Abstract
The antibacterial activity and mechanism of berberine against Streptococcus agalactiae were investigated in this study by analyzing the growth, morphology and protein of the S. agalactiae cells treated with berberine. The antibacterial susceptibility test result indicated minimum inhibition concentration (MIC) of berberine against Streptococcus agalactiae was 78 μg/mL and the time-kill curves showed the correlation of concentration-time. After the bacteria was exposed to 78 μg/mL berberine, the fragmentary cell membrane and cells unequal division were observed by the transmission electron microscopy (TEM), indicating the bacterial cells were severely damaged. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) study demonstrated that berberine could damage bacterial cells through destroying cellular proteins. Meanwhile, Fluorescence microscope revealed that berberine could affect the synthesis of DNA. In conclusion, these results strongly suggested that berberine may damage the structure of bacterial cell membrane and inhibit synthesis of protein and DNA, which cause Streptococcus agalactiae bacteria to die eventually.
Keywords: Berberine, Streptococcus agalactiae, antibacterial activity, SDS-PAGE, TEM
Introduction
Streptococcus agalactiae (S. agalactiae), known as group B Streptococcus (GBS), can infect terrestrial mammals [1,2], S. agalactiae are also the predominant cause of invasive bacterial disease, which can cause septicaemia, meningitis, and pneumonia in neonates. Besides, it can lead to mortality or morbidity in non-pregnant adults, particularly in elderly persons and those with underlying diseases [3-5].
However, in recent years, the increased indiscriminate use of commercial antimicrobial drugs leads to the development of antibiotic resistance in pathogenic bacteria [6]. So it is in great need developing effective antibacterial agents with high efficacy and low toxicity to combat this problem [7-9]. Otherwise, the herbs have a strong antibacterial activity against pathogenic bacteria. It is reported that Coptis has a strong antibacterial activity in vitro against S. agalactiae [10].
Therefore, drugs that can either inhibit the growth of pathogenic bacteria or kill them without damaging host cells are considered as the first candidates. In recent years, berberine, as a broad-spectrum anti-microbial agent has attracted more and more interests [11,12]. Berberine is an isoquinoline derivative alkaloid isolated from Cortex phellodendri and Rhizoma coptidis [13]. In Chinese pharmacopoeia, Cortex phellodendri and Rhizoma coptidis have the ‘heating-removing’ effect on their fever to reduce therapeutic application [14]. Berberine has anti-inflammatory [15,16], antimicrobial [17,18], and antiviral [19] effects. Berberine also has good antibacterial effect on S. agalactiae. Previous reports mainly focused on the effects of berberine on Escherichia coli, few studies tried to investigate antibacterial activity and mechanism of berberine on S. agalactiae, or to continue in-depth exploration.
To evaluate the antibacterial activity of berberine against S. agalactiae and elucidate its mechanism, we studied the inhibitory effect of berberine on bacterial growth, membranous structure and synthesis of protein and DNA.
Materials and methods
Microbial strain and chemicals
Streptococcus agalactiae (CVCC 1886 strain, obtained from the Microbiological Lab of Sichuan Agricultural University, Ya’an, China) was cultivated on trypticase soy agar (TSA) which contained 0.5% calf serum (GIBCO). Inoculum were incubated for 24 h at 37°C in trypticase soy broth (TSB) which contained 0.5% calf serum, then diluting with TSB to approximately achieve the concentration of 1×108 CFU/mL. Berberine hydrochloride was obtained from China Control Institute of veterinary bio-products and pharmaceuticals, Beijing. The berberine was dissolved in 6.25% DMSO.
Antibacterial susceptibility test
Minimum inhibition concentration (MIC) value of S. agalactiae was determined by broth dilution method described in the National Committee for Clinical Laboratory Standards [20]. The berberine was added into TSB to achieve concentrations ranging from 5 mg/mL to 0.078 mg/mL. Then, the bacterial inocula were added into 10 mL tube containing 2 mL TSB (containing different concentrations of berberine) as the medium to approximately achieve an initial inoculum of 1×107 CFU/mL. 6.25% DMSO was used as negative control. The OD600 values of each tube were measured by UV spectrophotometer before incubation, then after incubation at 37°C for 24 h. The OD600 values of each tube were measured again. The test tube with the same OD600 value after 24 h, showing that there were no S. agalactiae to grow and that is the value of MIC.
