Abstract
Background: The failure of intestinal mucosal barrier may induce multiple organ dysfunction and systemic inflammatory response syndrome, but little work has been done on whether hypobaric hypoxia related to the failure of intestinal mucosal barrier. Aims: To study the expression of hypoxia-inducible factor 1α (HIF-1α), inducible nitric oxide synthase (iNOS) and morphological changes of intestinal mucosa in albino rats at different altitude. Methods: 30 male Wistar rats raised in plain for one month were randomly divided into 3 groups: Plain 500 m group (n=10), High-altitude (HA) 3842 m group (n=10) and HA4767 m group (n=10). Each group was delivered to different altitude area at the same shipping time and executed after 3 days’ exposure to different altitude. Intestinal segments with the same location of all rats were removed for morphological analyses. Morphologic parameters (villous height, crypt depth, mucosal wall thickness and villous surface area) were measured by optical and scanning electron microscope. The expression of iNOS and HIF-1α were detected by immunohistochemistry. Results: Morphological indexes in higher altitude groups were exacerbated obviously compared with those of lower altitude groups. While the expression of iNOS and HIF-1α in higher altitude groups were significantly increased than those of lower altitude groups. Linear correlation analysis showed that the expression of iNOS was positively correlated with that of HIF-1α. Conclusions: Hypobaric hypoxia increases the expression of HIF-1α and iNOS in intestinal mucosa, however exacerbates the mucous morphologic parameters with altitude increasing. HIF-1α may regulate the expression of iNOS and be involved in the damage of intestinal mucosa.
Keywords: High-altitude, hypoxia, intestinal mucosal barrier, inducible nitric oxide synthase, hypoxia inducible factor-1α
Introduction
High altitude affects the human body because of oxygen deprivation. Other environmental factors, such as severe cold, squall, and intense solar radiation are also involved in the damnification [1]. The pathophysiologic changes in intestinal blood vessel and mucosa, caused by hypoxia of high altitude are severe. The intestine is not only the digestion and absorption viscera, but also immunity and incretion viscera. Hypobaric hypoxia always causes symptom of various gastric-intestinal reactions, but little work has been done on whether hypobaric hypoxia related to the failure of intestinal mucosal barrier. Which posses various structural and molecular regulated mechanisms and abundant biological functions, together with each signal pathway to protect against exogenous factors attack. The failure of intestinal mucosal barrier may cause symptom of primary affection, induce multiple organ dysfunction and systemic inflammatory response syndrome, finally may result in a vicious circle, even die.
Some papers suggested that the pattern of high altitude exposure-associated damage resembles ischemia/reperfusion injury [2]. The intestinal mucosa with a high density of capillary network and a high metabolic rate, so that depends on a high demand of blood oxygen supply. The small intestinal mucosal villi are very sensitive to ischemia/reperfision. On the other hand, hypoxia triggers various systemic, cellular, and metabolic responses necessary for tissues to adapt to low oxygen conditions [3].
Hypoxia-inducible factor 1 (HIF-1) plays as an important role in the coordination of oxygen supply and cellular metabolism [4]. When humans are exposed to hypoxia, systemic and intracellular changes operate together to minimize hypoxic injury and restore adequate oxygenation. The emerging role of HIF in systemic physiology, described here in terms of the response to high altitude, in fact translates to any clinical scenario in which hypoxia is a feature.
Hypoxia increases the expression of inducible nitric oxide synthase (iNOS). Nitric oxide (NO) generated from NOS is also involved in many physiological and pathological processes, including hypoxic damnification [6]. In mammals including humans, NO is an important cellular signaling molecule. The endothelium (inner lining) of blood vessels uses nitric oxide to signal the surrounding smooth muscle to relax, thus resulting in vasodilation and increasing blood flow as well as oxygen supply. The production of nitric oxide is elevated in populations living at high altitudes, which helps these people avoid hypoxia by aiding in pulmonary vasculature vasodilation. The dual role of NO as a cytoprotective or a cytotoxic factor depended upon the concentration of NO, which has been noted in various types of pathophysiological conditions, including the digestive system. The low level and basal NO production are important in minimizing mucosal and microvascular barrier dysfunction associated with reperfusion of postischemic intestine [7]. Oppositely, the high level of NO can aggravate to ischemia- reperfusion injury. Sustained upregulation of NO production in the intestine can lead to intestinal epithelial injury [8]. Moreover, the NO production may also accelerated by ischemia-reperfusion of small intestine and then participate in the breakdown of intestinal mucosa after ischemia-reperfusion insult [9,10].
