Abstract
Background: A large number of studies demonstrated that microRNAs play important roles in the progression and development of human cancers. However, the expression level of miR-107 and its biological function in hepatocellular carcinoma (HCC) remains unclear. Method: Quantitative real-time PCR (qRT-PCR) was used to evaluate the expression level of miR-107 in HCC tissues and cell lines. Then, we explored the function of miR-107 to determine its potential roles on HCC cell proliferation in vitro. Luciferase reporter assay was used to confirm the target gene of miR-107, and the results were validated in cell lines. Results: miR-107 was significantly up-regulated in HCC tissues and cell lines. The enforced expression of miR-107 was able to promote cell proliferation in HepG2 cells. At the molecular level, our results suggested that expression of Axin2 was negatively regulated by miR-107. Conclusion: Our observations suggested that miR-107 could promote HCC cells proliferation via targeting Axin2 and might represent a potential therapeutic target for HCC.
Keywords: Hepatocellular carcinoma, miR-107, Axin2, proliferation
Introduction
Hepatocellular carcinoma (HCC), which accounts for about 95% of all primary liver cancers, has become the third most frequent cause of cancer-related mortality worldwide [1,2]. Each year, more than 700,000 patients are diagnosed with HCC and more than 600,000 cases dead due to this malignancy around the world [3]. Due to local invasion and intrahepatic metastasis, HCC patients have a high incidence of recurrence even after curative therapy and the 5-year survival rate of HCC patients is only approximately 5% [4]. Therefore, revealing the molecular mechanism for the tumorigenesis of HCC is indispensable for developing effective therapy.
MicroRNAs (miRNAs) are a class of endogenous expressed, small non-coding RNAs (18-25 nucleotides in length) that can regulate gene expression at a post-transcriptional level [5]. Increasing evidence suggests that microRNAs play important roles in the regulation of diverse biological processes, such as cell proliferation, cell cycle distribution, apoptosis, migration and invasion [6,7]. For example, Luo et al revealed that miRNA-212 could inhibit osteosarcoma cell proliferation and invasion [8]. Yu et al demonstrated that miRNA-34a suppressed angiogenesis in bladder cancer by directly targeting CD44 [9]. Zhang et al suggested that miRNA-301a promoted migration and invasion by targeting TGFBR2 in human colorectal cancer [10]. However, understanding involvement of miRNAs in tumor carcinogenesis remains to be resolved.
Accumulating evidence has suggested that miR-107 might play critical roles in human cancers. For example, He et al showed that miR-107 was down-regulated in glioma and up-regulation of miR-107 could suppress glioma cell growth through direct targeting of SALL4 [11]. Datta et al found that miRNA-107 functions as a tumor-suppressor in head and neck squamous cell carcinoma by down-regulation of protein kinase Cε [12]. Yamakuchi et al indicated that forced expression of miR-107 inhibited colon cancer growth by suppressing HIF-1β [13]. However, there are inverse reports about the regulatory role of miR-107 in cancer progression. Chen et al suggested that up-regulation of miR-107 was associated with distant metastasis and poorer clinical outcome in breast cancer. Overexpression of miR-107 in mammary epithelial cells promotes epithelial-to-mesenchymal transition, leading to a highly metastatic phenotype via regulation of Dicer1 [14]. Li et al revealed that miR-107 was increased in gastric cancer and up-regulation of miRNA-107 could induce gastric cancer cell proliferation by targeting the transcription factor FOXO1 [15]. So, the role of miR-107 in cancer development seems to be complicated and highly tissue-specific. Unfortunately, the expression level of miR-107 and its biological significance in HCC has been rarely investigated.
In the present study, we focused on the expression and function of miR-107 in HCC. Our data revealed that miR-107 was up-regulated in HCC tissues and cell line, indicating that miR-107 might act as a tumor oncogene in HCC progression. We identified that Axin2 is one of direct target genes of miR-107. MiR-107 is able to promote proliferation of HCC cells by targeting Axin2.
Materials and methods
Tissue specimens
A total of 20 paired cancer tissue specimens and peripheral normal tissue samples were obtained from patients with primary hepatocellular carcinoma who received surgery at Department of General Surgery, Huaihe Clinical College of HeNan University. All samples were immediately frozen and stored in liquid nitrogen until use. None of the patients received preoperative treatment. This study was approved by the Ethics Committee of Huaihe Clinical College of HeNan University and written informed consent was obtained from all participants included in this study.
