Abstract
Background: Gastric carcinoma is one of the most aggressive malignancies with an extremely poor prognosis. Recent findings suggest decreasing PHLDA1 (pleckstrin-homologylike domain family A, member1) expression plays a significant role in inhibiting cell migration and tumor invasion. The clinicopathological significance of the expression of PHLDA1 in gastric carcinoma remains to be determined. Methods: PHLDA1 protein was investigated by immunohistochemistry for the expression status in 336 cases of gastric adenocarcinomas and 60 normal mucosa, and then the results were analyzed with the patient’s age, sex, tumor site, size and the histological grade, clinical stage as well as overall median survival time. Results: The expression of PHLDA1 protein was obviously decreased in 57.1% of gastric carcinomas. Carcinomas with loss of expression of PHLDA1 were significantly corresponding to with tumor size (P=0.037), grade (P=0.028), depth of invasion (P=0.001), lymph node metastasis (P=0.008) and stage (P=0.001) but not with age (P=0.194), sex (P=0.312), tumor site (P=0.287) and distal metastasis (P=0.331) respectively. Follow-up data showed that there was a significant difference in overall median survival time between the carcinomas with PHLDA1 negative expression (31.0 months) and those with positive expression (54.0 months) (P=0.001). Conclusions: Our findings suggest that the decreased expression of PHLDA1 may play an important role in tumor progression, and may become a new adjunct biomarker in the prognosis in gastric carcinoma. A potential role for PHLDA1 in the early detection/or therapy of gastric cancer warrants further investigation.
Keywords: Gastric carcinoma, PHLDA1, immunohistochemistry, prognosis
Introduction
Pleckstrin homology-like domain family A member 1 (PHLDA1) gene encodes a 401-amino acid protein that comprises a central pleckstrin homology domain common to proteins involved in intracellular signaling or as constituents of the cytoskeleton [1-3], a central polyglutamine tract, and 2C-terminal regions rich in proline-glutamine and proline-histidine repeats. The gene is expressed in a wide range of normal and cancer tissues [4,5]. PHLDA1 function varies with cell type and context, with several studies reporting a proapoptotic [6-9] or antiproliferative role [10]. PHLDA1 expression is induced by external stresses such as heat shock [8,9], and may be modulated by insulin-like growth factor I (IGF-I) [7] and extracellular-regulated kinase (ERK) pathways [9]. The expression of PHLDA1 protein was characterized and compared with other proposed markers of intestinal stem cells in the human small and large intestine. Sakthianandeswaren et al. [11] found the gene has been shown to be coexpressed with leucine-rich repeat containing G-protein coupled receptor 5 (Lgr5), the previously reported intestinal epithelial stem cells (ISC) in murine crypt base cells and further determined that the PHLDA1 expression mark the putative epithelial stem cells and contributes to intestinal tumorigenesis. PHLDA1 is overexpressed in human intestinal tumors of all stages and may play a role in cell migration, initially suggested by the increased staining and nuclear relocalization of the protein at the invasive front of intestinal carcinomas. Accordingly, colon cancer cells showed significantly reduced migratory behavior in response to PHLDA1 suppression. And in skin tumors, PHLDA1 is recognized as the follicular stem cell marker indicating that most are basal cell carcinomas and not trichoblastomas [12]. The constitutive expression of PHLDA1 protein by nevi in vivo [13] raises the possibility that it contributes to the benign nature of these tumors, maintaining growth regulation and apoptosis sensitivity to loss of survival signals provided by keratinocyte neighbors. Therefore, the progressive loss of PHLDA1 expression with malignant transformation may contribute to the loss of these characteristics in melanoma. Down-regulation of PHLDA1 protein has also been reported as a strong predictor of poor prognosis for breast cancer patients [4]. Since gastric cancer is the second leading cause of cancer-related death worldwide [14], and usually its precancerous process includes intestinal metaplasia, it is important to assess its possible significance according to the expression of some intestinal epithelial stem cell markers including PHLDA1. There is no report evaluating the expression of PHLDA1 in gastric carcinoma so far. We wonder if PHLDA1 is a putative tumor suppressor in gastric cancer and thus the significance of expression of PHLDA1 protein in gastric adenocarcinoma needs further explored.
