Abstract
Aim: To investigate the effect of Salvianolic acid B (Sal B) on the disease progress of NASH and change of intestinal barrier function. Methods: Sixty Sprague-Dawley (SD) rats were randomly divided into control group, model group and treated group, with the former given normal diet and the latter 2 groups rats fed high-fat diet. In treated group, rats were infused through the stomach with 1 mg/ml Sal B every day at a dose of 20 mL/kg body weight. All animals were killed at the 24th week and plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), endotoxin (ET) and diamine oxdase (DAO) were analyzed using the blood samples. The histopathology of liver was observed by H&E staining. The expression changes of tight junction protein occludin and ZO-1 were analyzed by immunocytochemistry. Ultrastructural morphology of small intestinal tissues was investigated by transmission electron microscopy. Results: Plasma levels of ALT, AST, TG, TC, ET and DAO were significantly higher in model group than those in both control group and group treated with Sal B. In model group, vacuolated swelling of the cytoplasm with aggregates of chronic inflammatory cells was observed in the liver tissue but not in Sal B-treated group. NAFLD Activity Score in the treated group was significantly lower than that in model group. Immunohistochemical staining showed that Sal B administration recovered the expression of occludin and ZO-1, which was downregulated in the model group. Transmission electron microscopy analysis demonstrated that cell surface microvilli and major intercellular junctional complex including tight junction, gap junction and adherens junction were restored in Sal B-treated group. Conclusion: Sal B exerted protective function against high-fat diet-induced liver damage by restoring healthy barrier function of intestine in NASH rat model.
Keywords: Salvianolic acid B, non-alcoholic steatohepatitis, intestinal mucosal barrier, tight junction
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic hepatic manifestation of the metabolic disease that affecting up to 95 million adults in the United States alone [1,2]. It includes a spectrum of liver pathologies ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis, hepatocellular carcinoma (HCC) and end-stage liver disease. Being the most extreme form of NAFLD, NASH can progress to cirrhosis and hepatocellular carcinoma and is potentially becoming the most common cause of advanced liver disease in coming decades [3].
In recent years many evidences have shown that gut-liver axis malfunction, which includes small intestinal bacterial overgrowth (SIBO), intestinal dysbiosis, and elevation of intestinal permeability (“leaky gut”), is another leading factor for the development of NAFLD and NASH [4,5]. The gut-liver axis refers to the intimate anatomical and functional interrelationship between the gastrointestinal tract and the liver. Imbalance of gut-liver axis may arise from changes in intestinal permeability and microbiome composition [6], which could be caused by disruption of the intestinal barrier function. Recognized as the important cause of the development of NASH, the physical intestinal barrier is supposed to be an ideal therapeutic target [7].
Salvianolic acid B (Sal B), the most abundant and bioactive member of the hydrophilic components isolated from the dried root of the well-known Chinese traditional medical agent Salvia miltiorrhiza, has shown anti-oxidative, anti-inflammatory, anti-fibrotic and anti-proliferative properties [8,9]. Salvia miltiorrhiza, recorded as a “super grade” drug in Shen-nung pen ts’ao ching, has been widely and successfully used in treating and preventing cardiovascular and cerebrovascular diseases, and cancers for thousand years [10]. Salvianolate, a highly concentrated form of Sal B, can decrease the serum endotoxin level in the portal vein and protect cirrhosis-associated intestinal mucosal damage in rats with CCl4-induced liver cirrhosis [11,12]. The current study was aimed to investigate the effect of Sal B on the disease progress of NASH and change of intestinal barrier function.
Materials and methods
Establishment of animal model
All animal work performed in this study was approved by the Animal Ethics Committee of Affiliated Zhongshan Hospital of Dalian University. 60 Sprague-Dawley (SD) rats with weight at about 150 g were purchased from the laboratory animal center of Dalian medical university. The animals were divided into 3 groups at random: Control group fed an ordinary diet, while model group and treatment group fed a high-fat diet. 2 rats of each group were executed at the 12th weekend to validate NASH. In the treatment group, rats were treated by infusing the stomach with 1 mg/ml Sal B (Sichuan Herbal plant Chemical Co., Ltd.) every day at a dose of 20 mL/kg body weight. At the 24th week the experimental period, all rats were anesthetized with 10% chloral hydrate at a dose of 2.0 ml/kg body weight and dissected. Blood samples from the portal vein, liver and small intestine tissue were obtained for further analysis. Liver index was calculated (liver weight as a percentage of body weight).