Cell culture and transfection
HCC cell lines (HepG2, HuH-7, and Hep3B) and normal human liver cell line L02 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in DMEM (Invitrogen, CA) supplemented with 10% FBS (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified incubator containing 5% CO2.
Transfection was performed using Lipofectamine 2000 transfection reagent (Invitrogen) following the manufacturer’s protocol. For miR-107 functional analysis, HepG2 cells were transfected with miR-107 mimics or negative control (miR-NC) (Invitrogen). For Axin2 functional analysis, the HepG2 cells were transfected with Axin2-specific interfering RNA (si-Axin2). The sequences of siRNA were as follows: Axin2: 5’-GCAGAGGGACAGGAATCAT-3’.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from tissues or cell lines using Trizol reagent (Invitrogen) according to the manufacturer’s instruction. RNA was then reversely transcribed into cDNA and the quantitative real-time PCR (qRT-PCR) was performed with SYBR Green master mix (Invitrogen) using a 7500 Fast Real-Time Sequence detection system (ABI, Foster City, CA). TaqMan microRNA assays were used to evaluate the expression level of miR-107. The relative expression level of miR-107 and Axin2 was normalized to that of the internal control U6 and GAPDH, respectively, by using the 2-ΔΔCt method. All experiments were conducted in triplicate.
MTT assay
The in vitro proliferation of HepG2 cells transfected with miR-107 mimics or si-Axin2 was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay according to the manufacturer’s protocol. Briefly, treated cells were seeded onto 96-well plates at a density of 2 × 103 cells per well and maintained at 37°C in DMEM supplemented with 10% FBS. Next, 20 μL of MTT (0.5 mg/ml, Sigma) was added to each well at different time (24 h, 48 h, 72 h, 96 h) after seeding and the plates were additional incubated for 4 h. Then, after removed the medium, 150 μL of DMSO (Sigma) was added to solubilize the crystals and the absorbance was measured at 450 nm. Each experiment was repeated in triplicate at least.
Flow cytometry
Cell apoptosis was measured by using FITC-Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instruction. Briefly, transfected cells were washed twice with cold PBS and then stained with FITC-Annexin V and PI (BD Biosciences). Apoptotic cells were analyzed by using by FACS Caliber flow cytometer (BD Bioscience). For cell cycle analysis, cells after tranfection were harvested by trypsinization, washed in ice-cold PBS and fixed in 75% ice-cold ethanol. Next, the fixed cells were stained with PI supplemented with RNaseA (Sigma) and analysis of cell cycle was performed by FACS Caliber (BD Bioscience) following the manufacturer’s guidelines. At least three independent experiments were performed.
Luciferase reporter assay
For luciferase reporter assay, the mutant-type 3’-UTR of Axin2 was generated by using Quik Change Site-Directed Mutagenesis kit (Stratagene, CA) according to manufactory protocol. The Axin2 3’-UTR was amplified from human genomic DNA and inserted into vector pGL3 using XhoI and NotI. HepG2 cells were plated into 24-well plate and incubated for 24, then co-transfected with miR-107 mimics or miR-NC and pGL3-Axin2-3’-UTR or pGL3-mutant-Axin2-3’-UTR vector by using Lipofectamine 2000 (Invitrogen). Following transfection for 48 h, cells were collected and the relative luciferase activity was determined with Dual-Luciferase Reporter System (Promega) in accordance with manufacture’s instruction. All assays were conducted in triplicate.
Western blotting
Cells were lysed using the mammalian protein extraction reagent RIPA (Beyotime) supplemented with a protease inhibitor cocktail (Roche) and phenylmethylsulfonylfluoride (Roche). Fifty micrograms of protein extracts were separated by 10% SDS-PAGE, transferred to 0.22 mm nitrocellulose (NC) membranes (Sigma) and incubated with specific antibodies. Autoradiograms were quantified by densitometry (Quantity One software, Bio-Rad). The Axin2 antibody (1:1000) was purchased from Abcam.
Statistical analysis
All data were expressed as the mean ± SD for three independent experiments. Statistical analyses of differences were performed by Student’s t test or one-way analysis of variance (ANOVA) using SPSS software (version 17; Chicago). P values less than 0.05 were considered to be statistically significant.