Materials and methods
Biopsy specimens
Paraffin embedded sections of 336 gastric carcinomas and 60 distal normal gastric tissues were obtained from the Department of Pathology, Chinese People’s Liberation Army (PLA) General Hospital (Beijing, China) from 1998 to 2006. Of these patients, 17 were grade I, 65 grade II and 254 grade III, according to histological grading; 66 were stage I, 77 stage II, 147 stage III and 46 stage IV, according to clinical TNM stage revised by UIAC in 2003; and 75 were tubular adenocarcinoma (well-moderately differentiated adenocarcinoma), 32 were mucinous adenocarcinoma, 183 were poorly differentiated adenocarcinoma, 35 were signet-ring cell carcinoma, 11 were other gastric carcinoma according to histological type, respectively. By March, 2008 (the time of data analysis), 251 patients were dead, and 85 patients were alive. The median survival time was 36 months (range, 0.17-120 months). Ethical approval for this study was not required by our institution as the experiments carried out did not relate to patient’s privacy, impairment or treatment.
Immunohistochemistry
All samples were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut 4 μm thick from wax blocks, mounted on to APES-coated glass slides. Slides were deparaffinized in xylene twice for 10 min, rehydrated through graded ethanols to distilled water before incubation for 10 min with 3% hydrogen peroxidase-methanol to inhibit endogenous peroxidase activity, and heated in Tris/EDTA buffer (pH 8.0) in a microwave oven for 5 min at 100°C after reaching boiling point for antigen retrieval. Then the slides were taken out of microwave oven to be cooled at room temperature for 15 min. After incubating for 20 min in a blocking solution containing 10% normal goat serum in PBS, sections were incubated at 4°C overnight in a humidified chamber with monoclonal mouse antibody against human PHLDA1 (Santa Cruz, CA, USA) diluted 1:50 in blocking solution. After exposure to primary antibody, the sections were allowed to react with the poly peroxidase-anti-mouse/rabbit IgG for 20 minutes by the standard non-biotin PV-6000 Polymer Detection System (Zymed Laboratories Inc., South San Francisco, CA). The sections were then washed in water, counter-stained with Mayer’s haematoxylin for one minute at room temperature, dehydrated, cleaned and finally mounted. Paraffin blocks of human breast invasive ductal carcinoma tissues were used as the positive controls. Negative controls were sections treated the same as above but with omission of the primary anti-body and replaced by 0.01M PBS. For immunohistochemical evaluation of PHLDA1, cytoplasmic and nucleic labeling of tumor cells was classified as positive. In scoring expression of PHLDA1, both the extent and intensity of immunopositivity were considered, according to Hao et al. [15]. The intensity of positivity was scored as follows: 0, negative; 1, weak; 2, moderate; 3, strong. The extent of positivity was scored as follows: 0, <5%; 1, >5-25%; 2, >25-50%; 3, >50-75%; 4, >75% of the cells in the respective lesions. The final score was determined by multiplying the intensity of positivity and the extent of positivity scores, yielding a range from 0 to 12. Scores ≥4 were defined as positive expression pattern. Scores <4 were recorded as negative expression pattern.
Statistical analysis
Fisher’s exact test, Pearson Chi square’s test, Spearman’s correlation coefficient test for trends in proportions and Kaplan-Meier method with Log rank test or Cox Regression method for univariate or multivariate overall survival analysis were used to assess the associations between PHLDA1 expression and pathological indices. A P<0.05 was considered statistically significant.
Results
PHLDA1 expression in normal gastric mucosa tissue and gastric carcinoma
PHLDA1 protein was expressed positive diffusely in the cytoplasm and nucleus of in crypt base cells that may suggest the putative epithelial stem cells in all sixty normal gastric mucosa (Figure 1). In carcinoma, PHLDA1 was expressed diffusely in the cytoplasm and nucleus of cancer cells in one hundred and forty-four out of three hundred and thirty-six cases (42.9%, Figure 2). The expression of PHLDA1 was negative in 192 (57.1%) cases of carcinoma. Most poorly-differentiated cancer cells were negative for PHLDA1 protein (Figure 3). There was a statistical difference in PHLDA1 staining between gastric carcinoma and normal mucosa (P<0.0001). Strong PHLDA1 nuclear staining at the invasive margin could be observed.