In order to study whether high altitude induced intestinal injury, we transported rats from plain to different altitude areas, and took a deep look at the morphological changes of intestines and the expression of HIF-1α and iNOS. To explore the relationship between hypoxia factors and intestinal mucosa injury may prepare good conditions for the continued clinic study.
Methods
Drugs and materials
The antibodies of iNOS (bs-0465R) and HIF-1α (bs-0162R) were purchased from Biosynthesis Biotechnology Company (Beijing, China). Healthy male Wistar rats, aged 8-12 weeks, weighted 250 g ± 22 g, were provided by the Reproduction and Experimental Animal Center (CREAL) of the Animal Center of the Forth Military Medical University (Xi’an, China).
Groups and experiments
All rats (n=30) were raised at 24 °C, with a 12 h light/dark cycle, and provided with food and water ad libitum for one month at the Department of Clinical Laboratory, Lanzhou General Hospital of Lanzhou Military Command. Then they were randomly divided into 3 groups: (1) Plain 500 m group: Rats were ascended to plain (Xi’an, China, 500 m of altitude, Patm =97.1 kPa, n=10); (2) HA3842 m group: Rats were ascended to plateau (Yuzhong County, Gansu, China, 3842 m of altitude, Patm =73.5 kPa, n=10); (3) HA4767 m group: Rats were ascended to plateau (Qinghai High Altitude Medical Research Center, China, 4767 m of altitude, Patm =50.2 kPa, n=10). All groups were delivered by car at 24 h ± 1 h and were killed on the 3rd day after the exposure to different altitude. The experiments were carried out in accordance with the guidelines of the Ethics Committee of our hospital. Small intestines were harvested, rinsed with normal saline, 1 cm×1 cm segments were obtained from the proximal ileum on the same area.
Observation
Segments of ileum were collected and cleaned with cold phosphate-buffered saline. Then the segments were immersed directly in a 4% buffered formaldehyde solution overnight as fixative. The fixed tissues were processed routinely into paraffin and 5 μm sections were stained routinely with hematoxylin and eosin. The tissue sections were observed under optical microscope. The ultra-structural evaluation of ileum was done by standard procedure. Desiccated samples were mounted on aluminum stub and sputter coated with gold palladium to produce a uniform and reproducible coating at a thickness of 200A° and examined under scanning electron microscope (SEM, JEOL JSM-6380LV at 30 kV).
Morphologic parameters
Hematoxylin-and-eosin-stained sections were setting under the optical microscope. Morphologic parameters (villous height, crypt depth, mucosal wall thickness and villous surface area) were measured by image measurement software (Image-Pro Plus 6.0).
Immunohistochemistry
Sections were dewaxed and endogenous peroxidase activity was quenched with methanol and 3% H2O2 for 15 minutes. Antigen retrieval was achieved by means of microwave treatment (two treatments for four minutes each for all pathological sections). The primary antibodies were applied overnight. After washing with Tris buffered saline (TBS), sections were incubated with a secondary rabbit antimouse antibody for 15 minutes and washed in TBS. The Kwik streptavidin peroxidase reagent was applied for 15 minutes and sections were again washed in TBS. The colour was developed by 15 minute incubation with DAB solution and the sections were weakly counterstained with hematoxylin. Negative controls were performed in all cases omitting primary antibody. Immunohistochemistry images were processed using an optical microscope and DP controller software. The mean optical density of iNOS and HIF-1α were obtained with image measurement software (Image-Pro Plus 6.0).
