Abstract
Glycyrrhizic acid (GA) is the bioactive compound of licorice and has been used as an herbal medicine because of its anti-viral, anti-cancer, and anti-inflammatory properties. This study was designed to investigate the effects of GA on leukemia cells growth, migration, and the mechanisms underlying the anti-cancer activities of GA. MTT test was used to detect the effect of GA on TF-1 leukemia cell growth. Wound closure assay and Transwell were adopted to assess the effect of GA on TF-1 migration and invasion. Migration and invasion related proteins including AKT and mTOR were detected by western blot assay. We further used western blot and immunofluorescence assay to evaluate the effect of GA on STAT3 phosphorylation in vitro. We also evaluated the anti-tumor effect of GA in TF-1 tumor bearing BALB/c mice model. The present study showed GA significant inhibit of TF-1 proliferation in a dose and time-dependent manner. GA could remarkably inhibit TF-1 cell migration and invasion; meanwhile effectively suppress AKT, mTOR, and STAT3 phosphorylation in TF-1 cells. GA in 100 mg/kg/ could inhibit the tumor growth in vivo and down-regulated AKT, mTOR, and STAT3 phosphorylation in TF-1 tumor tissues. Our findings suggest that GA is a promising therapeutic agent for leukemia that targets the AKT/mTOR/STAT3 pathway.
Keywords: Glycyrrhizic acid, AKT, mTOR, STAT3, leukemia
Introduction
Leukemia is the most common aggressive blood cancers and the leading cause of cancer-related deaths. Therefore, management of leukemia remains a significant clinical challenge and new therapeutic agents that inhibit leukemia cell growth and metastasis through multiple targeted pathways are urgently needed [1]. Notably, signaling of serine-threonine protein kinase (AKT)/mammalian target of rapamycin (mTOR) and signal transduction and activator of transcription (STAT3) are confirmed to be critical for leukemia cell survival and proliferation [2]. STAT3 is persistently activated by non-receptor tyrosine kinases such as AKT and mTOR. The AKT/mTOR pathway widely reported as a potent pro-survival and pro-metastatic signaling axis, and novel agents that specifically inhibit its activation offer a novel targeted therapeutic approach for many cancers. In addition, activation of STAT3 leads to tumor-promoting gene products, including cleavage caspase-3, cleavage poly ADP-ribose polymerase (PARP), cyclin D1, and survivin.
Glycyrrhizic acid (GA) is the bioactive compound of licorice and has been used as an herbal medicine because of its anti-viral, anti-cancer, and anti-inflammatory properties [3]. Glycyrrhizic acid was recently demonstrated to induce apoptosis in human endometrial cancer cells, and potently inhibited growth of the breast cancer stem/progenitor cells via inhibition of AKT/mTOR signaling [4]. However, the potential therapeutic effects of glycyrrhizic acid and its key molecular mechanisms have not yet been explored in leukemia [5]. Here, we show that GA suppressed STAT3 activation, associated with a reduction in activation of AKT/mTOR. Inhibition of STAT3 phosphorylation by GA reduced the expression of STAT3-regulated cell survival and proliferation factors such as cleavage caspase-3, cleavage PARP, cyclin D1, and survivin. Additionally, GA inhibited leukemia cells growth in vivo, potently suppressed STAT3 activation, and significantly intervention AKT/mTOR pathway in vivo. These data suggest that glycyrrhizic acid is a specific inhibitor of AKT/mTOR signaling and STAT3 activation, and may represent a viable therapeutic strategy for the treatment of advanced leukemia.
Materials and methods
Reagents and cells
Glycyrrhizic acid was purchased from Shanghai Win Herb Medical Science Corporation (Purity > 98%). Anti-phosphorylated Stat3 (p-Stat3; Ser727), anti-phosphorylated mTOR (p-mTOR; Ser2448), anti-phosphorylated AKT (p-AKT; Ser473), anti-cleaved caspase-3, anti-cleaved PARP, anti-cyclin D1, anti-survivin, and anti-GAPDH were purchased from Cell Signaling Technology, Inc. Anti-Stat3, anti-mTOR, and anti-AKT were all purchased from Santa Cruz Biotechnology. Human leukemia cell line TF-1 was from ATCC and was grown in RPMI-1640 supplemented with 10% fetal bovine serum (FBS).