Figure 1.
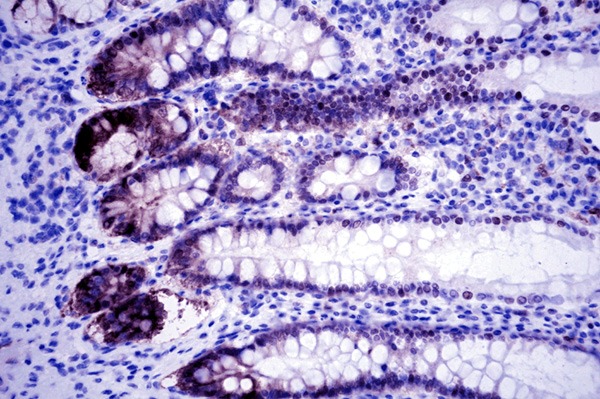
PHLDA1 expression in normal gastric mucosa tissue. PHLDA1 protein was expressed positive diffusely in the cytoplasm and nucleus of in crypt base cells that may suggest the putative epithelial stem cells (PHLDA1 × 400).
Figure 2.
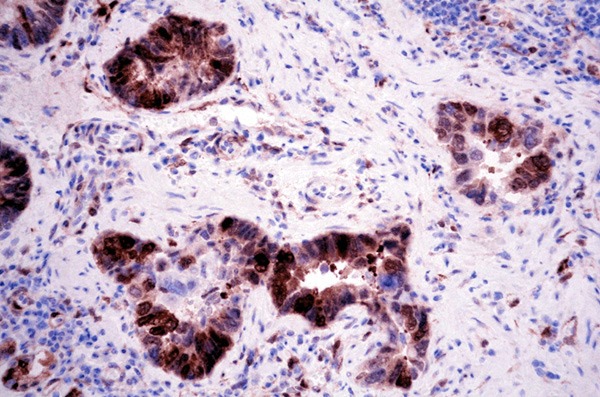
PHLDA1 expression in gastric adenocarcinoma. PHLDA1 was expressed diffusely in the cytoplasm and nucleus of cancer cells (PHLDA1 × 400).
Figure 3.
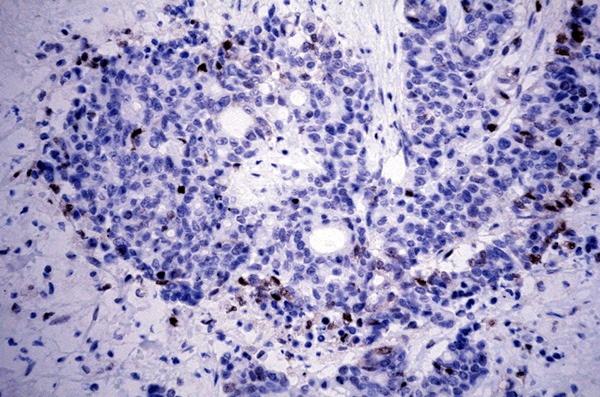
PHLDA1 expression in gastric adenocarcinoma. Most poorly-differentiated cancer cells were negative for PHLDA1 protein (PHLDA1 × 400).
Relationships between PHLDA1 expression and histological grade, clinical stage and prognosis
In this group of 336 gastric carcinomas, PHLDA1 expression was negatively correlative with tumor size (P=0.037), grade (P=0.028), depth of invasion (P=0.001), lymph node metastasis (P=0.008) and stage (P=0.001), but not with age (P=0.194), sex (P=0.312), tumor site (P=0.287) and distal metastasis (P=0.331) (Table 1). Follow-up data showed that there was a significant difference in overall median survival time between the carcinomas with PHLDA1 negative expression (31.0 months) and those with positive expression (54.0months) (Log rank=11.232; P=0.001) (Figure 4). In the result of multivariate analysis by Cox Regression, PHLDA1 expression was not an independent prognostic factor (P=0.220).