Time-kill curve study
The berberine was added into TSB to achieve concentrations ranging from 4MIC to MIC. Then, inocula were added into 10 ml tube containing 2 ml TSB (containing different concentrations of berberine) as the medium to approximately achieve an initial inoculum of 1×107 CFU/mL. All samples were maintained at 37°C. After cultivating 0, 2, 4, 8, 12, 24 and 36 h , 0.1 ml was removed from each tube for colony counting. At least, two replications were performed for each sample.
Observation of the action of berberine on the membrane structure of S. agalactiae
Different volume of TSB medium, berberine solutions, and S. agalactiae were added to 10 ml cultures to achieve final MIC concentration of berberine and 108 cfu/ml S. agalactiae. Control experiment was conducted without berberine. The cultures were incubated at 37°C with shaking at 150 rpm for 4 h and 8 h. The S. agalactiae suspensions were centrifuged in sterile plastic centrifuge tubes at 8000 g for 15 min at 4°C and then were washed with saline for three times. Then the supernatant was discarded and the pellet was fixed with 2.5% glutaraldehyde in phosphate buffer (pH 7.2) overnight at 4°C. After the cells were dehydrated, embedded and stained, they were observed by TEM [21,22].
SDS-PAGE assay
108 CFU/mL S. agalactiae grew on TSB medium containing MIC concentration of berberine. Control experiment was conducted in absence of berberine. After the cultures were incubated at 37°C with shaking at 150 rpm for 2 h, 4 h, 8 h and 12 h, the samples were centrifuged for 10 min at 6,000 g. The supernatant was discarded. Then 150 μL ddH2O and 50 μL DTT were added to the pellet. Samples were boiled for 10 min and then 10 μL of each sample was loaded on the gel. Electrophoresis was performed at 80 V through the stacking gel (5%), and at 120 V through the separation gel (12%).
Detection of the effect of berbine on fluorescence intensity of S. agalactiae DNA
108 cfu/mL S. agalactiae were added to TSB containing MIC concentration of berberine. Control experiment was conducted in absence of berberine. The cultures were incubated at 37°C with shaking at 150 rpm for 12 h. After 1 μg/mL DAPI and 1 mL supernatant respectively were mixed in the dark for 1 h, a drop of the mixture was put on the glass slide and then directly observed under fluorescence microscope.
Results
Antibacterial activity of berberine
The MIC value of berberine against S. agalactiae was 0.78 μg/mL.
Time-kill curve of berberine against S. agalactiae
Time-kill curves of berberine (Figure 1) showed that the growth curves of S. agalactiae without berberine included four phases: lag phase, exponential phase, stationary phase and death phase. Treated with 0.5× MIC of berberine, S. agalactiae had the integral growth cycle except for the decline phase in the first two hours. But treated with 1× MIC and 2× MIC of berberine, S. agalactiae directly experienced decline phase without adjustment phase, logarithmic phase and stable phase. All the bacterial cells of S. agalactiae were killed by berberine at 1× MIC within 8 h and 2× MIC within 4 h.
Figure 1.
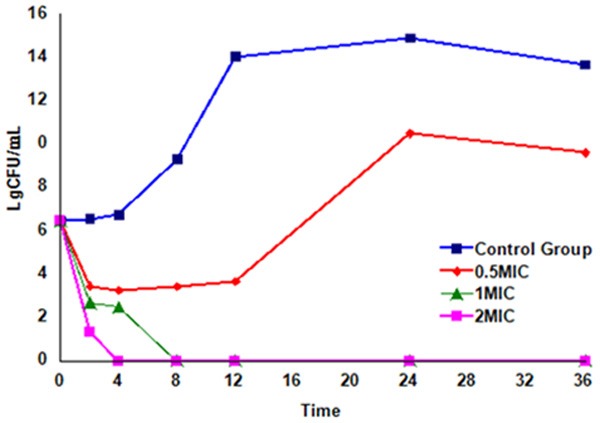
Time-kill curves of berberine against S. agalactiae.
Action of berberine on the structures of S. agalactiae cells
It shows typical structure of normal S. agalactiae cells, which are shaped cells with intact cell walls, smooth membranes, a uniformly distributed cytoplasm and clear nuclear area in the middle of cells. Besides, cells stained evenly (Figure 2A, 2B).
Figure 2.