Blood testing
Plasma levels of ALT, AST, TG and TC in blood samples were analyzed on a Vitros® 5.1 FS chemistry system (Ortho-Clinical Diagnostics, Johnson & Johnson, Beerse, Belgium). Chromogenic Limulus amebocyte lysate (LAL) assay was performed to measure venous blood endotoxin (ET). Diamine oxdase (DAO) activity was determined in blood sample using spectrophotometer.
Histopathological analysis of liver
Tissue samples were taken and washed three times with cold physiological saline and fixed in 10% formalin solution. After fixation, tissue specimens were dehydrated and embedded in paraffin. Sections from each sample were stained with hematoxylin and eosin. The hepatocyte fatty change was scored as follows: 0 (< 5%), 1 (5%~33%), 2 (34%~66%) and 3 (> 66%). Intralobular inflammatory reaction was scored as follows: 0 (None), 1 (< 2), 2 (2~4), 3 (> 4). Hepatocellular ballooning was scored as follows: 0 (None), 1 (Rare), 2 (Common). Above 3 parameters constitute NAFLD activity score (NAS), and NASH can be diagnosed with NAS scoring exceeding 4.
Immunohistochemical analysis of small intestine
The small intestine tissues from each group were retrieved and fixed with 10% formalin and embedded in paraffin. For immunostaining, deparaffinized sections were incubated separately with the following primary antibodies: Occludin (1:100, Abcam, Hong Kong), ZO-1 (1:100, Boshide, Wuhan, China) followed by secondary antibody (Maixin, Fuzhou, China). Then the samples were incubated in diaminobenzidine (DAB) until a brown reaction product could be observed. The quantitative analysis was based on average optical density in a single visual field and was performed independently by two observers. 5 visual fields were randomly selected and analyzed with ImagePro Plus 6.0 software.
Transmission electron microscopy
Intestine samples were separated and fixed with 2.5% glutaric dialdehyde for 2 h and 1% osmic acid for another 2 h. Then the samples were dehydrated and embedded in resin. Ultrathin sections were cut and stained with uranyl acetate. Samples were examined using a transmission electron microscope (FEI Tecnai Spirit, 120 kV) and analyzed by an electron microscope image analyzer.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 (Chicago, IL, United States). Group comparison was performed by one-factor analysis of variance. Data were expressed as the means ± SD. P value of < 0.05 was considered statistically significant.
Results
Blood biochemistry
We compared the liver index (liver weight as a percentage of body weight), plasma levels of ALT, AST, TG, TC, ET and DAO from three groups. As illustrated in Table 1, the liver index and all of these markers were significantly higher in model group than those in both control group and group treated with Sal B.
Table 1.
Analysis of liver index and plasma markers (X̅ ± S)
| Control group | Model group | Treated group | F value | P value | |
|---|---|---|---|---|---|
| Number | 18 | 18 | 18 | ||
| Liver index (%) | 2.33 ± 0.52 | 3.91 ± 1.37 | 3.18 ± 2.01 | 37.94 | 0.002 |
| TG (mmol/L) | 0.32 ± 0.21 | 0.93 ± 0.13 | 0.40 ± 0.06 | 15.06 | 0.005 |
| TC (mmol/L) | 0.91 ± 0.03 | 1.50 ± 0.06 | 1.13 ± 0.04 | 106.71 | 0.000 |
| ALT (U/L) | 45.23 ± 2.13 | 94.14 ± 3.28 | 52.12 ± 1.57 | 60.25 | 0.001 |
| AST (U/L) | 82.53 ± 6.77 | 131.33 ± 3.13 | 85.31 ± 6.53 | 105.76 | 0.000 |
| ET (EU/mL) | 0.10 ± 0.02 | 0.57 ± 0.06 | 0.24 ± 0.03 | 135.27 | 0.000 |
| DAO (U/mL) | 0.46 ± 0.08 | 1.62 ± 0.10 | 0.90 ± 0.12 | 74.32 | 0.000 |
TG, triglycerides. TC, total cholesterol. ALT, alanine aminotransferase. AST, aspartate aminotransferase. ET, endotoxin. DAO, diamine oxidase.