Proliferation assay
Cell proliferation was measured with Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega), which contains MTS. 96-well plates were seeded with 3,000 cells per well in RPMI-1640 supplemented with 1% FBS. After overnight incubation, cells were treated with varying concentrations of glycyrrhizic acid or DMSO control. After treatment, MTS was added to the cells according to the manufacturer’s protocol and absorbance was measured at 490 nm using an automated ELISA plate reader (Molecular Devices).
Wound healing migration and transwell invasion assay
TF-1 cells were allowed to grow into full confluence in 24-well plates and then wounded by scratching with pipette tips and washed with PBS. Fresh 1640 was added with different concentrations of glycyrrhizic acid or vehicle. Images were taken by an OLYMPUS inverted digital camera after 24 hours incubation. Transwell assay was conducted as described previously with some modifications [6]. Matrigel diluted in serum-free medium was added to the top chamber of 24-well transwell plate (Millipore). After Matrigel polymerization, the bottom chambers were filled with 600 μL 1640 medium containing various growth factors. The top chambers were seeded with 100 μL 1640 medium and TF-1 cells (4×104 cells per well). Immediately, 100 μL 1640 medium with various concentrations of GA was added to the upper chamber. After 24 hours, invasion was stopped by scraping non-migrated cells on the top chamber with a cotton swab. Migrated cells were fixed in 4% paraformaldehyde and stained with 0.05% Crystal Violet [7]. Images were taken using a ZEISS digital microscope and invading cells were counted by manual couning. The assays were replicated 3 times.
Western blot
Total protein (20 μg) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinyllidene difluoride membrane. Membranes were blocked for 1 hour at ambient room temperature (ART) in 10% non-fat dry milk in TBST (16TBS with 0.1% Tween 20) followed by an overnight incubation at 4uC with primary antibodies in TBST with 5% BSA. Horseradish peroxidase-labeled antimouse or anti-rabbit secondary antibodies were added for 1 hour at ART and detected with Super Signal West Pico substrate (Pierce). Bands were measured as optical density using ImageJ software. The optical density of each band was normalized by GAPDH optical density.
Immunofluorescence
After the treatment of GA (10 μM) for 4 h or 24 h, cells were fixed with 4% paraformaldehyde in PBS for 20 min, permeabilized with 0.5% Triton X-100 for 20 min, and blocked with 3% bovine serum albumin (BSA) for 1 h. Nuclei were counterstained with Hoechst 33258 (Biotime Biotech, Haimen, China). FITC conjugated goat antirabbit secondary antibody was used to probe the samples for 1 h to analyze p-Stat3Ser727.
In vivo experiments
Female BALB/c mice (7-8 week old) were purchased from NCI. Animal use procedures were approved by the institutional committee of the Beckman Research Institute at City of Hope Medical Center. Mice were implanted s.c. with 2.5×106 TF-1 cells. After tumors reached 5 to 7 mm in diameter, GA or vehicle (DMSO) control was administered peritumorally once every other day at 100 mg/kg body weight [8]. Tumor growth was monitored every other day with digital caliper measurements.
Statistical analysis
The data were presented as mean ± SD. Differences in the results of two groups were evaluated using either two-tailed Student’s t test or one-way ANOVA followed by post-hoc Dunnett’s test. The differences with P < 0.05 were considered statistically significant.
Results
GA inhibits proliferation in leukemia cells
To determine whether GA (Figure 1A) has direct anti-tumor effects in leukemia cells, doseresponse and time course studies were peformed in human TF-1 cells. Cells treated with GA showed significant inhibition of cell proliferation in a dose (Figure 1B) and time-dependent manner (Figure 1C). IC50 value was calculated, showing that GA exerted 50% inhibition at 16 μM after 24 h treatment (Figure 1B). Representative images of cell morphology showed that GA significantly killed TF-1 cells (Figure 1D). Collectively, these data indicate that GA has potent anti-tumor effects on human leukemia cells.