Statistical analysis
Data were presented as means ± SE and analyzed with the PASW Statistics for Windows release 18.0 (SPSS, Inc., Chicago, Illinois). Statistical differences between groups were analyzed using one-way analysis and the Student-Newman-Keuls’s post hoc test, as appropriate. Differences were considered to be significant at P < 0.05. The linear trends were measured by corresponding test.
Results
Optical microscope features of intestinal mucosa
Pathological changes were not found in the plain group (Figure 1A), but they did occur in the two higher altitude groups. Intestinal villus appeared as swelling, shortening, thickening, lodging, integration or even exfoliation. Lamina propria were exposed and inflammatory granulocyte could be seen (Figure 1B).
Figure 1.
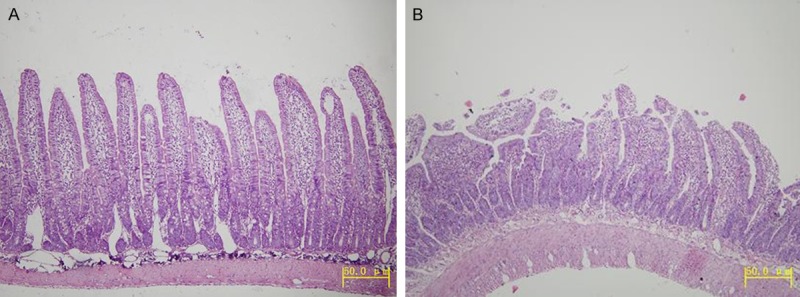
A. The small intestine villi in the plain 500 m group (HE, Original magnification ×100). B. The small intestine villi in the HA4767 m group (HE, Original magnification ×100).
SEM features of intestinal mucosa
The small intestine villi in plain group was about 150 μm × 50 μm, and its surface was smooth and integrated (Figure 2A). However, the surface in the two higher altitude groups was swelling, shortening and structural disorganization. Many epithelia showed inflammatory exudation, necrotic changes or dissolved of the epithelia, where honeycomb-like structure could be found (Figure 2B).
Figure 2.
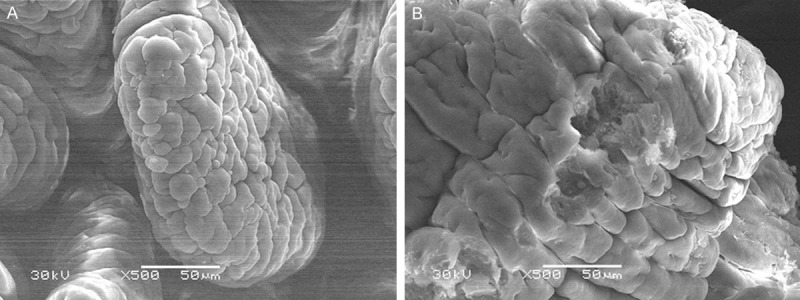
A. The intestine mucosa in the plain 500 m group (SEM, Original magnification ×500). B. The intestine mucosa in the HA4767 m group (SEM, Original magnification ×500).
There was significant injury in morphologic parameters with the increase of altitude (Table 1).
Table 1.
Small intestine morphologic parameters (µm) of the rats exposured to different high altitude
| Group | Mucosal wallthickness (µm) | Crypt depth (µm) | Villous heigh (µm) | Villous surface area (µm2) |
|---|---|---|---|---|
| Plain | 322.00 ± 12.68 | 127.70 ± 7.24 | 447.50 ± 21.93 | 0.101 ± 0.010 |
| HA3842 | 292.60 ± 9.834a | 106.80 ± 10.79a | 395.80 ± 12.40a | 0.071 ± 0.014a |
| HA4767 | 235.80 ± 19.54a,b | 76.10 ± 7.58a,b | 311.90 ± 18.38a,b | 0.047 ± 0.008a,b |
Significantly different compared to the plain 500 m group; P < 0.05;
Significantly different from the HA3842 m group; P < 0.05.