Histopathological change of liver tissue
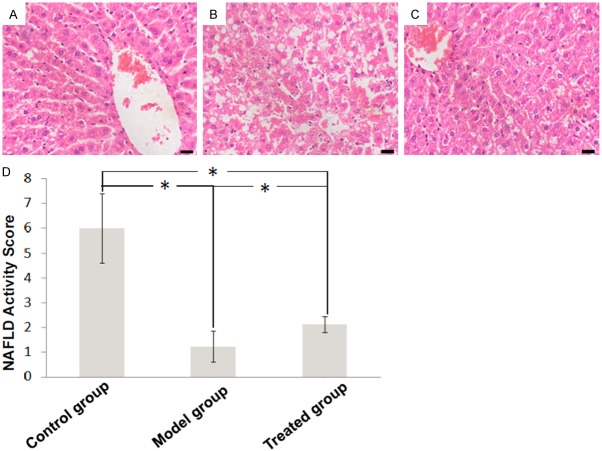
In the control group, H&E staining showed that normal liver consists of network of hepatocytes without infiltration of inflammatory cells (Figure 1A). Vacuolated swelling of the cytoplasm was observed in model group, with aggregates of chronic inflammatory cells (Figure 1B). These abnormalities were ameliorated in the treated group (Figure 1C). NAFLD Activity Score (NAS) in model group was significantly higher than that in the control group, and was lowered in the treated group (Figure 1D).
Figure 1.
Liver histopathology of (A) control group, (B) model group and (C) treated group (hematoxylin and eosin staining, × 400). (D) NAFLD Activity Score (NAS) analysis.
Immunohistochemistry of tight junction markers among intestinal epithelial cells
In untreated groups, immunoreactivity for occludin was evenly and continuously distributed at the cell borders (Figure 2A). In contrast, occludin was almost negative in the intestinal epithelial cells of model group, and moderately expressed in treated group (Figure 2B, 2C). The quantification of expression level of occludin disclosed significant differences between model group and two other groups.
Figure 2.
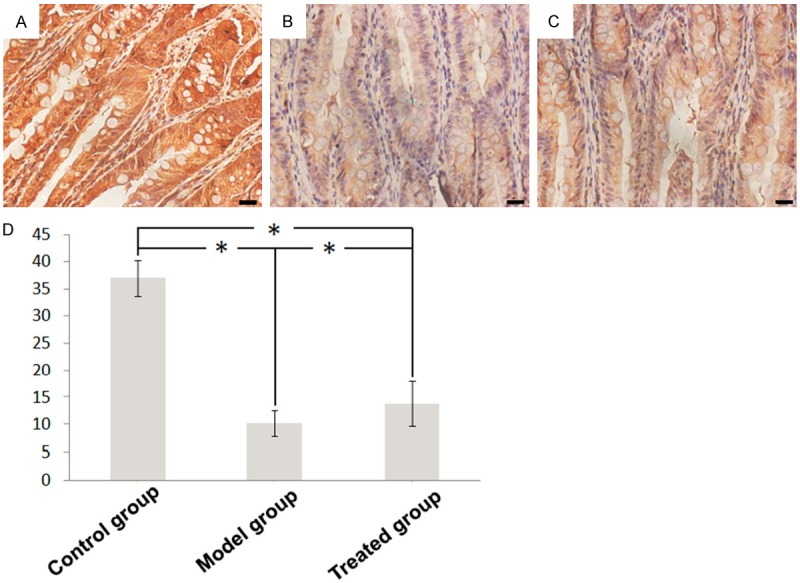
Immunohistochemical staining for occludin in intestinal tissues of (A) control group, (B) model group and (C) treated group (× 400). (D) The quantification results of expression level.