Figure 1.

GA inhibits TF-1 cells proliferation. A, Structure of GA; B. TF-1 cells were exposed to indicated concentrations of GA (1, 5, 10, 20, 30, 40 μM) for 24 h; C. TF-1 cells were treated with 10 μM GA for 6, 12, 24, 36, 48, and 72 h respectively. Cell viability was determined by One solution cell proliferation assay; D. Representative images of cell morphology in TF-1 cells were taken after 48 h treatment with or without 20 μM GA. Columns, mean (n = 3, in triplicate); bars, SD. *P < 0.05; **P < 0.01; ***P < 0.001.
GA inhibits leukemia cells migration and invasion
Cell migration is essential for leukemia cells in tumor growth and metastasis. We performed wound healing assays to investigate the effects of GA on cell migration and observed GA at 20 μM strongly inhibited the migration of TF-1 cells (Figure 2A). Cell invasion is essential for leukemia cells in metastasis, so we performed transwell assays to evaluate the ability of TF-1 cells to pass through the Matrigel and membrane barriers of the transwell in the presence of various concentrations of GA or vehicle. As shown in Figure 2B, GA significantly inhibited the invasion activities of TF-1 cells in a concentration-dependent manner.
Figure 2.
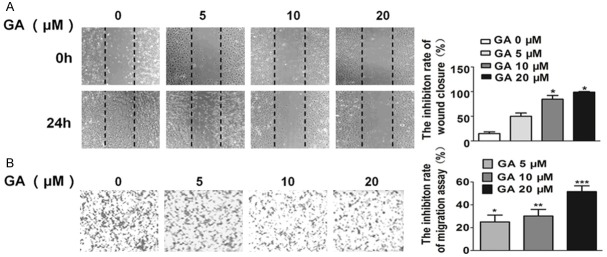
Effects of GA on TF-1 migration and invasion. A. GA inhibited TF-1 migration in wound healing assay. Cells were wounded by the pipette and then treated with various concentrations of compounds for 24 hours. Migrated cells were quantified by manual counting; B. GA inhibited TF-1 cells invasion in transwell assay. The bottom chambers of the transwells were filled with 600 μl 1640 containing various growth factors while the top chambers were seeded with 4×104 TF-1 in 1640 and treated with different concentrations of GA for 24 hours. Cells invaded through the membrane were stained and quantified. Columns, mean (n = 3, in triplicate); bars, SD. *P < 0.05; **P < 0.01; ***P < 0.001.
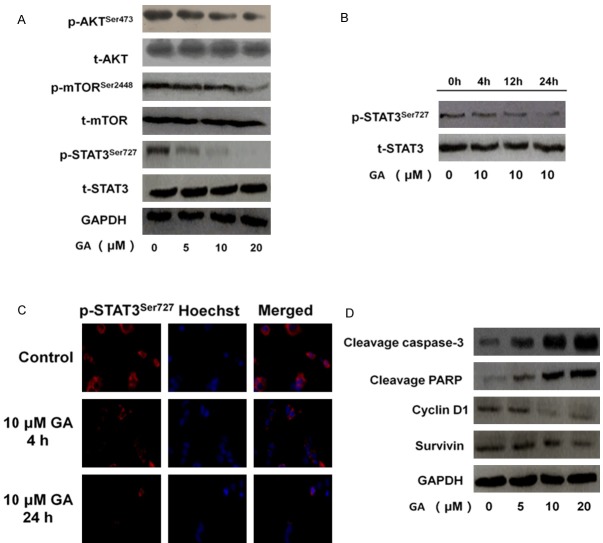
GA inhibits AKT/mTOR/STAT3 signaling in leukemia cells
To explore the underlying mechanisms regulating the effects of GA on TF-1 cells, we examined the most important oncogenic signaling pathway AKT/mTOR/STAT3, which is associated with leukemia cells growth and migration [9]. Western blot analysis showed that GA dose-dependently decrease the phosphor-AKT (Ser473) and phosphor-mTOR (Ser2448) without total protein level changed in TF-1 cells (Figure 3A). Given that STAT3 is constitutively activated in diverse cancers, including leukemia, we assessed whether GA-induced tumor growth and migration inhibition was associated with STAT3 inhibition. Although GA had no effects on total STAT3 protein levels in TF-1 cells, it inhibited activated STAT3 as early as 4 hours after GA treatment, with continued inhibition of STAT3 activation after 24 hours (Figure 3B and 3C).