Table 1.
The relationship between expression of PHLDA1 and clinicopathological features in gastric adenocarcinoma
| Clinicopathological features | PHLDA1 | P | |
|---|---|---|---|
|
| |||
| - | + | ||
| Age | |||
| ≤40 | 24 | 10 | 0.120 |
| 41-65 | 113 | 85 | |
| >65 | 55 | 49 | |
| Sex | |||
| Man | 153 | 121 | 0.312 |
| Woman | 39 | 23 | |
| Tumor size (cm) | |||
| <4 | 36 | 41 | 0.037 |
| 4-7 | 119 | 82 | |
| ≥8 | 37 | 21 | |
| Tumour stage | |||
| T1 | 8 | 15 | 0.001 |
| T2 | 53 | 50 | |
| T3 | 110 | 77 | |
| T4 | 21 | 2 | |
| Lymph node metastasis | |||
| 0 | 50 | 61 | 0.008 |
| 1-6 | 76 | 52 | |
| 7-15 | 50 | 22 | |
| >15 | 16 | 9 | |
| Distant metastasis | |||
| Negative | 181 | 139 | 0.333 |
| Positive | 11 | 5 | |
| Grade | |||
| 1 | 5 | 12 | 0.028 |
| 2 | 34 | 31 | |
| 3 | 153 | 101 | |
| TNM stage | |||
| I-II | 67 | 76 | 0.0001 |
| III-IV | 125 | 68 | |
Figure 4.
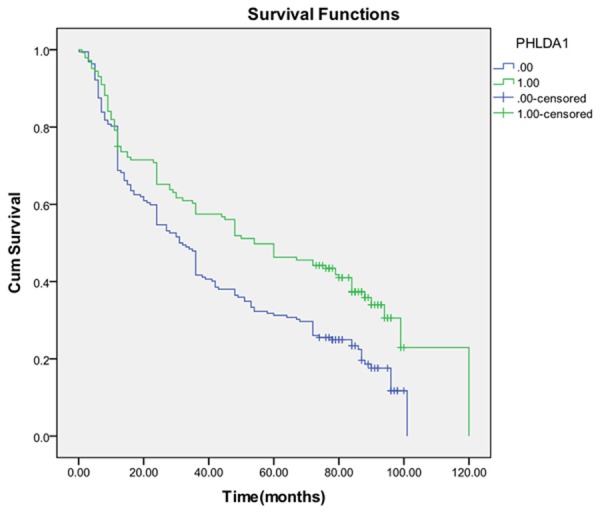
Kaplan-Meier survival analysis by PHLDA1 status (n=336). The y-axis represnts the percentage of patients; the x-axis, their survival in months. The green line represents PHLDA1-positive patients with a trend of better survival than the blue line representing PHLDA1-negative patients (Log rank=11.232; P=0.001. Overall median survival (OS) time was 54.0 months for the PHLDA1-positive group and 31.0 months for the PHLDA1-negative group.