Results
MiR-107 is up-regulated in HCC tissues and cell lines
The expression level of miR-107 in HCC tissues and adjacent non-tumor tissues were determined by qRT-PCR. Our results showed that the expression of miR-107 was significantly increased in HCC tissues compared with that of adjacent non-tumor tissues (P<0.05, Figure 1A). Then, miR-107 expression was also explored in HCC cell lines (HepG2, HuH-7, and Hep3B) and normal human liver cell line L02. We found that miR-107 was significantly evaluated in HCC cell lines than that in normal cell line L02 (P<0.05, Figure 1B). These results indicated that miR-107 was involved in the progression of HCC.
Figure 1.
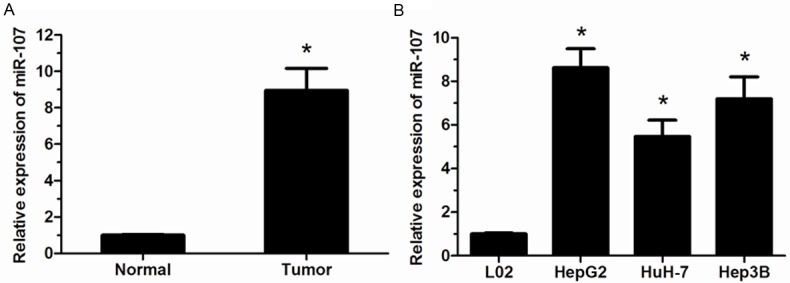
miR-107 is up-regulated in HCC tissues and cell lines. A. miR-107 was significantly up-regulated in HCC tissues (Tumor) compared with adjacent non-tumor tissues (Normal) determined by using qRT-PCR. B. miR-107 level was significantly higher in HCC cell lines than that in normal human liver cell line L02. Data were represented as means ± SD at least three independent experiments. *P<0.05.
MiR-107 promotes the proliferation of HCC cells
To determine the biological role of miR-107 in HCC progression, HepG2 cells were transfected with miR-107 mimics or miR-NC and the miR-107 expression was confirmed by qRT-PCR (Figure 2A). MTT assay indicated that overexpression of miR-107 significantly promoted the proliferation of HepG2 cells (Figure 2B). Then, we supposed that the promoted proliferation of HCC cells was correlated with cell apoptosis and cell cycle distribution. Flow cytometry showed a markedly decrease in the percentage of apoptosis cells tranfected with miR-107 mimics than that in HepG2 cells tranfected with miR-NC (Figure 2C). For cell cycle analysis, our results revealed that miR-107 overexpressing cells had a significantly lower percentage of cells in the G1/G0 phase and increased percentage of cells in the S phase compared to the miR-NC transfected cells (Figure 2D).
Figure 2.
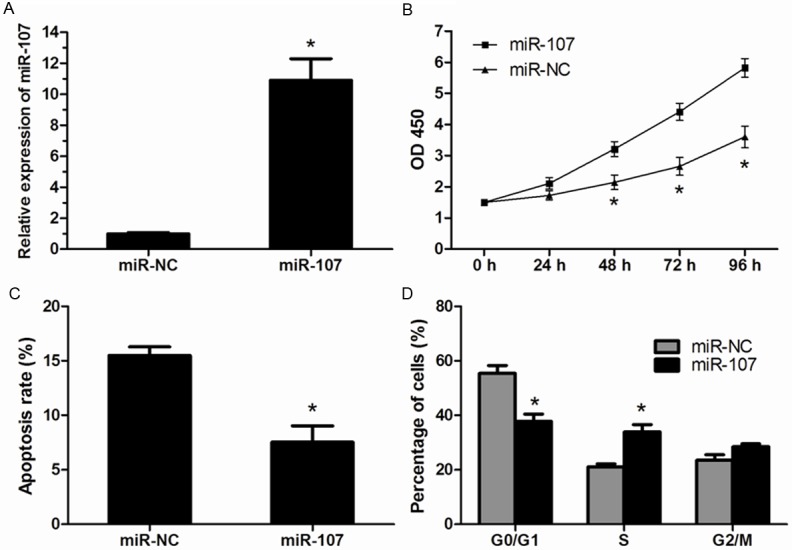
MiR-107 promotes the proliferation of HCC cells. A. Overexpression of miR-107 in HepG2 cells was confirmed by using qRT-PCR. B. Cell proliferation of HepG2 cells was significantly increased after transfected with miR-107 mimics compared with that in cells transfected with miR-NC. C. The apoptosis rate was markedly decreased in HepG2 cells transfected with miR-107 mimics. D. Transient transfection of miR-107 mimics resulted in a significant decrease in the cellular population in G0/G1 phase but a sharp increase in S phase. Data were represented as means ± SD at least three independent experiments. *P<0.05.