Figure 3.
Effects of GA on major oncogenic signaling pathways in TF-1 cells. A. GA reduced AKT/mTOR/STAT3 signaling. TF-1 cells were treated with GA at indicated concentrations for 24 h. Total cell lysates were prepared and western blots were performed using relevant antibodies to detect total protein levels, with GAPDH used as the loading control; B. To assess whether GA inhibits the phosphorylation of STAT3 at Ser727, TF-1 cells were treated with GA for 4, 12, 24 h. Then were subjected to western blot for measuring protein level of phosphor-STAT3 (Ser727). Anti-GAPDH monoclonal antibody was used as a loading control; C. Pre-incubation of GA inhibited the phosphorylation of STAT3 (Ser727). TF-1 cells were treated with vehicle, 10 μM GA for 4h or 24 h, and then cells were fixed and incubated with primary antibodies against p-STAT3Ser727. TF-1 cells were immunostained with anti-rabbit FITC-conjugated secondary antibody and then stained with Hoechst 33258. The specimens were visualized and photographed using a fluorescence microscope (400×, scale bar represents 50 μm). D. TF-1 cells were treated with 0, 5, 10 or 20 μM GA, and then were subjected to western blot for measuring protein levels of cyclin D1, cleavage caspase-3, cleavage PARP and survivin, respectively.
We further assessed the potential effects of GA on the STAT3 downstream factors in TF-1 cells [10]. The results showed that GA treatment of TF-1 cells reduced expression of several key anti-apoptotic and pro-proliferative proteins, including cyclin D1, and survivin (Figure 3D). To confirm proliferation inhibition in GA-treated TF-1 cells, we detected the levels of activated caspase-3 and PARP cleavage and the results showed that GA increased expression of several key apoptotic proteins including cleavage caspase-3 and cleavage PARP in TF-1 cells (Figure 3D).
GA inhibited leukemia cells growth in vivo
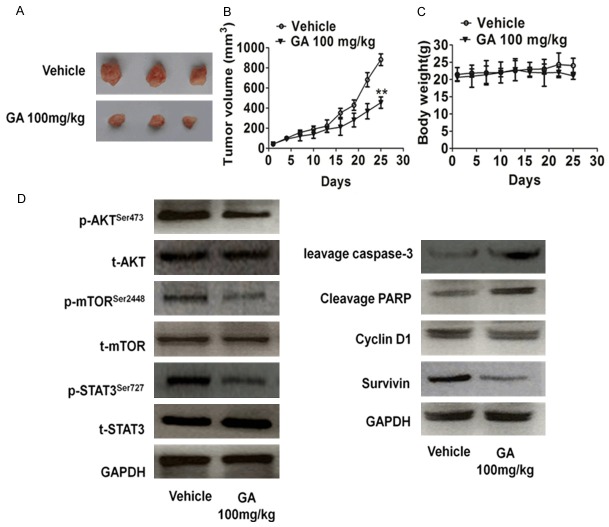
We next assessed whether GA inhibits tumor growth in vivo. GA treatment (100 mg/kg) of resulted in potent inhibition of TF-1 tumor growth (Figure 4A and 4B), which correlated with a reduction in AKT, mTOR, and STAT3 activity in tumors (Figure 4D). Moreover, body weight loss was not observed in mice treated with GA. At the end of the experiment, in the GA group, the body weight was 23.3 ± 0.25 g, which is comparable to the vehicle group 22.5 ± 0.49 g. There was no statistical difference between GA treated and vehicle group (Figure 4C). Taken together, these data indicate that GA resulting in significantly reduced leukemia cells growth in vivo.
