Abstract
Aims: To observe the effect of bevacizumab on human A549 cells and explore its mechanism. Methods: After different concentrations (0 μM, 1 μM, 5 μM, 25 μM) of bevacizumab treating in A549 cells, CCK8 assay detect the impact of bevacizumab on A549 cell proliferation and flow cytometry determine the effect of bevacizumab on human A549 cells apoptosis. Real-time PCR and Western blotting detect the changing expression of the target gene (CHOP, caspase-4, IRE1, XBP-1) on mRNA and Protein level. Results: Treatment with bevacizumab for 24-hr have induced cell death in a does-dependent manner dramatically (P<0.05). In terms of the mRNA level, expression of XBP-1 has increased obviously in each group (1 μM, 5 μM, 25 μM) (P<0.01); the expression of CHOP (25 μM) and caspase-4 (5 μM) have increased slightly (P<0.05). In terms of the protein level, the expression of CHOP has increased obviously in each group (1 μM, 5 μM, 25 μM) when compared with the control group (0 μM) (P<0.05). As for caspase-4 (5 μM, 25 μM), the expression have increased slightly when compared with the control group (0 μM) (P<0.05). Conclusion: Bevacizumab can induce A549 cell apoptosis through the mechanism of endoplasmic reticulum stress.
Keywords: Bevacizumab, endoplasmic reticulum stress, A549 cell, apoptosis
Introduction
Lung cancer is one of the most common tumors worldwide. Though our treatment technology have improved a lot, the therapeutic methods are so limited and the effects are not so pleasing,especially non-small cell lung cancer (NSCLC) [1]. Under such circumstances, it is extremely urgent for researchers to take active part in developing some new drugs to cure this disease. Bevacizumab, is a recombined human monoclonal antibody researched by Genentech which has been bought by Roche. As a new generation of molecularly targeted antineoplastic drugs, bevacizumab has been widely used in clinically first-line treatment of the tumors in middle and advanced stage, including NSCLC by inhibiting the biological activity of vascular endothelial growth factor (VEGF). By cutting off the biological activity of VEGF, bevacizumab will bring about ischemia and hypoxia of the tumor cells and thus affect the growth, the attack and the proliferation of a cancer [2-4]. According to the latest research [5], VEGF can adjust cells’ endoplasmic reticulum stress (ERS) by way of directly activating this signal channel. Thus we can speculate that the reason why bevacizumab will play a role in treating cancers is that it can accelerate cell apoptosis through the mechanism of ERS. On the basis of our experimental results, we can come to the conclusion that bevacizumab do induce A549 cell apoptosis through the mechanism of ERS.
Materials and methods
Experimental materials
A549 cells come from Central Laboratory, Central South University, China. Bevacizumab is from Roche, Switzerland. Fetal bovine serum (FBS), tryptan-EDTA, RNA enzyme, trypsin and Triton X-100 are all from Gibco, America. RPMI-1640, PBS buffer solution comes from Hyclone, America. CCK-8 solution is from Seven Sea biology Ltd. CO, Shanghai, China. Annexin V, Propidium Iodide (PI), Ambion RNA-queous kit and cDNA archive kit are all from Invitrogen, America. The antibodies including caspase-4, CHOP, IRE1 and XBP-1 all come from Santa Cruz, America.
Bevacizumab interfere A549 cells
A549 cells are cultivated by RPMI-1640 which containing 10% of FBS and are put in the incubator whose temperature is 37°C and the concentration of CO2 is 5%. Bevacizumab is attenuated by sterile water for injection. The cells are interfered respectively for 12-hr, 48-hr and 72-hr with Bevacizumab of 0 μM, 1 μM, 5 μM and 25 μM [2].
CCK-8 assay detects cell proliferation
We inoculate A549 cells in a plate with 96 holes. Then we respectively use bevacizumab in different concentrations such as 0 μM, 1 μM, 5 μM, 25 μM to process the cells for corresponding 12-hr, 24-hr, 48-hr, 72-hr. Next, we add CCK-8 solution (10 μL) to each hole. Finally the absorption value at 450 nm is measured by a plate reader after the plate being put in an incubator for another 4 hours [6]. We repeat the procedure for three times and then calculate the average value.