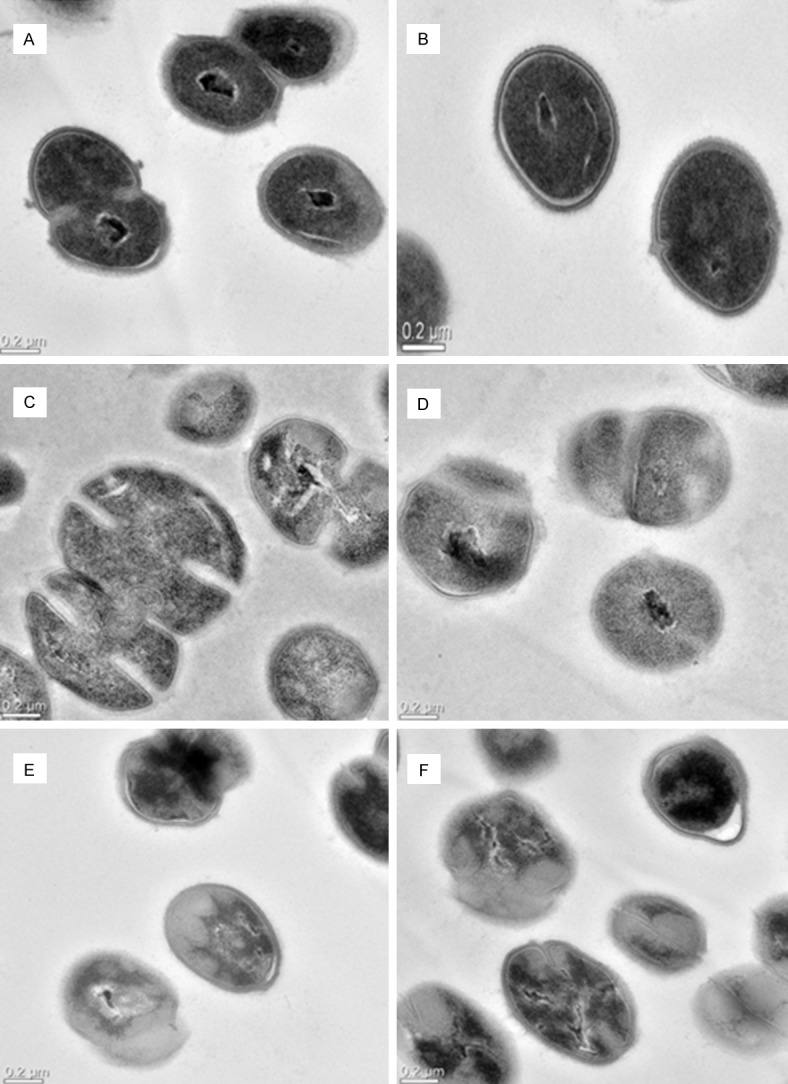
TEM diagrams of S. agalactiae cells treated and untreated with berberine at 0.2 μm scale. A and B are untreated S. agalactiae cells. C and D are treated cells with berberines at concentrations 1× MIC for 4 h. E and F are treated cells with berberine at concentrations 1× MIC for 8 h.
The S. agalactiae cells treated with berberine at 1× MIC for 4 h and 8 h were very different from those untreated cells. After 4 h incubation with berberine, some cell walls and membranes were dissolved and the shape of cells became irregular; cells unequal division could be seen (Figure 2C, 2D). Besides, some cells stained slightly and nuclear areas were on the edge of cells (Figure 2C).
After treatment for 8 h, cells were seriously damaged (Figure 2E, 2F); there was loss of cell integrity and the cytoplasmic contents were leaking out of the cells; the shape of cells became more irregular (Figure 2E, 2F). Besides, some cells stained unevenly and nuclear areas were straggling in the cells (Figure 2E, 2F).
Protein analysis of S. agalactiae cells treated with berberines
SDS-PAGE profiles of proteins from treated and untreated S. agalactiae cells are shown in Figure 3. Lane1, 6 were the Marker and control. Lane 2-5 were protein patterns of S. agalactiae treated with berberine for 2 h, 4 h, 8 h and 12 h, respectively. The protein profiles of bacteria treated with berberine differed from those of the control. The protein profiles of bacteria treated with berberine for different times were also different. Protein bands observed for untreated S. agalactiae were more than the treated cells. There were less kinds and amount of bands between 66.4 KDa and 29 KDa than control. Protein bands of lane 2 were almost the same as lane 3. The change of protein bands (approximately 66.4 kDa) in lane 2-5 was apparent. The more time the bacteria were treated, the lower the intensities of the protein bands were observed.