Figure 4.
GA inhibited growth on TF-1 leukemia cell xenografts. A. Nude mice bearing TF-1 cells were treated daily with the vehicle or GA at 100 mg/kg/day by intraperitoneal administration for 25 days. Representative mice with TF-1 xenografts and tumor masses were shown; B. It was found that treatment with GA obviously suppressed tumor volumes compared to the vehicle control group, indicating that GA could significantly inhibit the TF-1 tumor growth in vivo (values represent means ± SD, n = 3, *P < 0.05 versus vehicle group); C. GA resulted in little toxicity effects in vivo. No significant differences of body weights were detected among all the groups; D. Tumor tissues were prepared for western blot with specific antibodies against AKT, mTOR, STAT3, cyclin D1, cleavage caspase-3, cleavage PARP and survivin. It was found that GA could obviously decrease p-AKT, p-mTOR and p-STAT3 in comparison with vehicle treatment. Meanwhile, GA treatment could obviously attenuate expressions of cyclin D1, survivin and increase cleavage caspase-3, cleavage PARP expression, further demonstrating that GA played an important role in suppressing tumor growth at least in part via AKT/mTOR/STAT3 signaling pathways in vivo.
Discussion
Cell proliferation, migration and invasion were closely related to cancer progression, therefore, inhibition of tumor cell growth and migration might be important method to prevent tumor progression [11]. Gycyrrhizic acid (GA), which is the main and sweet component of licorice, has been investigated for its ability to cause hypermineralocorticoidism with sodium retention and potassium loss, edema, increased blood pressure, as well as depression of the renin-angiotensin-aldosterone system. However, only a small number of studies have been reported on the anticancer activity of GA and the mechanism of the antitumor activity of GA in leukemia cells was not evaluated. Leukemia cells proliferation plays an important role in the process of leukemia, therefore, we examined whether GA showed anti-proliferative effects on leukemia cells. The results showed that GA markedly inhibited TF-1 leukemia cell proliferation. In this study, we also demonstrate that GA could effectively inhibit TF-1 leukemia cells migration and invasion. Moreover, the effect of GA on TF-1 cells may be mediated by AKT/mTOR/STAT3 pat-hway.
The AKT/mTOR/STAT3 pathway is a master intracellular signaling pathway which is important in tumor cell growth and migration [12]. Phosphorylated AKT and mTOR are translocated into the nucleus to transmit extracellular signals that regulate cell growth, differentiation, proliferation, apoptosis, and migration functions [13]. STAT3 was regarded as a prominent regulatory factor in the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation [14]. Activation of the STAT3 has been shown to regulate leukemia cells several functions such as migration and proliferation. Therefore, targeted drugs of AKT/mTOR/STAT3 pathway might be helpful for treatment of cancers [15]. In current studies, we found that AKT/mTOR/STAT3 pathway was inactivated by GA treatment.
Besides inhibiting TF-1 leukemia cell growth, migration and invasion, GA also had a direct inhibitory effect on TF-1 growth in vivo. GA inhibited the migration and invasion of TF-1 cells. Nude mice bearing TF-1 tumor were treated daily with the vehicle or GA at 100 mg/kg/day by intraperitoneal administration. It was found that treatment with GA obviously suppressed tumor volumes, indicating that GA could significantly inhibit the TF-1 tumor growth in vivo. Meanwhile, western blot results show that GA treatment could obviously attenuate expressions of p-AKT, p-mTOR, and p-STAT3 in tumor tissue. Overall, our study indicated that GA at non-toxic dosages exerted potent anti-tumor growth activities via specifically targeting AKT/mTOR/STAT3 axis.
Disclosure of conflict of interest
None.