Flow cytometry technology to detect cells apoptosis
A549 cells are inoculated in a 60-mm-plate overnight. We respectively use bevacizumab in different concentrations (0 μM, 1 μM, 5 μM, 25 μM) to process the cells for 24-hr, and then use PBS buffer solution to wash them for 3 times as well as using tryptan-EDTA (0.25%) to digest. After being centrifuged, the cells will suspend in PBS (0.5 ml). And the cells are not only dyed by Annexin V and Propidium Iodide (PI) but also processed by RNA enzyme and Triton X-100 (0.1%) at indoor temperature for 30 minutes. In the end, flow cytometric analysis can be carried out by using fluorescence activated cell sorter [7].
Real-time quantitative PCR to detect RNA expression
The extraction of total mRNA were from cells treated by different concentration (0 μM, 1 μM, 5 μM, 25 μM) of bevacizumab with using the Ambion RNA-queous kit. The high capacity cDNA archive kit used to get the first chain cDNA of mRNA. The mRNA level of CHOP, caspase-4, IRE1 and XBP-1 were determined through specific gene kit and real-time polymerase chain reaction (Real-time PCR) detection system (Eppendorf, Hamburg, Germany). PCR Primer sequence: CHOP, sense strand, 5’-CTGAATCTGCACCAAGCATGA- 3’, antisense strand, 5’-AAGGTGGGTAGTGTGGCCC-3’; capase-4, sense strand, 5’-TGGAGAAGGACTTCATTG-3’, antisense strand, 5’-GAAGCATGTGATGAGTTG-3’; IRE1, sense strand, 5’-GACTCCATG CTTAAGGAC-3’, antisense strand, 5’-GGGATAGGTGATGATGAA-3’; XBP1, sense strand, 5’-GAACC AGGAG TT-AAGACA-3’, antisense strand, 5’-CTCACTTCATTCCCCTTG-3’ [7].
Western blotting technology to detect protein expression
The expression of protein (CHOP, caspase-4) are detected by Western blotting. Briefly, after cytolysis, we collect the supernate and then extract the plasmosin or nucleoprotein in an already-known-way [8]. At the voltage of 120 V, hydrochloric acid polyacrylamide gel can make protein transfer to the blotting membrane, on which we use specific polyclonal antibody to probe, of PVDF. We can conduct electrophoretic analysis of equal quantity from protein lysate with b-actin being the internal reference.
Statistical analysis
All the experiments are conducted at least for three times and all the measured parameters are expressed as average value ± standard deviation (x ± s). Variance is adopted to analyze the repeatedly measured data from the experiments of time-varying bevacizumab’s inhibition action on the proliferation of A549 cells. While as for the comparisons between bevacizumab’s treatment groups in different concentration (0 μM, 1 μM, 5 μM, 25 μM), t-test method is adopted.
Results
Bevacizumab inhibits proliferation of A549 cells
Bevacizumab treat A549 cells for 12 hours, showing the inhibition of cell proliferation mild, but after 24 hours showing significant induction of cell apoptosis in a dose dependent manner (Figure 1A; Table 1). Conversely, bevacizumab (0 μM) which was as a control group showed no significant inhibition of cell lines at any time. These data suggest that bevacizumab inhibits proliferation of A549 cells (Figure 1B and 1C).
Figure 1.

Effect of bevacizumab on A549 cells growth. A. The figures were obtained by microscope with 200×amplification. Cells were treated with bevacizumab (0 μM, 1 μM, 25 μM) for 24 hr. B. Time-OD value curves of bevacizumab in each group A549 cells. C. Bevacizumab treatment A549 cells for 24 hours, 48 hours effects on cell survival. The effects of bevacizumab on cell relative survival rate in A549 cells. Cell relative survival rate (%) = experimental group OD value/control group OD value ×100%, bevacizumab (0 μM) which was as a control group. Statistically significant, #P<0.05, t-test vs. vehicle control.