Expression of HIF-1α and iNOS
In the high altitude groups, HIF-1α mainly expressed in epithelial cells and vascular endothelial cells of intestinal tissue (Figure 3B), while rarely expressed or absent in the plain group (Figure 3A). The positive granules of HIF-1α were mainly located in cytoplasm or/and membrane diffusely.
Figure 3.
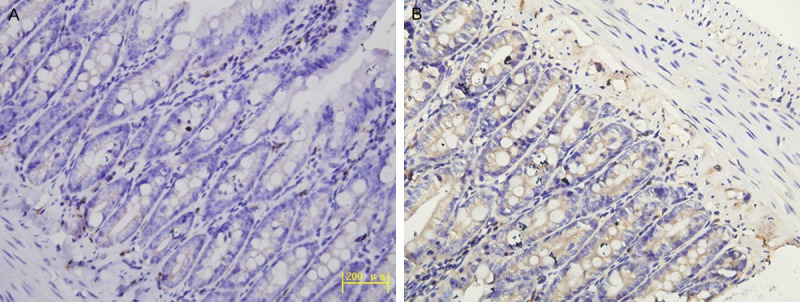
A. Expression of HIF-1α in the plain 500 m group (IHC, Original magnification ×400). B. Expression of HIF-1α in the HA4767 m group (IHC, Original magnification ×400).
The expression of iNOS mainly located in intestinal lumen, glandular epithelium and inflammatory cells (lymphocytes and macrophages). The positive presentation of iNOS in the high altitude groups was brown or brown-yellow particles in nucleus or cytoplasm (Figure 4B), While iNOS was almost negative in the plain group (Figure 4A).
Figure 4.
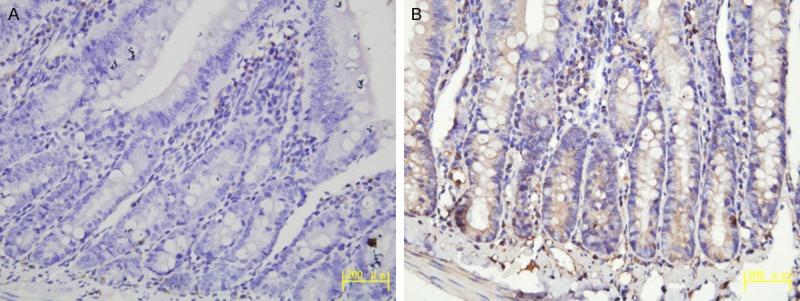
A. Expression of iNOS in the plain 500 m group (IHC, Original magnification ×400). B. Expression of iNOS in the HA4767 m group (IHC, Original magnification ×400).
There was significant increase in the mean optical density (MOD) of HIF-1α and iNOS with the altitude increasing. (r=0.912, P < 0.05) (Table 2).
Table 2.
Expression of iNOS and HIF-1α during rats exposed to different altitude
| Group | HIF-1α | iNOS |
|---|---|---|
| Plain | 0.128 ± 0.030 | 0.068 ± 0.022 |
| HA3842 | 0.312 ± 0.036a | 0.164 ± 0.040a |
| HA4767 | 0.445 ± 0.032a,b | 0.365 ± 0.022a,b |
Significantly different compared to the plain 500 m control group; P < 0.05;
Significantly different from the HA3842 m group; P < 0.05.
Discussion
People who are used to reside at or near sea level may have the possibility to develop high-altitude diseases when fleetly ascending to highland over 2500 m [11]. The majority hypobaric hypoxia related diseases that previous researchers focused on are acute mountain sickness, high-altitude pulmonary edema, and high-altitude cerebral edema. Little was known about the dysfunction or injury in digestive tract. Unfortunately, gastrointestinal problems at high altitude are very common [12]. When fleetly ascending to highland, the increase of inferior vena cava flow and decrease of blood flow into the mucosa of stomach and duodenum were found, so that aggravate ischemia injury in both organs. Our data showed that high-altitude related intestinal injury was aggravated with altitude increasing, such as the villi of ileum mucosa were swollen, shorten, broken and destroyed. But limited finding was seen in a hypoxic cell model [13].