References
- 1.Zhao XF, Gojo I, York T, Ning Y, Baer MR. Diagnosis of biphenotypic acute leukemia: a paradigmatic approach. Int J Clin Exp Pathol. 2009;3:75–86. [PMC free article] [PubMed] [Google Scholar]
- 2.C Chiarini F, Lonetti A, Teti G, Orsini E, Bressanin D, Cappellini A, Ricci F, Tazzari PL, Ognibene A, Falconi M, Pagliaro P, Iacobucci I, Martinelli G, Amadori S, McCubrey JA, Martelli AM. A combination of temsirolimus, an allosteric mTOR inhibitor, with clofarabine as a new therapeutic option for patients with acute myeloid leukemia. Oncotarget. 2012;3:1615–28. doi: 10.18632/oncotarget.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Zhu JH, Cao LP, Sun Q, Liu HD, Li WD, Li JS, Hang CH. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci. 2014;35:1115–20. doi: 10.1007/s10072-014-1661-4. [DOI] [PubMed] [Google Scholar]
- 4.Raphael TJ, Kuttan G. Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals. Immunopharmacol Immunotoxicol. 2008;30:243–55. doi: 10.1080/08923970701675044. [DOI] [PubMed] [Google Scholar]
- 5.H Hibasami H, Iwase H, Yoshioka K, Takahashi H. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int J Mol Med. 2006;17:215–19. [PubMed] [Google Scholar]
- 6.Wang N, Wang ZY, Mo SL, Loo TY, Wang DM, Luo HB, Yang DP, Chen YL, Shen JG, Chen JP. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat. 2012;134:943–55. doi: 10.1007/s10549-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chueh FS, Hsiao YT, Chang SJ, Wu PP, Yang JS, Lin JJ, Chung JG, Lai TY. Glycyrrhizic acid induces apoptosis in WEHI-3 mouse leukemia cells through the caspase- and mitochondria-dependent pathways. Oncol Rep. 2012;28:2069–76. doi: 10.3892/or.2012.2029. [DOI] [PubMed] [Google Scholar]
- 8.Meng XG, Yue SW. Dexamethasone disrupts cytoskeleton organization and migration of T47D human breast cancer cells by modulating the AKT/mTOR/RhoA pathway. Asian Pac J Cancer Prev. 2014;15:10245–10250. doi: 10.7314/apjcp.2014.15.23.10245. [DOI] [PubMed] [Google Scholar]
- 9.Bai HW, Badaboina S, Park CH, Choi BY, Na YH, Chung BY. Centipedegrass extract induces apoptosis through the activation of caspases and the downregulation of PI3K/Akt and MAPK phosphorylation in leukemia cells. Int J Mol Med. 2015;35:511–8. doi: 10.3892/ijmm.2014.2012. [DOI] [PubMed] [Google Scholar]
- 10.Qiu XM, Bai X, Jiang HF, He P, Wang JH. 20-(s)-ginsenoside Rg3 induces apoptotic cell death in human leukemic U937 and HL-60 cells through PI3K/Akt pathways. Anticancer Drugs. 2014;25:1072–1080. doi: 10.1097/CAD.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 11.Zeng D, Wang J, Kong P, Chang C, Li J, Li J. Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 2014;7:2172–2178. [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto KM, Grant S, Saleiro D, Crispino JD, Hijiya N, Giles F, Platanias L, Eklund EA. Targeting novel signaling pathways for resistant acute myeloid leukemia. Mol Genet Metab. 2015;114:397–402. doi: 10.1016/j.ymgme.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CH, Chen AZ, Yen GC. Protective effects of glycyrrhizic acid and 18β-glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J Agric Food Chem. 2015 doi: 10.1021/jf505471a. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Jia X, Yang W, Han J, Xiong H. Effects of lentivirus mediated STAT3 silencing on human chronic myeloid leukemia cells and leukemia mice. Int J Clin Exp Med. 2014;7:4031–4037. [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan NJ, Dutkowski CM, Barrow D, Mottram HJ, Hutcheson IR, Nicholson RI, Guichard SM, Gee JM. Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res. 2014;16:R12. doi: 10.1186/bcr3604. [DOI] [PMC free article] [PubMed] [Google Scholar]