Table 1.
Bevacizumab impact on A549 cell proliferation (x ± s, n=9)
| Groups | Concentration (μM) | 12 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|
|
| |||||
| OD value | OD value | OD value | OD value | ||
| Control Experimental | 0 | 0.486 ± 0.026 | 0.491 ± 0.019 | 0.496 ± 0.027 | 0.502 ± 0.023 |
| 1 | 0.487 ± 0.016 | 0.479 ± 0.017 | 0.469 ± 0.021 | 0.464 ± 0.031 | |
| 5 | 0.464 ± 0.014 | 0.440 ± 0.016 | 0.425 ± 0.020 | 0.408 ± 0.027 | |
| 25 | 0.446 ± 0.023 | 0.425 ± 0.022 | 0.360 ± 0.035 | 0.327 ± 0.031 | |
Note: CCK 8 method is used to detect the OD value of each group A549 cells. Data are presented as the mean ± S. E. Data using analysis of variance of repeated measurement data.
Bevacizumab can induce A549 cells apoptosis
We adopt flow cytometry to assess the influence on A549 cells apoptosis exerted by bevacizumab (Figure 2A). The results show that bevacizumab processed for 24-hr can obviously induce cells apoptosis which further indicates that bevacizumab participates the signal channel (Figure 2B).
Figure 2.

Effect of bevacizumab on the induction of apoptosis in A549 cells as determined by fiow cytometry. A. A549 cells were treated with bevacizumab at indicated concentrations for 12 hr, then stained with annexin V and propidium iodide (PI), followed by detection using fiow cytometry. B. The percentage of cells with early apoptosis and late apoptosis are shown. Statistically significant, #P<0.05, ##P<0.05, t-test vs. vehicle control.
Bevacizumab activate signal pathway of endoplasmic reticulum stress induced apoptosis
We further study the possibility that Bevacizumab induces A549 cell apoptosis through ERS. In the unfolded protein response (UPR) caused by ERS [9,10], we choose inositol requiring enzyme 1 (IRE-1) and X-box binding protein 1 (XBP1) as our test index while in the signal channel of endoplasmic reticulum stress-induced apoptosis (ERSIA) [11], we select C/EBP-homologous protein (CHOP) and caspase-4 as our test index. We can come to the conclusion that, in terms of the RNA level, expression of XBP-1 has increased obviously in each group (1 μM, 5 μM, 25 μM) (P<0.01), the figures were 2.196 ± 0.101, 4.605 ± 0.413, 2.532 ± 0.185; the expression of CHOP (25 μM) and caspase-4 (5 μM) have increased slightly (P<0.05), the figures were 2.191 ± 0.112, 1.588 ± 0.167 (Figure 3A). In terms of the protein level, the expression of CHOP has increased obviously in each group (1 μM, 5 μM, 25 μM) when compared with the control group (0 μM) (P<0.05), the figures were 0.67 ± 0.08, 1.52 ± 0.24, 1.32 ± 0.31. As for caspase-4 (5 μM, 25 μM), the expression have increased slightly when compared with the control group (0 μM) (P<0.05), the figures were 0.65 ± 0.15, 0.63 ± 0.23 (Figure 3B). Our data shows that bevacizumab activates cascade reaction of the endoplasmic reticulum stress induced apoptosis.
Figure 3.
