaminobenzidine (Vector, CA).
The sections were examined independently by two experienced pathologists. Results were expressed as previously reported [16] based on the proportion of positively stained cells and intensity of staining. Positively stained cell proportion was scored as follows: 0 (< 5% positive cells), 1 (5~25% positive cells), 2 (25~50% positive cells), 3 (50~75% positive cells), and 4 (> 75% positive cells). The intensity of staining was graded as follows: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). Staining index was calculated as the intensity score and the proportion score: 1~2 (+ = weak positive), 3~5 (++ = moderate positive), and 6~7 (+++ = strong positive).
16S rDNA quantitation in feces
Fresh fecal samples were collected from all participants, frozen immediately and stored at -80°C until further processing.
DNA was extracted from feces using QIAGENQIAamp® DNA stool mini kits (Qiagen Ltd., UK) described by Skrivanova et al [17]. The 16S rDNA gene was amplified by using the universal bacterial Primers of Bifidobacterium (5’-GATTCTGGCTCAGGATGAA CGC-3’), Lactobacillus (5’-AGCAGTAGGGAATCTTCCA-3’), Escherichia coli (5’-CATGCCGCGTGTATG AAGAA-3’) and Enterococcus (5’-GCAAGCGTTGT CCGGATTTAT-3’).
DNA was amplified in a reaction mixture consisting of 5.0 mmol of MgCl2 and 0.25 mmol of each primer in a final volume of 20 μl. The reaction conditions for amplification of DNA were 95°C for 5 min, followed by 30 cycles of 95°C for 45 s, 52°C for 30 s and 72°C for 40 s with a final extension at 72°C for 10 min. Real time PCR was performed in duplicates for each standard dilution and sample and mean CT value of the duplicate PCRs was determined and used for the calculations. A standard curve was created from serial dilutions. Copy numbers of the samples were calculated from the standard curve. Results were expressed as Log10 16S rDNA copy number/g feces.
LPS detection in plasma
Plasma LPS were measured by ELISA according to instruction (Shanghai Elisa Biotech Co. Ltd., China) [18].
Statistics
Data are presented as the means ± SE. Significant differences were evaluated by Student’s t-test or ANOVA. The Spearman coefficient of correlation was used to examine the correlation. A p value < 0.05 was considered significant.
Results
Integrity of intestinal tight junctions is impaired in AIH patients
In healthy control group, we observed normal duodenal villus, intact epithelial cells arranged regularly. In contrast, in AIH group, the duodenal villus was small, scarce irregular in arrangement, and the tight junctions were disrupted. Inflammatory cells were visible in the lamina propria. Also, the morphological changes with advanced stages of disease were observed in AIH patients (Figure 1).
Figure 1.
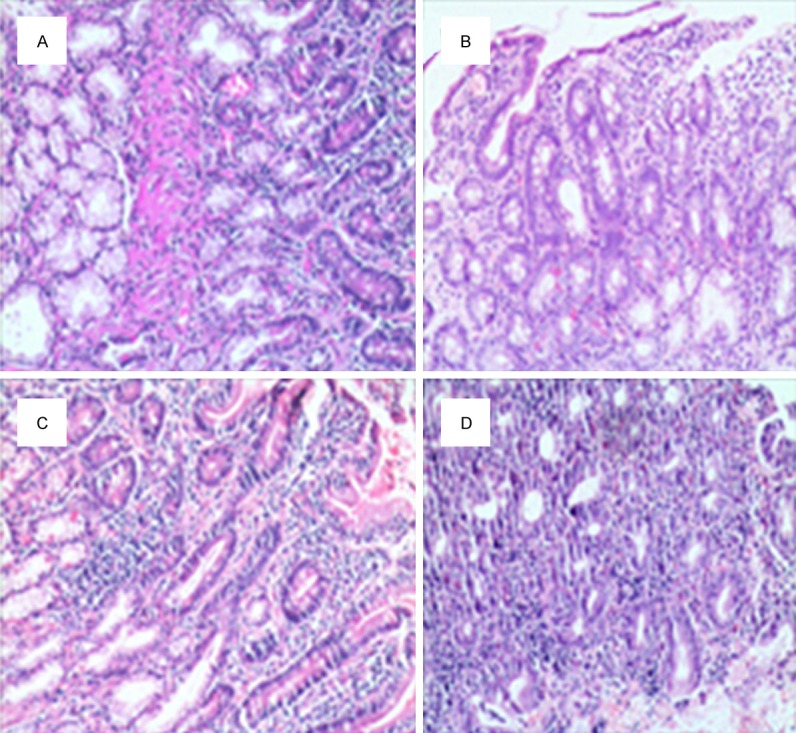
Pathology studies of the duodenum. H&E-staining of the duodenum in AIH and controls. A. Displayed the normal duodenal mucosa in healthy control group. B-D. Showed disruption of the architecture of the duodenal mucosa in AIH; B. AIH normal liver function group; C. AIH abnormal liver function group; D. AIH cirrhosis group.