The expression of ZO-1 exhibit similar pattern among three groups. In normal condition, ZO-1 was strongly expressed at the cell borders (Figure 3A). However, in the model group ZO-1 level was downregulated (Figure 3B). After treatment, ZO-1 expression was recovered (Figure 3C, 3D).
Figure 3.
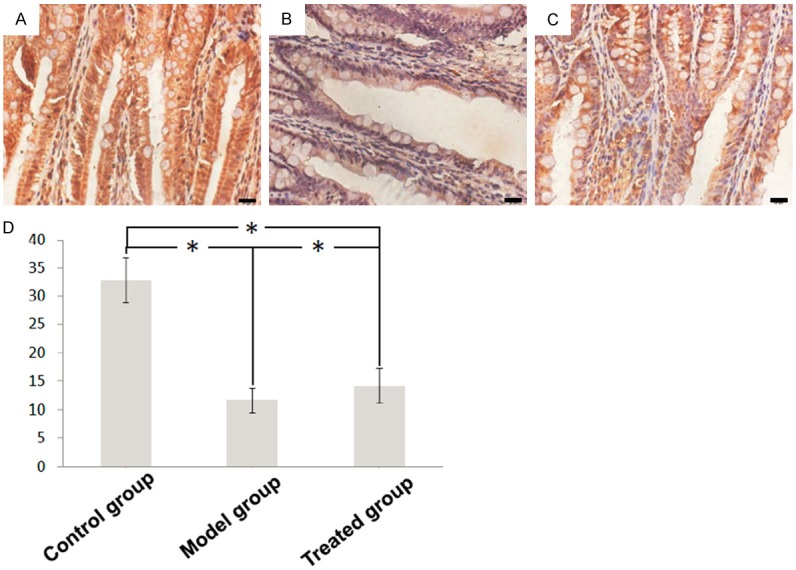
Immunohistochemical staining for ZO-1 in intestinal tissues of (A) control group, (B) model group and (C) treated group (× 400). (D) The quantification results of expression level.
Transmission electron microscopy (TEM)
We used TEM to observe the intestinal epithelial cells and their connections. The surface of intestinal epithelial cells was enriched with microvilli. Cell-to-cell junction was visible at the luminal side of cell-cell contact, which contained long tight junctions (TJ), small gap junctions (GJ), distinct adherens junctions (AJ) and desmosomes (Figure 4A). In the model group, cell surface microvilli displayed sparse and deformed morphology, shortened TJ, widened GJ with no AJ and desmosome in the area (Figure 4B). In the treated group, cell surface microvilli recovered to nearly normal state, with visible TJ, GJ, AJ but no desmosome (Figure 4C).
Figure 4.
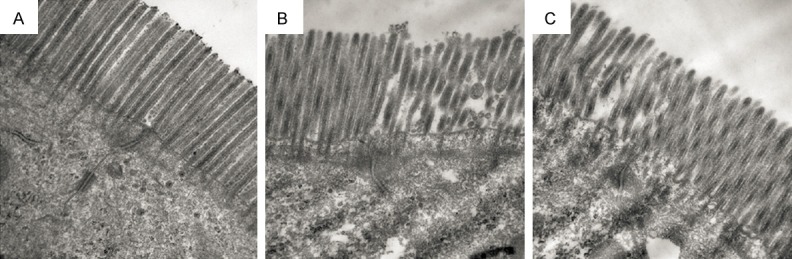
Transmission electron microscopy (TEM) analysis of intestinal tissues of (A) control group, (B) model group and (C) treated group (× 40000).
Discussion
The current study is to our knowledge the first to prove that Sal B can decrease the plasma level of markers representative of liver dysfunction and ameliorate the intestinal mucosal injury through restoring inter-cell junction structures in NASH model rats.