Hypoxia activates hypoxia-inducible factor (HIF-1), a transcription factor essential in regulating hypoxia-induced gene expression [14]. Therefore, HIF-1 is a special factor of pathological response for hypoxia adoption. For example, HIF-1 is the key regulator in the formation of erythropoietin (EPO), which elevates tissue O2 concentration to counteract the injury of hypoxia. Under hypoxic conditions, O2-dependent hydroxylation of HIF-1α is decreased [15], which means the activation of HIF-1α. Numerous studies showed that hypoxia induced HIF-1α via the PI3-kinase/AKT/mTOR pathway [16,17], but the exact mechanisms are not fully understood. In our study, the expression of HIF-1α was seen in the high altitude groups, but rarely in plain group. The expression level of HIF-1α was positively correlated with altitude increasing and tissue injuring.
Consist with previous studies [18,19], our data also showed an upregulation in the expression of iNOS expression in intestinal tissue. Compared with the plain group, the levels of iNOS in the HA3842 m and the HA4767 m groups were significantly elevated in our study. Some researches [10,20] have shown that sustaining upregulation in the expression of iNOS and NO are associated with the increased nitrosative stress in the intestinal epithelium, presumably as a result of the formation of peroxynitrite. Peroxynitrite is considered as the causes of enterocyte apoptosis and increase of intestinal permeability, which results in the exposure of intestinal basement membrane and further more facilitate bacterial adherence and penetration [21,22]. Bacteria induce the formation of inflammatory mediators, amplify the local inflammatory responses and worsen the intestinal environment. These findings confirm a detrimental role for iNOS-derived NO overproduction during reperfusion. But its protective or detrimental role remains still question of debate.
In conclusion, our study firstly demonstrated that high altitude hypoxia in rats severely injure mucosa and up-regulates the expression of both iNOS and HIF-1α in intestine. Statistical results revealed that the expression of HIF-1α and iNOS are positively correlated. Other study indicated that hypoxia increased iNOS gene’s expression through inducing HIF-1 in rat’s pulmonary artery endothelial cells [23]. It means that HIF-1α and iNOS may play a critical role in the pathogenesis of hypoxic injury. We speculated that HIF-1α may regulate the expression of iNOS and be involved in the damage of intestinal mucosa. The exact relationship between HIF-1α and iNOS is uncertain. Its mechanism still need for further study.
Acknowledgements
The study was supported by the 11th Five-Year Plan Fund of the PLA (Project No. 06MA089).
Disclosure of conflict of interest
None.
References
- 1.Leissner KB, Mahmood FU. Physiology and pathophysiology at high altitude: considerations for the anesthesiologist. J Anesth. 2009;23:543–553. doi: 10.1007/s00540-009-0787-7. [DOI] [PubMed] [Google Scholar]
- 2.Dosek A, Ohno H, Acs Z, Taylor AW, Radak Z. High altitude and oxidative stress. Respir Physiol Neurobiol. 2007;158:128–131. doi: 10.1016/j.resp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BF, Clegg DJ. Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol. 2014;397:51–58. doi: 10.1016/j.mce.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Vadlapatla RK, Vadlapudi AD, Mitra AK. Hypoxia-inducible factor-1 (HIF-1): a potential target for intervention in ocular neovascular diseases. Curr Drug Targets. 2013;14:919–935. doi: 10.2174/13894501113149990015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffrey Man HS, Tsui AK, Marsden PA. Nitric oxide and hypoxia signaling. Vitam Horm. 2014;96:161–192. doi: 10.1016/B978-0-12-800254-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 6.Uusijarvi J, Eriksson K, Larsson AC, Nihlén C, Schiffer T, Lindholm P, Weitzberg E. Effects of hyperbaric oxygen on nitric oxide generation in humans. Nitric Oxide. 2015;44:88–97. doi: 10.1016/j.niox.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol. 1993;264:G143–149. doi: 10.1152/ajpgi.1993.264.1.G143. [DOI] [PubMed] [Google Scholar]