ZO-1 and occludin expression in duodenal tissue was examined by immunohistochemistry. As shown in Figure 2 and Table 1, both ZO-1 and occluding appeared as a continuous band localized at the intracellular border in healthy controls, but with displacement of the protein away from the cellular border and increased frequency of strand breaks. The levels of ZO-1 and occludin expression were significantly decreased in AIH patients compared with healthy controls. There was a significant association of decreased expression of ZO-1 and occludin with advanced stages of the disease (P < 0.05).
Figure 2.
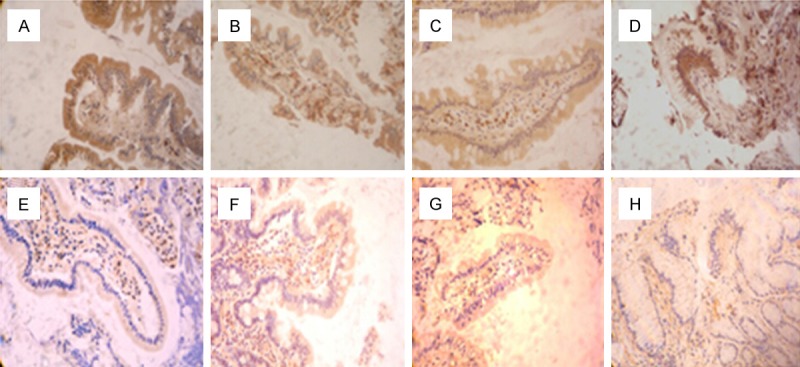
Immunohistochemistry analysis of ZO-1 and occludin expression on intestinal mucosa of AIH and controls. The expression of ZO-1 (A-D) and occludin (E-H) in the duodenal biopsy specimens from AIH patients were examined. A, E. Healthy control group; B, F. AIH normal liver function group; C, G. AIH abnormal liver function group; D, H. AIH cirrhosis group.
Table 1.
Expression of ZO-1 and occludin in duodenal tissues
| Group | n | ZO-1 | occludin | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| +++ | ++ | ++ | +++ | ++ | ++ | ||
| Healthy control | 8 | 4 | 3 | 1 | 5 | 2 | 1 |
| AIH normal liver function | 8 | 2 | 4 | 2 | 3 | 4 | 1 |
| AIH abnormal liver function | 8 | 1 | 5 | 2 | 1 | 5 | 2 |
| AIH cirrhosis | 8 | 0 | 5 | 3 | 1 | 3 | 4 |
| χ2 | 14.775 | 16.351 | |||||
| P | 0.0389 | 0.0216 | |||||
AIH: autoimmune hepatitis; ZO-1: zona occludens-1.
Changes in the enteric microbiome by 16S rDNA in AIH patients
To assess whether or not changes occur in the enteric microbiome of patients with AIH, we quantitatively analyzed major enteric bacteria. Compared with the healthy controls, a reduced quantitative amounts of anaerobes (represented by the Bifidobacterium and Lactobacillus) were found (P < 0.05), while aerobes (represented by Escherichia coli and Enterococcus) were unchanged in AIH. Bifidobacteria/Escherichia coli (B/E) which indicated a balance of intestinal flora was decreased (P < 0.05) (Figure 3; Table 2). The results demonstrate the intestinal microbiome dysbiosis occurred in patients with AIH, but not in healthy control group, and indicates that the integrity of intestinal tight junctions is impaired in AIH patients.
Figure 3.