Axin2 is a direct target of miR-107
To explore the underlying mechanisms through which miR-107 promote the proliferation of HCC cells, we used publicly available algorithms (TargetScan 6.2) to predict the potential targets of miR-107 and Axin2 was predicted to be one of its targets (Figure 3A). Then, luciferase assay results showed that miR-107 overexpression could suppress the luciferase activity of pGL3-Axin2-3’-UTR reporter, while the luciferase activity of pGL3-mut-Axin2-3’-UTR reporter didn’t show a significant change compared to control (Figure 3B). In addition, Western blot showed that the expression of Axin2 was notably decreased in cells transfected with miR-107 mimics (Figure 3C). These data demonstrated that miR-107 might acts its oncogenic role in HCC via inhibiting the expression of Axin2.
Figure 3.
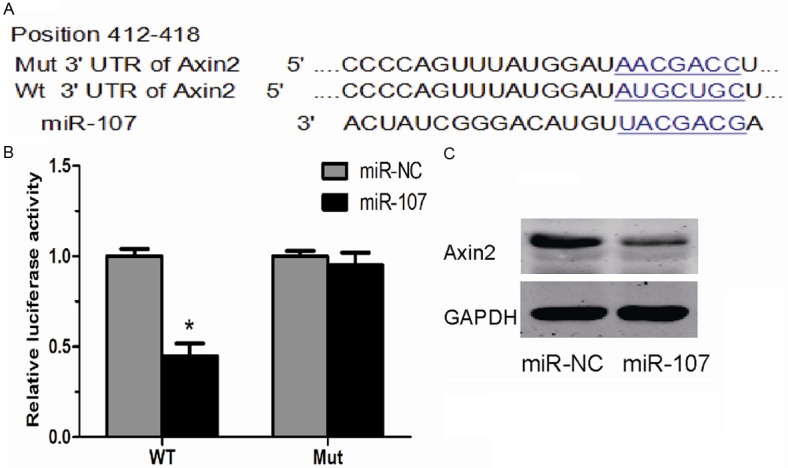
Axin2 is a direct target of miR-107. A. A schematic representation of the Axin2 3’-UTR that showed the putative miRNA target site. B. A luciferase reporter assay showed the inhibitory effect of miR-107 on Axin2 3’-UTR luciferase activity in HepG2 cells. C. The protein level of Axin2 in HepG2 cells after transfected with miR-107 mimics or miR-NC. Data were represented as means ± SD at least three independent experiments. *P<0.05.
Axin2 knockdown increases the proliferation of HCC cells
To further verify the role of Axin2 in the progression of HCC, Axin2-specific interfering RNA (si-Axin2) and its negative control (si-NC) were transfected into HepG2 cells, respectively. After transfected with si-Axin2, the expression of Axin2 was dramatically decreased in HepG2 cells (Figure 4A). The MTT assay revealed that the knockdown of Axin2 significantly promoted the proliferation of HepG2 cells (Figure 4B). Cell apoptosis assay showed that silencing of Axin2 significantly inhibited apoptosis of HepG2 cells (Figure 4C). Furthermore, Cell cycle distribution analysis showed that down-regulated expression of Axin2 resulted in a significant decreased in percentage of cells in G0/G1 phase and higher percentage of cells in the S phase (Figure 4D). These results further confirmed that Axin2 is a target of miR-107 in HCC cells.
Figure 4.