Figure 3.
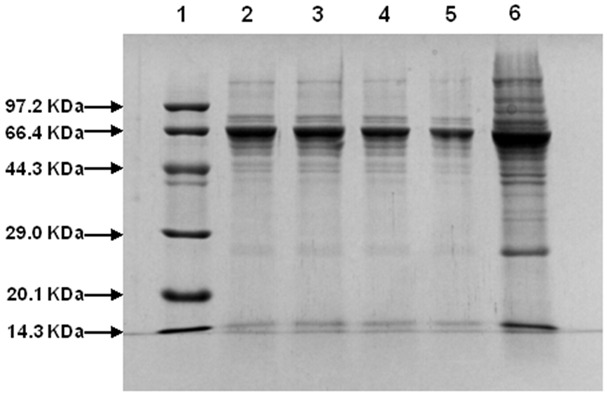
SDS-PAGE whole protein profiles from bacteria treated and untreated with berberines. Lanes 1 and 6 are marker and untreated cells of S. agalactiae, respectively. Lanes 2-5 are treated cells with berberines at concentrations 1× MIC for 2 h, 4 h, 8 h and 12 h, respectively.
Effect of berberine on fluorescence intensity of S. agalactiae DNA
It showed the fluorescence intensity of DNA of untreated and treated S. agalactiae (from Figure 4). The fluorescence intensity of treated S. agalactiae DNA were weaker than untreated S. agalactiae DNA.
Figure 4.
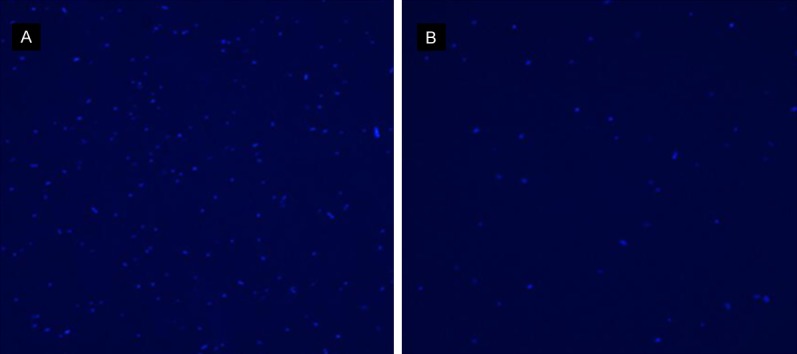
The fluorescence intensity of Streptococcus agalactiae DNA. A is S. agalactiae DNA untreated with berberine. B is S. agalactiae DNA treated with berberine.
Discussion
In this study, the growth curves of S. agalactiae exposure to berberine indicated that berberine could inhibit the growth and reproduction of S. agalactiae (Figure 1). A minor concentration (39 μg/mL) of berberine could prolong the lag phase of S. agalactiae. When the concentration of berberines was up to 78 μg/mL, 106 CFU/mL S. agalactiae was completely inhibited within 8 h. When the concentration of berberine was 2MIC (156 μg/ml), all bacteria were completely inhibited in 4 h. It is suggested that high concentration of berberine could kill the bacteria more quickly. Other study has shown the berberine against E.coli at 0.582 mg/mL and against Staphylococcus aureus at 0.952 mg/mL would cause 50% decrease of the bacterial growth rate constant [23].
To understand the antibacterial mechanism, we observed the ultrastructure of S. agalactiae through the TEM. The TEM results showed that micro-morphology of the treated S. agalactiae has changed and the out membrane has diffused compared to the untreated cells. The out membrane plays an important role in maintaining the morphology and protecting the cell. Normal metabolism and growth of bacteria could be affected by broken cell membrane and wall [24,25]. It is reported that some drugs, such as Heartleaf Houttuynia Herb, Lonicera japonica Thunb and so on, inhibit the growth of bacteria by damaging the structure of bacteria [26]. After treatment, cell membrane and walls were damaged seriously, this could lead to the increasing permeability of membrane and reduce some protein materials in cells. These results suggested that membrane of bacteria would be served as an important action site for drugs. But it is still a mystery where the damage takes place.