The gut and the liver are closely intercorrelated through frequent bidirectional communication mediated by the hormones, inflammatory mediators, bile acids and digestion products. In the present study, the plasma level of not only markers for liver function as ALT, AST, TG, TC, but also markers for intestinal mucosal barrier function as endotoxin and DAO were significantly higher in model group than in control group. This suggested that intestinal mucosal barrier have direct or indirect effects on liver physiology and disease progression. During the development of NASH, disrupted intestinal mucosal barrier can lead to invasion of enteric bacteria into blood, which could contribute to the liver pathogenesis by stimulating gut luminal ethanol production, consuming dietary choline and producing LPS, which may enhance proinflammatory cytokine production in luminal epithelial cells and liver macrophages [13]. The notion that pathologic changes of the intestinal barrier could trigger bacterial translocation across the gut mucosa was also demonstrated in human experiment, in which increased gut permeability was more distinct in NAFLD patients than in healthy subjects [14].
In present study, Sal B was shown to possess the ability to improve the liver histopathological changes in NASH rats, suggesting that Sal B may exert action through protecting the mucosal barrier integrity. Intestinal mucosal barriers are mainly comprised of mechanical, chemical, immunologic barrier and biology barrier [15,16]. As the essential component of the intestinal barrier, intercellular junctional complex including tight junctions (TJ), gap junctions (GJ), adherens junctions (AJ) and desmosomes is crucial for the maintenance of barrier integrity. They regulate the barrier function of the epithelium by controlling the entry of nutrients, ions, and water while restricting pathogen access to underlying tissue compartments. As shown in current results, Sal B treatment rescued the intestinal expression of occludin, the important integral membrane TJ protein regulating the gate and fence functions [17]. Disruption of occludin was demonstrated to account for barrier dysfunction in intestinal inflammation [18]. The intestinal expression of another membrane TJ protein, ZO-1, which directly binds the cytoplasmic tails of occludin and forms the scaffolding to interact with the actin cytoskeleton [19], was also shown to be elevated under Sal B treatment. As the most important plaque protein to regulate TJ, ZO-1 can also bind directly to F-actin and cause reorganization of the cytoskeleton [20].
The increased expression level of the occludin and ZO-1 of treated group compared to those in model group appeared to be in consistence with increased intensity of intercellular junctional complex proteins observed by transmission electron microscopy. These results well supported the suggestion that Sal B could promote the recovery of intestinal barrier level by upregulating the expression and function of junction proteins. With a phenolic hydroxyl group in its molecular structure, Sal B exhibited ability to serve as strong scavengers for free radical, which could underlie its anti-apoptotic effects [21]. It has been demonstrated that brain and heart can be protected by Sal B from ischemia-reperfusion injury by reducing the lipid peroxidation and superoxide anion production [22,23]. Other studies indicated that Sal B might block platelet aggregation induced by high shear stress [24], attenuates inflammatory and atherosclerotic factors [25], prevent low-density lipoprotein from oxidation, and prevent tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation via suppression of NAD (P) H oxidase-derived reactive oxygen species [26]. These progresses suggest that multifaceted function of Sal B could support its beneficial effect on the recovery of intestinal barrier. More recently and relevantly, Sal B was found to antagonize the downregulatory effect of tight junction-associated proteins occludin and claudin-5 by VEGF and decrease the permeability of the rabbit aortary endothelial cells [27]. In spinal cord injury scenario, blood-spinal cord barrier permeability of rat was significantly reduced by administration of Sal B [28]. Although others’ and our data indicated the direct role of Sal B on the intestinal mucosal barrier function, other possible pathway could not be excluded. Theoretically, the anti-inflammatory function of Sal B can antagonize the negative effect of inflammatory factors on the expression of tight junction proteins [29].
Taken together, Sal B can protect high-fat diet-induced liver deterioration by restoring healthy barrier function of intestine in NASH rat model. These beneficial effects on NASH needed to be corroborated in clinical settings, and further investigation into the underlying mechanisms could offer more insight for their application in disease treatment.
Disclosure of conflict of interest
None.