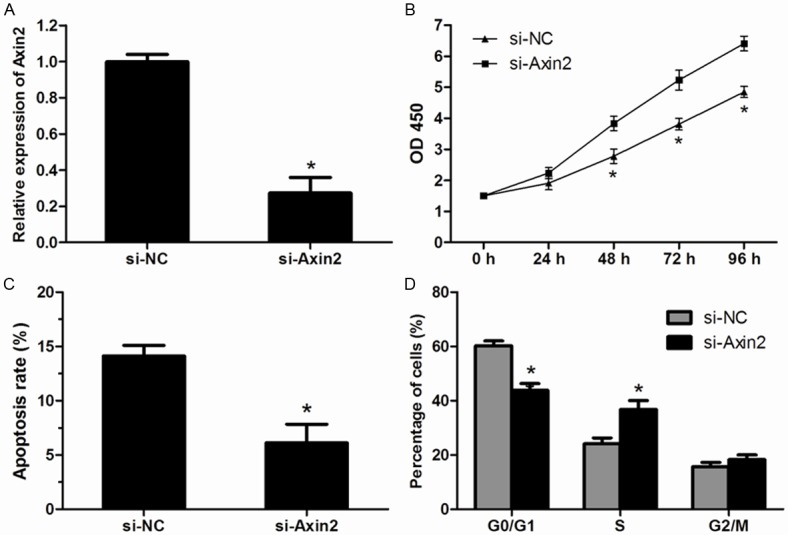
Effect of si-Axin2 on the proliferation of HCC cells. A. Expression of Axin2 was determined by qRT-PCR in HepG2 cells after transfected with si-Axin2. B. Cell proliferation of HepG2 cells was significantly increased after transfected with si-Axin2 compared with that in cells transfected with si-NC. C. The apoptosis rate was markedly decreased in HepG2 cells transfected with si-Axin2. D. Down-regulated expression of Axin2 resulted in a significant decrease in the cellular population in G0/G1 phase but a sharp increase in S phase. Data were represented as means ± SD at least three independent experiments. *P<0.05.
Discussion
Hepatocellular carcinoma (HCC) is one of the most frequent human malignant tumors in the world, especially in China, where the incidence of HCC is much higher than that in other Asian countries [16]. Accumulating evidence suggested that expression levels of various miRNAs are altered in HCC and miRNAs may play important roles in the development and progression of HCC. For example, miRNA-206 was reported to down-regulated in HCC and overexpression of miRNA-206 could suppress cell proliferation, invasion, and migration, but promote cell apoptosis in HepG2 cells [17]. Yu et al demonstrated that miR-543 was increased in HCC. They found that forced expression of miR-543 could promote the proliferative and invasive potential of HCC cells [18]. In addition, Lin et al showed that miRNA-141 suppressed the proliferation and invasion of HepG2 cells by repressing its target HNF-3β [19].
In the present study, we focused on the role of miR-107 in HCC. Fist, we analyzed miR-107 expression in HCC tissues and cells lines by using qRT-PCR. Our results showed that the expression level of miR-107 was significantly increased in HCC, indicating that miR-107 is involved in the progression of HCC. Then, to explore the biological function of miR-107 in HCC cells progression, HepG2 cells were transfected with miR-107 mimics. Our data revealed that over-expression of miR-107 significantly promoted cell proliferation of HepG2 cells. These results suggested that miR-107 may act as a tumor oncogene in the progression of HCC.
As we known, miRNAs exert their function by interacting with the 3’-UTR of the target mRNA. To further understand the underlying mechanism of miR-107 in HCC, we explored the potential target of miR-107. As a member of β-catenin/T-cell factor14 dependent genes, Axis inhibition protein 2 (Axin2) has complementary sequences of miR-107 core region. So we hypothesis that Axin2 may be a candidate target of miR-107 and the results of luciferase assay and western blot suggested that Axin2 is a direct target of miR-107.
Axin2 is a negative regulator of the canonical Wnt/β-catenin signaling pathway, and functions as a tumor suppressor in a number of human cancers [20]. Koinuma et al showed that epigenetic silencing of Axin2 is specifically associated with carcinogenesis in colorectal carcinomas [21]. Wei et al found that Axin2 expression was lower in ameloblastomas and Axin2 may play an important role in the tumorigenesis and progression of ameloblastoma [22]. In our study, we found that Axin2 knockdown significantly increased cell proliferation in HepG2 cells. These results further confirmed that Axin2 is a target of miR-107 in HCC.
Taken together, our study demonstrated that miR-107 was evaluated in HCC tissues and cell lines, and its ectopic expression of miR-107 could promote HCC cell proliferation. Furthermore, we identified Axin2 as a direct target of miR-107 in HCC. These results indicated that miR-107 might involve in the tumorigenesis of HCC and represent a potential therapeutic target for the HCC treatment.