Additionally, the study showed that berberine had the effect on some proteins of S. agalactiae measured by SDS-PAGE which is a powerful tool to dissociate proteins into individual chains and separate them according to their molecular weight [27,28]. SDS-PAGE is therefore an ideal technique to use for demonstrating antimicrobial effectivity and has previously been used to study resistance mechanisms in bacteria [29]. Cloete and his co-workers [30] observed the disappearance of protein bands after exposure of Pseudomonas aeruginosa to halide anolyte. Zinkevich and his co-workers [31] also found the disappearance of protein bands after exposing E. coli to an anolyte solution with an ORP of 1000 mV. The SDS-PAGE results showed some protein bands of treated bacteria became low and even disappeared, suggesting that berberine could cause bacterial death by completely destroying proteins or partially degrading proteins.
Moreover, berberine could also inhibit DNA synthesis. He and his co-workers [32] found that a new type of polysaccharide from Streptomyces can inhibit plasmid DNA synthesis of bacteria. The mechanism of many antibacterial and antitumor drugs has relationship with DNA topoisomerase [33,34]. Our experiment results suggested that berberine might inhibit DNA synthesis by affecting the activity of DNA topoisomerase.
In conclusion, berberine had antibacterial activities against S. agalactiae by damaging the membrane and inhibiting synthesis of protein and DNA. Nevertheless, the further mechanism of interaction of berberine with S. agalactiae still need to be explored in future research.
Acknowledgements
This study was supported by the International cooperation projects of Sichuan Province (2014HH0058, 2013HH0042), the Sichuan Youth Science and Technology Innovation Research Team for waterfowl disease prevention and control (2013TD0015) and the National Natural Science Foundation of China (Grant No. 31372477).
Disclosure of conflict of interest
None.
References
- 1.Brochet M, Couve E, Zouine M, Vallaeys T, Rusniok C, Lamy MC, Buchrieser C, Trieu-Cuot P, Kunst F, Poyart C, Glaser P. Genomic diversity and evolution within the species Streptococcus agalactiae. Microbes Infect. 2006;8:1227–1243. doi: 10.1016/j.micinf.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen UB, Poulsen K, Ghezzo C, Margarit I, Kilian M. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. MBio. 2010;1:1–9. doi: 10.1128/mBio.00178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuchat A. Epidemiology of group streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nizet V, Rubens CE. Pathogenic mechanisms and virulence factors of group B streptococci. In: Nizet V, Rubens CE, editors. Gram-positive pathogens. Washington: DC; 2000. pp. 125–136. [Google Scholar]
- 5.Farley MM. Group B streptococcal disease in non-pregnant adults. Clin Infect Dis. 2001;33:556–561. doi: 10.1086/322696. [DOI] [PubMed] [Google Scholar]
- 6.Zewdu E, Cornelius P. Antimicrobial resistance pattern of Salmonella serotypes isolated from food items and personnel in Addis Ababa, Ethiopia. Trop Anim Health Prod. 2009;41:241–249. doi: 10.1007/s11250-008-9181-y. [DOI] [PubMed] [Google Scholar]
- 7.Haydon DJ, Stokes NR, Ure R, Galbraith G, Bennett JM, Brown DR Baker PJ, Barynin VV, Rice DW, Sedelnikova SE, Heal JR Sheridan JM, Aiwale ST, Chauhan PK, Srivastava A, Taneja A, Collins I, Errington J, Czaplewski LG. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science. 2008;321:1673–1675. doi: 10.1126/science.1159961. [DOI] [PubMed] [Google Scholar]
- 8.Sommer MOA, Dantas G, Church GM. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009;325:1128–1131. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlitni S, Brown ED. Drug discovery: Not as fab as we thought. Nature. 2009;458:39–40. doi: 10.1038/458039a. [DOI] [PubMed] [Google Scholar]
- 10.Peng LC, Yin ZQ, Jia RY, Li L, Dai RY, Qu J, Liu MH, Chen P. Effects of twenty traditional Chinese medicine extracts against Streptococcus agalactiae in vitro. Journal of South China Agricul. 2014;35:22–25. [Google Scholar]
- 11.Yang Y, Ye XL, Li XG, Zhen LS. Anti-microbial effect of four alkaloids from Coptidis rhizoma . Med Mater Med Res. 2007;18:3013–3014. [Google Scholar]