Discussion
In mechanism, PHLDA1 is an Aurora A substrate [16], Aurora A directly phosphorylates PHLDA1 leading to its degradation. PHLDA1 also negatively regulates Aurora A, by promotes Aurora A degradation, thereby triggering a feedback loop. The underlying mechanisms by which PHLDA1 upregulation strongly antagonizes Aurora-A-mediated oncogenic pathways, therefore revealing PHLDA1 degradation as an important mechanism by which Aurora A promotes gastric malignancy [17-20]. Thus, although the mechanisms involved in the down-regulation of PHLDA1 expression remain unknown, the loss of PHLDA1 expression may possibly contribute to the development of apoptosis resistance in gastric carcinomas. In this study we retrospectively analyzed the relationships between the expression of PHLDA1 and clinicopathological indicators in 336 Chinese patients with gastric cancer. Our results indicated that the loss of PHLDA1 expression was correlative with the histological degree (P= 0.0001) and the clinical stage (P=0.0001). Compared with the previous results in colorectal cancer by Sakthianandeswaren et al. [11], in which there was no correlation between PHLDA1 staining and adenocarcinoma grade or clinical stage, our different results suggest PHLDA1 may play an important role in the evolution and development of gastric carcinoma. We also found the phenomenon of the increased staining and nuclear relocalization of the PHLDA1 protein at the invasive front of cancer cells, the similar result present in colorectal cancer by Sakthianandeswaren et al. [11], also suggesting the evidence for a novel role for PHLDA1 in cell migration. However, the mechanism for the different functions between nuclear and cytoplasmic pools of PHLDA1 still remains to be elucidated. Of interest, the polyglutamine tract in PHLDA1 is a feature common to several transcription factors, suggesting a possible role as a transcription factor or coactivator [21]. Follow-up data showed that there was a significant difference in overall median survival time between the gastric carcinoma patients with PHLDA1 negative expression (31 months) and those with positive expression (54 months) (Log rank=11.232; P=0.001). Our results are also corresponding to previously published results in breast cancer and malignant melanoma [4,5]. It is suggested that PHLDA1 expression might be a potential prognostic factor in gastric adenocarcinoma.
In conclusion, PHLDA1 protein may play an important role in the carcinogenesis, progress and prognosis of Chinese gastric carcinoma and, PHLDA1 expression detected by immunohistochemistry may be a simple and useful molecular biomarker to predict the prognosis in gastric carcinoma patients. The association between PHLDA1 and other important biomarkers in gastric cancer development needs to be further investigated, from which PHLDA1 targeting therapy may be applied in gastric cancer patients.
Acknowledgements
The authors thank all colleagues (Department of Pathology, Chinese People’s Liberation Army General Hospital) who have done much work on PHLDA1.
Disclosure of conflict of interest
None.
References
- 1.Haslam RJ, Koide HB, Hemmings BA. Pleckstrin domain homology. Nature. 1993;363:309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- 2.Ingley E, Hemmings BA. Pleckstrin homology (PH) domains in signal transduction. J Cell Biochem. 1994;56:436–443. doi: 10.1002/jcb.240560403. [DOI] [PubMed] [Google Scholar]
- 3.Saraste M, Hyvonen M. Pleckstrin homology domains: a fact file. Curr Opin Struct Biol. 1995;5:403–408. doi: 10.1016/0959-440x(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 4.Nagai MA, Fregnani JH, Netto MM, Brentani MM, Soares FA. Down-regulation of PHLDA1 gene expression is associated with breast cancer progression. Breast Cancer Res Treat. 2007;106:49–56. doi: 10.1007/s10549-006-9475-6. [DOI] [PubMed] [Google Scholar]
- 5.Neef R, Kuske MA, Prols E, Johnson JP. Identification of the human PHLDA1/TDAG51 gene: down-regulation in metastatic melanoma contributes to apoptosis resistance and growth deregulation. Cancer Res. 2002;62:5920–5029. [PubMed] [Google Scholar]