Disclosure of conflict of interest
None.
References
- 1.Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–S26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Du R, Wu S, Lv X, Fang H, Kang J. Overexpression of brachyury contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:105. doi: 10.1186/s13046-014-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L, Cai X. Up-regulation of miR-9 expression predicate advanced clinicopathological features and poor prognosis in patients with hepatocellular carcinoma. Diagn Pathol. 2014;9:1000. doi: 10.1186/s13000-014-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takizawa D, Kakizaki S, Sohara N, Sato K, Takagi H, Arai H, Katakai K, Kojima A, Matsuzaki Y, Mori M. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci. 2007;52:3290–3295. doi: 10.1007/s10620-007-9808-2. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 8.Luo XJ, Tang DG, Gao TL, Zhang YL, Wang M, Quan ZX, Chen J. MicroRNA-212 inhibits osteosarcoma cells proliferation and invasion by down-regulation of sox4. Cell Physiol Biochem. 2014;34:2180–2188. doi: 10.1159/000369661. [DOI] [PubMed] [Google Scholar]
- 9.Yu G, Yao W, Xiao W, Li H, Xu H, Lang B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J Exp Clin Cancer Res. 2014;33:779–779. doi: 10.1186/s13046-014-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Zhang T, Jin R, Zhao H, Hu J, Feng B, Zang L, Zheng M, Wang M. MicroRNA-301a promotes migration and invasion by targeting TGFBR2 in human colorectal cancer. J Exp Clin Cancer Res. 2014;33:780. doi: 10.1186/s13046-014-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Zhang W, Zhou Q, Zhao T, Song Y, Chai L, Li Y. Low-expression of microRNA-107 inhibits cell apoptosis in glioma by upregulation of SALL4. Int J Biochem Cell Biol. 2013;45:1962–1973. doi: 10.1016/j.biocel.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Datta J, Smith A, Lang JC, Islam M, Dutt D, Teknos TN, Pan Q. microRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cε. Oncogene. 2012;31:4045–4053. doi: 10.1038/onc.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, Huso D, Lowenstein CJ. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A. 2010;107:6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PS, Su JL, Cha ST, Tarn WY, Wang MY, Hsu HC, Lin MT, Chu CY, Hua KT, Chen CN, Kuo TC, Chang KJ, Hsiao M, Chang YW, Chen JS, Yang PC, Kuo ML. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2011;121:3442–3455. doi: 10.1172/JCI45390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Liu B, Gao Y, Liu Y, Xu Y, Tong W, Zhang A. Upregulation of microRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1. FEBS Lett. 2014;588:538–544. doi: 10.1016/j.febslet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 17.Yunqiao L, Vanke H, Jun X, Tangmeng G. MicroRNA-206, down-regulated in hepatocellular carcinoma, suppresses cell proliferation and promotes apoptosis. Hepatogastroenterology. 2014;61:1302–1307. [PubMed] [Google Scholar]
- 18.Yu L, Zhou L, Cheng Y, Sun L, Fan J, Liang J, Guo M, Liu N, Zhu L. MicroRNA-543 acts as an oncogene by targeting PAQR3 in hepatocellular carcinoma. Am J Cancer Res. 2014;4:897. [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L, Liang H, Wang Y, Yin X, Hu Y, Huang J, Ren T, Xu H, Zheng L, Chen X. microRNA-141 inhibits cell proliferation and invasion and promotes apoptosis by targeting hepatocyte nuclear factor-3beta in hepatocellular carcinoma cells. BMC Cancer. 2014;14:879. doi: 10.1186/1471-2407-14-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koinuma K, Yamashita Y, Liu W, Hatanaka H, Kurashina K, Wada T, Takada S, Kaneda R, Choi Y, Fujiwara S. Epigenetic silencing of AXIN2 in colorectal carcinoma with microsatellite instability. Oncogene. 2005;25:139–146. doi: 10.1038/sj.onc.1209009. [DOI] [PubMed] [Google Scholar]
- 22.Wei ZH, Zhong M, Guo Y, Wang Y, Ren M, Wang Z. Original papers Expression of β-catenin and AXIN2 in ameloblastomas. Wspolczesna Onkol. 2013;17:250–256. doi: 10.5114/wo.2013.35278. [DOI] [PMC free article] [PubMed] [Google Scholar]