- 12.Braissant O, Wirz D, Göpfert B, Daniels AU. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol Lett. 2010;303:1–8. doi: 10.1111/j.1574-6968.2009.01819.x. [DOI] [PubMed] [Google Scholar]
- 13.Ikram M. A review on the chemical and pharmacological aspect of genus Berberis. Planta Med. 1975;28:353–358. doi: 10.1055/s-0028-1097869. [DOI] [PubMed] [Google Scholar]
- 14.Huang KC, Williams WM. The pharmacology of Chinese Herbs. New York. NY: CRC Press; 1999. Antibacterial, antiviral, and antifungal herbs; pp. 381–383. [Google Scholar]
- 15.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expressing of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006;38:599–605. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- 17.Yi ZB, Yan Y, Liang YZ, Bao Zeng. Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J Pharm Biomed Anal. 2007;44:301–304. doi: 10.1016/j.jpba.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Yan D, Jin C, Xiao XH, Dong XP. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. J Biochem Biophys Methods. 2008;70:845–849. doi: 10.1016/j.jbbm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17:1562–1564. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, PA. Ninth International Supplement. 2008:M100–S9. [Google Scholar]
- 21.Liu YF, Luan C, Xia X, An S, Wang Y. Antibacterial Activity, Cytotoxicity and Mechanisms of action of Cathelicidin Peptides against Enteric Pathogens in Weaning Piglets. Int J Pept Res Ther. 2011;17:175–184. [Google Scholar]
- 22.Tao C, Wei Q, Yin ZQ. Antifungal activity of the essential oil from Cinnamomum longepaniculatum leaves against three species of fungi. Chin Vet Sci. 2011;41:89–93. [Google Scholar]
- 23.Kong WJ, Xing XY, Xiao XH, Zhao YL, Wei JH, Wang JB, Yang RC, Yang MH. Effect of berberine on Escherichia coli, Bacillus subtilis, and their mixtures as determined by isothermal microcalorimetry. Appl Microbiol Biotechnol. 2012;96:503–510. doi: 10.1007/s00253-012-4302-y. [DOI] [PubMed] [Google Scholar]
- 24.Rasooli I, Rezaei MB, Allameh A. Growth inhibition and morphological alterations of Aspergillus niger by essential oils from Thymus eriocalyx and Thymus x-porlock. Food Control. 2006;17:359–364. [Google Scholar]
- 25.Sangetha S, Zuraini Z, Suryani S, Sasidharan S. In situ TEM and SEM studies on the antimicrobial activity and prevention of Candida albicans biofilm by Cassia spectabilis extract. Micron. 2009;40:439–443. doi: 10.1016/j.micron.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Wu GJ. The mechanism of the antibacterial medicine. Chin J Vet Med. 2007;43:42–43. [Google Scholar]
- 27.Walker JM. In: The protein protocols handbook. Walker JM, editor. Springer; 1996. [Google Scholar]
- 28.Jason JC, Ryden L. In: Protein Purfication: Principles, High Resolution Methods, and Applications. John Wiley Sons., editor. New York: 1998. pp. 463–493. [Google Scholar]
- 29.Brozel VS, Cloete TE. Bacterial resistance to conventional water treatment biocides. Biodeterior Abstracts. 1993;7:387–393. [Google Scholar]
- 30.Cloete TE, Thantsha MS, Maluleke MR, Kirkpatrick R. The anti-microbial mechanism of electrochemically activated water against Pseudomonas aeruginosa and Escherichia coli as determined by SDS-PAGE analysis. J Appl Microbiol. 2009;107:379–384. doi: 10.1111/j.1365-2672.2009.04233.x. [DOI] [PubMed] [Google Scholar]
- 31.Zinkevich V, Beech IB, Tapper R, Bogdarina I. The effect of super-oxidized water on Escherichia coli. J Hosp Infect. 2000;46:153–156. doi: 10.1053/jhin.2000.0819. [DOI] [PubMed] [Google Scholar]
- 32.He F, Yang Y, Yang G, Yu LJ. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control. 2010;21:1257–1262. [Google Scholar]
- 33.Yunman L, Haojie Z, Guoqing L. Shikonin Inhibiting the catalytic activity of DNA Topoisomerase I and inducing the apoptosis of K562 leukemia cells. Chin J Nat Med. 2003;1:165–167. [Google Scholar]
- 34.Yang F, Chen Y, Duan W, Zhang C, Zhu H, Ding J. SH-7, a new synthesized shikonin de-rivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int J Cancer. 2006;119:1184–1193. doi: 10.1002/ijc.21943. [DOI] [PubMed] [Google Scholar]