- 6.Park CG, Lee SY, Kandala G, Lee SY, Choi Y. A novel gene product that couples TCR signaling to Fas (CD95) expression in activation-induced cell death. Immunity. 1996;4:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- 7.Hossain GS, van Thienen JV, Werstuck GH, Zhou J, Sood SK, Dickhout JG, de Koning AB, Tang D, Wu D, Falk E, Poddar R, Jacobsen DW, Zhang K, Kaufman RJ, Austin RC. TDAG51 is induced by homocysteine, promotes detachment-mediated programmed cell death, and contributes to the cevelopment of atherosclerosis in hyperhomocysteinemia. J Biol Chem. 2003;278:30317–27. doi: 10.1074/jbc.M212897200. [DOI] [PubMed] [Google Scholar]
- 8.Hayashida N, Inouye S, Fujimoto M, Tanaka Y, Izu H, Takaki E, Ichikawa H, Rho J, Nakai A. A novel HSF1-mediated death pathway that is suppressed by heat shock proteins. EMBO J. 2006;25:4773–4783. doi: 10.1038/sj.emboj.7601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberst MD, Beberman SJ, Zhao L, Yin JJ, Ward Y, Kelly K. TDAG51 is an ERK signaling target that opposes ERK-mediated HME16C mammary epithelial cell transformation. BMC Cancer. 2008;8:189. doi: 10.1186/1471-2407-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyoshima Y, Karas M, Yakar S, Dupont J, Lee Helman, LeRoith D. TDAG51 mediates the effects of insulin-like growth factor I (IGF-I) on cell survival. J Biol Chem. 2004;279:25898–25904. doi: 10.1074/jbc.M400661200. [DOI] [PubMed] [Google Scholar]
- 11.Sakthianandeswaren A, Christie M, D’Andreti C, Tsui C, Jorissen RN, Li S, Fleming NI, Gibbs P, Lipton L, Malaterre J, Ramsay RG, Phesse TJ, Ernst M, Jeffery RE, Poulsom R, Leedham SJ, Segditsas S, Tomlinson IP, Bernhard OK, Simpson RJ, Walker F, Faux MC, Church N, Catimel B, Flanagan DJ, Vincan E, Sieber OM. PHLDA1 expression marks the putative epithelial stem cells and contributes to intestinal tumorigenesis. Cancer Res. 2011;71:3709–3719. doi: 10.1158/0008-5472.CAN-10-2342. [DOI] [PubMed] [Google Scholar]
- 12.Sellheyer K, Nelson P. Follicular stem cell marker PHLDA1 (TDAG51) is superior to cytokeratin-20 in differentiating between trichoepithelioma and basal cell carcinoma in small biopsy specimens. J Cutan Pathol. 2011;38:542–550. doi: 10.1111/j.1600-0560.2011.01693.x. [DOI] [PubMed] [Google Scholar]
- 13.Neef R, Kuske MA, Pröls E, Johnson JP. Identification of the human PHLDA1/TDAG51 gene: down-regulation in metastatic melanoma contributes to apoptosis resistance and growth deregulation. Cancer Res. 2002;62:5920–5929. [PubMed] [Google Scholar]
- 14.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 15.Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP. Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer Res. 2000;60:18–21. [PubMed] [Google Scholar]
- 16.Johnson EO, Chang KH, Pablo Y, Ghosh S, Mehta R, Badve S, Shah K. PHLDA1 is a crucial negative regulator and effector of aurora A kinase in breast cancer. J Cell Sci. 2011;124:2711–2722. doi: 10.1242/jcs.084970. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Zhang N, Wang J, Bu XM, Zhao CH. Inhibition of proliferation, viability, migration and invasion of gastric cancer cells by Aurora-A deletion. Asian Pac J Cancer Prev. 2011;12:2717–2720. [PubMed] [Google Scholar]
- 18.Honma K, Nakanishi R, Nakanoko T, Ando K, Saeki H, Oki E, Iimori M, Kitao H, Kakeji Y, Maehara Y. Contribution of Aurora-A and -B expression to DNA aneuploidy in gastric cancers. Surg Today. 2014;44:454–461. doi: 10.1007/s00595-013-0581-x. [DOI] [PubMed] [Google Scholar]
- 19.Katsha A, Soutto M, Sehdev V, Peng D, Washington MK, Piazuelo MB, Tantawy MN, Manning HC, Lu P, Shyr Y, Ecsedy J, Belkhiri A, El-Rifai W. Aurora kinase A promotes inflammation and tumorigenesis in mice and human gastric neoplasia. Gastroenterology. 2013;145:1312–1322. doi: 10.1053/j.gastro.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehdev V, Katsha A, Arras J, Peng D, Soutto M, Ecsedy J, Zaika A, Belkhiri A, El-Rifai W. HDM2 regulation by AURKA promotes cell survival in gastric cancer. Clin Cancer Res. 2014;20:76–86. doi: 10.1158/1078-0432.CCR-13-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alba MM, Santibanez-Koref MF, Hancock JM. The comparative genomics of polyglutamine repeats: extreme differences in the codon organization of repeat-encoding regions between mammals and Drosophila. J Mol Evol. 2001;52:249–259. doi: 10.1007/s002390010153. [DOI] [PubMed] [Google Scholar]