References
- 1.Mouzaki M, Allard J. Non-alcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Ann Gastroenterol. 2012;25:207–217. [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314–5324. [PubMed] [Google Scholar]
- 5.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461–468. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehal WZ. The gut-liver axis: a busy two-way street. Hepatology. 2012;55:1647–1649. doi: 10.1002/hep.25704. [DOI] [PubMed] [Google Scholar]
- 7.Căruntu FA, Benea L. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. J Gastrointestin Liver Dis. 2006;15:51–56. [PubMed] [Google Scholar]
- 8.Chen YL, Hu CS, Lin FY, Chen YH, Sheu LM, Ku HH, Shiao MS, Chen JW, Lin SJ. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem. 2006;98:618–631. doi: 10.1002/jcb.20793. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HS, Wang SQ. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol. 2006;41:138–148. doi: 10.1016/j.yjmcc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 11.Yang DH, Ye ZY, Jin B, He XJ, Zhang Q, Zhou WM, Xu WJ, Lu HX. Salvianolate inhibits cytokine gene expression in small intestine of cirrhotic rats. World J Gastroenterol. 2011;17:1903–1909. doi: 10.3748/wjg.v17.i14.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang DH, Ye ZY, Xie YJ, He XJ, Xu WJ, Zhou WM. Effect of salvianolate on intestinal epithelium tight junction protein zonula occludens protein 1 in cirrhotic rats. World J Gastroenterol. 2012;18:7040–7047. doi: 10.3748/wjg.v18.i47.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miele L, Beale G, Patman G, Nobili V, Leathart J, Grieco A, Abate M, Friedman SL, Narla G, Bugianesi E, Day CP, Reeves HL. The Kruppel-like factor 6 genotype is associated with fibrosis in nonalcoholic fatty liver disease. Gastroenterology. 2008;135:282–291. doi: 10.1053/j.gastro.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi T, Brand S, Reinecker HC. Mucosal barrier and immune mediators. Curr Opin Gastroenterol. 2001;17:573–577. doi: 10.1097/00001574-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–694. doi: 10.1097/00075197-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 20.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 21.Liu CS, Cheng Y, Hu JF, Zhang W, Chen NH, Zhang JT. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb 761) Acta Pharmacol Sin. 2006;27:1137–1145. doi: 10.1111/j.1745-7254.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Du GH, Zhang JT. Salvianolic acid B protects brain against injuries caused by ischemia-reperfusion in rats. Acta Pharmacol Sin. 2000;21:463–466. [PubMed] [Google Scholar]
- 23.Kong R, Gao Y, Sun B, Chen H, Wang G, Wang X, Zhu H, Pan S, Xue D, Jiang H. The strategy of combined ischemia preconditioning and salvianolic acid-B pretreatment to prevent hepatic ischemia-reperfusion injury in rats. Dig Dis Sci. 2009;54:2568–2576. doi: 10.1007/s10620-008-0681-4. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Zhao C, Wong RN, Goto S, Wang Z, Liao F. Inhibition of shear-induced platelet aggregation in rat by tetramethylpyrazine and salvianolic acid B. Clin Hemorheol Microcirc. 2004;31:97–103. [PubMed] [Google Scholar]
- 25.Chen YL, Hu CS, Lin FY, Chen YH, Sheu LM, Ku HH, Shiao MS, Chen JW, Lin SJ. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem. 2006;98:618–631. doi: 10.1002/jcb.20793. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HS, Wang SQ. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol. 2006;41:138–148. doi: 10.1016/j.yjmcc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Ba J, Peng H, Chen Y, Gao Y. Effects and mechanism analysis of vascular endothelial growth factor and salvianolic acid B on 125I-low density lipoprotein permeability of the rabbit aortary endothelial cells. Cell Biochem Biophys. 2014;70:1533–1538. doi: 10.1007/s12013-014-0089-z. [DOI] [PubMed] [Google Scholar]
- 28.Fan ZK, Lv G, Wang YF, Li G, Yu DS, Wang YS, Zhang YQ, Mei XF, Cao Y. The protective effect of salvianolic acid B on blood-spinal cord barrier after compression spinal cord injury in rats. J Mol Neurosci. 2013;51:986–993. doi: 10.1007/s12031-013-0083-8. [DOI] [PubMed] [Google Scholar]
- 29.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]