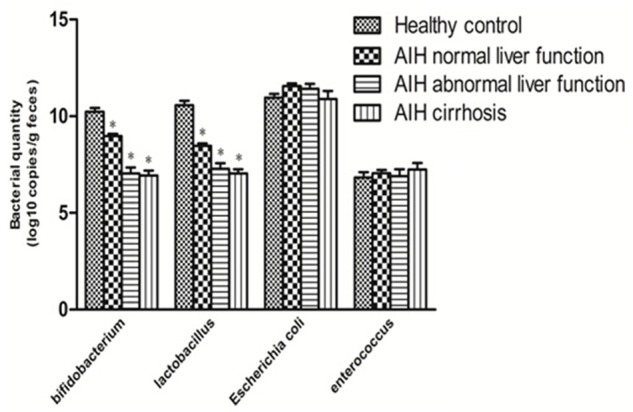
Changes in the enteric microbiome. Compared with the healthy controls, a reduced quantitative amount of Bifidobacterium and Lactobacillus in different stages of the disease (*P < 0.05 vs Healthy control), the amount of Escherichia coli and Enterococcus were unchanged in AIH. Bifidobacteria/Escherichia coli (B/E) which indicated a balance of intestinal flora declined (P < 0.05).
Table 2.
Changes in the enteric microbiome (Log10 copies/g feces)
| Group | Bifidobacterium | Lactobacillus | E. coli | Enterococcus |
|---|---|---|---|---|
| Healthy control | 10.24±0.54 | 10.57±0.62 | 10.96±0.55 | 6.84±0.76 |
| AIH normal liver function | 8.97±0.37* | 8.47±0.35* | 11.56±0.42 | 7.06±0.47 |
| AIH abnormal liver function | 7.05±0.83* | 7.29±0.78* | 11.43±0.72 | 6.90±1.02 |
| AIH cirrhosis | 6.93±0.73* | 7.04±0.63* | 10.89±1.16 | 7.25±0.93 |
AIH: autoimmune hepatitis,
p < 0.05 compare with Healthy control.
Elevated plasma LPS levels in AIH patients
To assess the presence of bacterial translocation, plasma LPS levels were analyzed by ELISA. As shown in Figure 4, the level of plasma LPS was significantly increased in AIH patients compared with healthy controls (P < 0.05). We also found a strong association of increased level of LPS associated with advanced stages of the disease (P < 0.05). These results indicate that bacterial translocation may occur in AIH patients.
Figure 4.
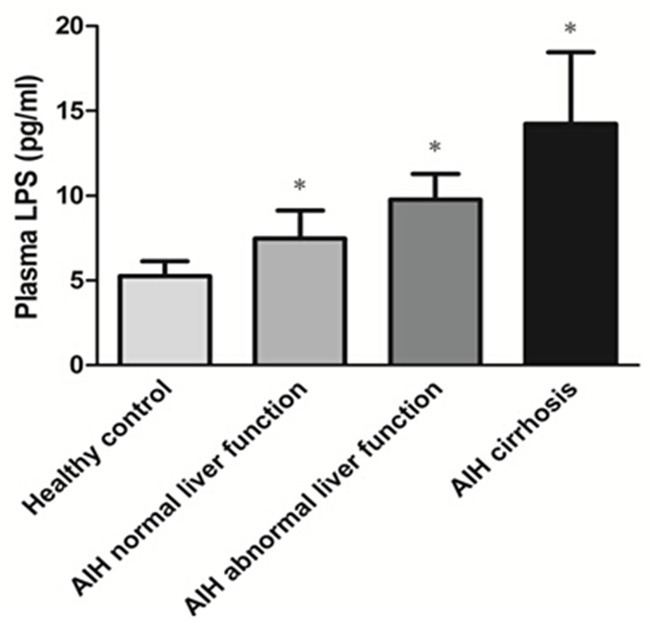
Plasma LPS levels of AIH. The level of plasma LPS was detected by ELISA in the patients with different stages of AIH. The LPS level of each group of the AIH patients was compared with that of healthy control, respectively, *P < 0.05.
Discussion
Data reported for this study show that leaky gut and microbiome dysbiosis are present in patients with AIH and correlate with the severity of disease. Histologic assessments show that disruption of the architecture of the duodenal mucosa and the intensity of duodenal ZO-1 and occludin staining by immunohistochemistry are significantly lower in patients with AIH compared with healthy controls, suggesting that disruption of tight junction integrity may result from the increased permeability in these patients.