- 8.Leitao RF, Brito GA, Oria RB, Braga-Neto MB, Bellaguarda EA, Silva JV, Gomes AS, Lima-Junior RC, Siqueira FJ, Freire RS, Vale ML, Ribeiro RA. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 2011;11:90. doi: 10.1186/1471-230X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li XL, Zou XM, Nie G, Song ML, Li G. Roles of neuronal nitric oxide synthase and inducible nitric oxide synthase in intestinal transplantation of rats. Transplant Proc. 2013;45:2497–2501. doi: 10.1016/j.transproceed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Yurdakan G, Tekin IO, Comert M, Acikgoz S, Sipahi EY. The presence of oxidized low-density lipoprotein and inducible nitric oxide synthase expression in renal damage after intestinal ischemia reperfusion. Kaohsiung J Med Sci. 2012;28:16–22. doi: 10.1016/j.kjms.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Shin T. High Altitude Illnesses in Hawai’i. Hawaii J Med Public Health. 2014;73(Suppl 2):4–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Adak A, Maity C, Ghosh K, Pati BR, Mondal KC. Dynamics of predominant microbiota in the human gastrointestinal tract and change in luminal enzymes and immunoglobulin profile during high-altitude adaptation. Folia Microbiol (Praha) 2013;58:523–528. doi: 10.1007/s12223-013-0241-y. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Qiu Y, Wang W, Xiao W, Liang H, Zhang C, Yang H, Teitelbaum DH, Sun LH, Yang H. Adenosine A2B receptor modulates intestinal barrier function under hypoxic and ischemia/reperfusion conditions. Int J Clin Exp Pathol. 2014;7:2006–2018. [PMC free article] [PubMed] [Google Scholar]
- 14.Schumacker PT. Hypoxia-inducible factor-1 (HIF-1) Crit Care Med. 2005;33(Suppl 12):S423–425. doi: 10.1097/01.ccm.0000191716.38566.e0. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 16.Marhold M, Tomasich E, El-Gazzar A, Heller G, Spittler A, Horvat R, Krainer M, Horak P. HIF-1alpha Regulates mTOR Signaling and Viability of Prostate Cancer Stem Cells. Mol Cancer Res. 2015;13:556–64. doi: 10.1158/1541-7786.MCR-14-0153-T. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Jee JG, Bae JS, Liu KH, Lee YM. A Group of Novel HIF-1alpha Inhibitors, Glyceollins, Blocks HIF-1alpha Synthesis and Decreases Its Stability via Inhibition of the PI3K/AKT/mTOR Pathway and Hsp90 Binding. J Cell Physiol. 2015;230:853–862. doi: 10.1002/jcp.24813. [DOI] [PubMed] [Google Scholar]
- 18.Pietzcker J, Kluthea C, Klinghammer K, Bührer C, Rüstow B, Guthmann F. Developmental delay in hypoxia-induced HO-1 expression predisposes to gut injury. J Perinat Med. 2012;40:191–197. doi: 10.1515/jpm.2011.117. [DOI] [PubMed] [Google Scholar]
- 19.Engebretsen BJ, Irwin D, Valdez ME, O’Donovan MK, Tucker A, van Patot MT. Acute hypobaric hypoxia (5486 m) induces greater pulmonary HIF-1 activation in hilltop compared to madison rats. High Alt Med Biol. 2007;8:312–321. doi: 10.1089/ham.2007.1031. [DOI] [PubMed] [Google Scholar]
- 20.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 21.Allen RG, Lafuse WP, Galley JD, Ali MM, Ahmer BM, Bailey MT. The intestinal microbiota are necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain Behav Immun. 2012;26:371–382. doi: 10.1016/j.bbi.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chokshi NK, Guner YS, Hunter CJ, Upperman JS, Grishin A, Ford HR. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32:92–99. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JR, Zhou Y, Sang K, Li MX. [Association between pulmonary vascular remodeling and expression of hypoxia-inducible factor-1alpha, endothelin-1 and inducible nitric oxide synthase in pulmonary vessels in neonatal rats with hypoxic pulmonary hypertension] . Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:138–144. [PubMed] [Google Scholar]