The gastrointestinal tract contains the body’s largest interface between a person and the external environment, characterized by a high complexity in terms of functions. It is not only a passive organ of alimentary substances absorption, but also the first-line barrier to the gut’s harmful entities. It prevents exposure of the gut microbiota to maintain the host immune homeostasis [19-21]. Because of the anatomical construction and its unique vascular character, a close relationship exists between the gut and liver, named “gut-liver axis” [22]. In healthy conditions, only very small amounts of bacteria or their products can reach the liver, where they are cleansed and detoxified by the liver immune system. However, disruption of gut barrier by various pathological conditions can lead the entry of large amount of gut-derived toxic factors, e.g., LPS, into the liver [23], and induce aberrant activation of immune system, which can trigger harmful or chronic inflammations in the liver [24,25]. Currently, there have been various studies providing strong evidences that the liver-gut axis plays an essential role in the pathogenesis of chronic liver disease. Miele et al found that the patients with non-alcoholic fatty liver disease are associated with increased gut permeability, which are caused by impaired intercellular tight junctions in the small intestine [26]. Bacterial translocation induced by impairment of the function of tight junction [27,28] and bacterial proliferation [29] plays an important role in the pathogenesis of alcoholic liver disease, which activates immune cells to release various pro-inflammatory cytokines [30]. The derangement of gut flora [31] and endotoxemia are frequently found in hepatic cirrhosis, and the degree of endotoxemia is correlated with the degree of liver failure [31]. Nevertheless, no data assessing intestinal barrier and the enteric microbiome in AIH patients have been reported.
The maintenance of gut barrier integrity depends on villus, tight junctions, and normal intestinal flora [33]. We found that duodenal villus in AIH was small, scarce, and irregular in arrangement. Inflammatory cells were visible in the lamina propria as well. The integrity of tight junctions within the gut was assessed by immunohistochemical analysis of ZO-1 and occludin expression in duodenal specimens. Tight junctions normally connect together at the apicolateral membranes of adjacent epithelial cells, forming a mucosal barrier against luminal bacteria, byproducts, and noxious substances [34,35]. They are consisted of a series of transmembrane proteins, including occludin, claudins, and junctional adhesion molecules [36], which are linked to the actin and myosin filaments through cytoplasmic plaque proteins, including ZO-1 [37]. It is widely considered that the correct and intact linkage of transmembrane and cytoplasmic proteins is essential for maintaining a functional gut barrier [38]. On the whole, impaired gut villus and lower expression of tight junction proteins may result in structural deficiencies that enable bacterial translocation.
Gut microbiota forms an integral biological barrier in the maintenance of intestinal homeostasis. The microbiot performs defensive function supported by the synergistic commensal relationship, including inhibition of pathogens, metabolism of toxic compounds, prevention against penetration and immunological clearance mechanisms [39]. Derangement of the gut flora leads to a break in the gut barrier, increased intestinal permeability and consequent endotoxemia that triggers inflammation. Our analysis of fecal microbiome by 16S rDNA quantitative PCR demonstrated a significant decrease of Bifidobacterium and Lactobacillus in AIH patients compared with healthy subjects, while no changes was observed in Escherichia coli and Enterococcus. The imbalance in the composition of anaerobes/aerobes classes can impair the gut barrier and promote bacterial translocation.
Bacterial translocation is defined as the passage of viable endogenous bacteria or their products, such as LPS and bacterial DNA, from the gut to the mesenteric lymph nodes, systemic circulation and extraintestinal organs [40]. LPS is the outer membrane molecule derived from gram-negative bacteria. As a marker of bacterial translocation, LPS is a potent activator of immune responses, which may induce liver damage [41]. In our study, plasma LPS levels were increased in AIH, and the degree of increase level was significantly related to the severity of disease.
According to these results, we hypothesize that leaky gut and microbiome dysbiosis may be causally linked to pathogenesis of AIH. Alteration of gut microbiota and impaired intestinal barrier which eventually induce bacterial translocation and portal endotoxemia and increase exposure of the liver to gut-derived bacterial products: LPS and unmethylated CpG DNA. These products break in the liver immune tolerance, aberrantly activate innate immune receptors--namely Toll-like receptors, which may trigger “harmful inflammation” that contributes to liver inflammation and tissue destruction. Therefore, it is conceivable that the impaired intestinal barrier function and may be part of the initiation of the autoimmune attack in AIH.
In summary, our results have been demonstrated that AIH is associated with impaired intestinal barrier and microbiome dysbiosis and that these changes are associated with the severity of the disease. We found evidences of “leaky” intestine with tight junction disruption in AIH may result in microbiome dysbiosis. These observations have important implications for understanding the linkage between gut-liver axis and the autoimmune reaction in AIH. Thus, restoration of intestinal barrier integrity may be a potential target therapy of AIH patients.
Acknowledgements
This study was partly supported by Chinese Foundation for Hepatitis Prevention and Control (Grant No. TQGB2011016, R.L.).
Disclosure of conflict of interest
None.
References
- 1.Gossard AA, Lindor KD. Autoimmune hepatitis: a review. J Gastroenterol. 2012;47:498–503. doi: 10.1007/s00535-012-0586-z. [DOI] [PubMed] [Google Scholar]
- 2.Mackay IR. Autoimmune hepatitis: what must be said. Exp Mol Pathol. 2012;93:350–353. doi: 10.1016/j.yexmp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Zeuzem S. Gut-liver axis. Int J Colorectal Dis. 2000;15:59–82. doi: 10.1007/s003840050236. [DOI] [PubMed] [Google Scholar]
- 4.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 5.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 6.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638–644. doi: 10.1007/s10620-013-2960-y. [DOI] [PubMed] [Google Scholar]
- 8.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 9.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232–244. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo G, Mandrekar P, Petrasek J, Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res. 2011;35:782–786. doi: 10.1111/j.1530-0277.2010.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouts DE, Torralba M, Nelson KE, Catalano D. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, Mandrekar P, Dolganiuc A, Kurt-Jones E, Szabo G. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Wu Z, Xu W, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693–703. doi: 10.1007/s00248-010-9801-8. [DOI] [PubMed] [Google Scholar]
- 15.Hennes EM, Zeniya M, Czaja AJ, Inui A, Fujisawa T. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990;21:607–612. doi: 10.1016/s0046-8177(96)90006-4. [DOI] [PubMed] [Google Scholar]
- 17.Skrivanova E, Worgan HJ, Pinloche E, Marounek M, Newbold CJ, McEwan NR. Changes in the bacterial population of the caecum and stomach of the rabbit in response to addition of dietary caprylic acid. Vet Microbiol. 2010;144:334–339. doi: 10.1016/j.vetmic.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Gong CY, Zhou AL, Mao JH, Hu YE, Geng JS. The role of Toll-like receptor 4 on inflammation and Abeta formation in cortex astrocytes. Sheng Li Xue Bao. 2014;66:631–638. [PubMed] [Google Scholar]
- 19.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 20.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett KE. New ways of thinking about (and teaching about) intestinal epithelial function. Adv Physiol Educ. 2008;32:25–34. doi: 10.1152/advan.00092.2007. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JC. The gut as a potential trigger of exercise-induced inflammatory responses. Can J Physiol Pharmacol. 1998;76:479–484. doi: 10.1139/cjpp-76-5-479. [DOI] [PubMed] [Google Scholar]
- 23.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 24.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 26.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 27.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 28.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanaugh MJ, Clark C, Goto M, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290–296. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S53–56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 31.Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol. 2006;12:1493–1502. doi: 10.3748/wjg.v12.i10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 33.Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10:415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- 34.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahidi L, Day AS, Lemberg DA, Leach ST. Differential effects of nutritional and non-nutritional therapies on intestinal barrier function in an in vitro model. J Gastroenterol. 2012;47:107–117. doi: 10.1007/s00535-011-0471-1. [DOI] [PubMed] [Google Scholar]
- 37.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montalto M, Maggiano N, Ricci R, Curigliano V, Santoro L, Di Nicuolo F, Vecchio FM, Gasbarrini A. Lactobacillus acidophilus protects tight junctions from aspirin damage in HT-29 cells. Digestion. 2004;69:225–228. doi: 10.1159/000079152. [DOI] [PubMed] [Google Scholar]
- 39.Marques TM, Wall R, Ross RP, Fitzgerald GF, Fitzgerald GF, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol. 2010;21:149–156. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Othman M, Aguero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11–16. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 41.French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20–27. doi: 10.1007/BF02255967. [DOI] [PubMed] [Google Scholar]