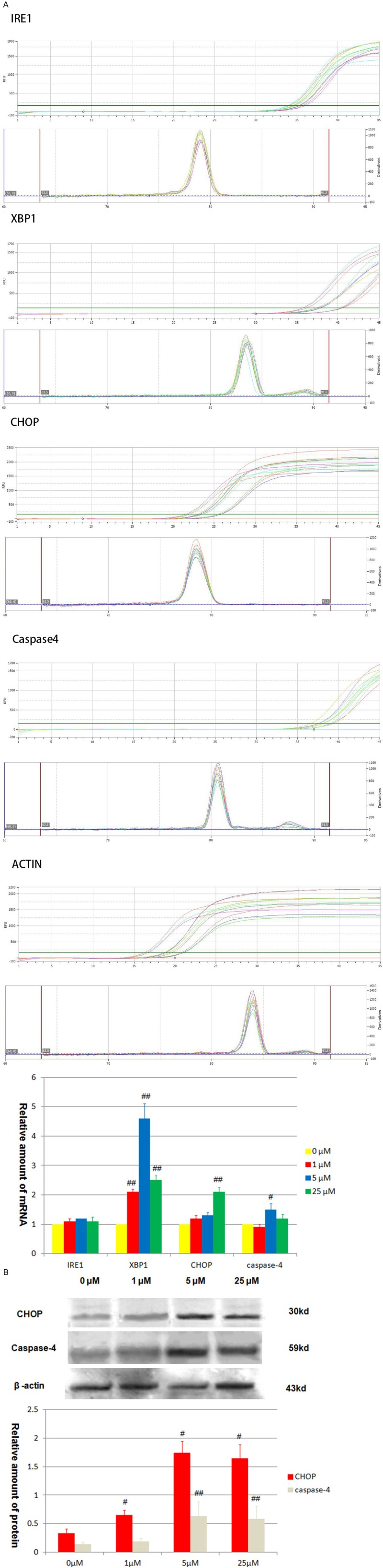
Effect of bevacizumab on endoplasmic reticulum stress pathway activation. A. The mRNA levels of IRE-1, XBP-1, CHOP and caspase-4 were examined. All RT-qPCR results were calculated and represented as the percent of vehicle control, and actin was used as a control for equal loading in the RT-qPCR experiments (#P<0.05, ##P<0.01, vs. vehicle group). B. A549 cells were incubated with bevacizumab at indicated concentrations for 24 hr. The protein of CHOP and caspase-4 were examined by Western blot. Actin was shown as a control for equal loading (#P<0.05, ##P<0.05, vs. vehicle group).
Discussion
As a new generation of molecularly targeted antineoplastic drugs, bevacizumab, whose mechanism of action is to inhibit the biological activity of VEGF and then cure the cancer, has been widely used in clinically first-line treatment of the tumors in middle and advanced stage [3]. Traditionally, we believed that the reason why bevacizumab can treat cancers by inhibiting the biological activity of VEGF is that bevacizumab can adjust the formation of tumor vessel on tissue level. While based on the recent findings, we can conclude that VEGE can adjust cells’ ERS by way of directly activating the signal channel of ERS. That is to say, bevacizumab’s anti-tumor effect can be fulfilled on cellular level without depending on the formation of tumor vessel on tissue level.
Endoplasmic reticulum, the place to process protein in a cell, takes part in the protein synthesis and protein folding of membrane protein and secretory protein. Pessimal stimulations such as oxidative stress, hypoxia, cytotoxic materials and nutrient deficiency will give rise to the dysfunction of ER and then increase the accumulation of misfolded protein and unfolded protein in the ER lumen, which will eventually cause ERS [12]. ERS is not only a significant defense mechanism for cells to resist the harmful stimulations but also a double-edged sword to determine the life or death of a cell. Moderate ERS is able to intensify the growth and transfer of a lung tumor and increase the resistance of a lung tumor cell against chemotherapeutics. While excessive ERS will accelerate a lung tumor cell apoptosis. A large number of drugs have been utilized in treating tumors clinically by inducing a lung tumor cell apoptosis [13,14]. ERS can regulate the life or death of a cell by its intensity and time, and it can even participate in the regulation of the development, senility and death of the organism. So ERS have played a significant role in the growth, attack and transfer of a tumor cell.
Under normal circumstances, protein folding in endoplasmic reticulum (ER) is in a state of dynamic equilibrium which will be broken by some sensitive elements such as drugs, ischemia, hypoxia and physicochemical factors and then therefore trigger ERS [15]. First, the dissociation and the activation of protein kinase R-like ER kinase (PERK), IRE-1, activating transcription factor 6(ATF6) and glucose regulate protein 78 (GRP78) on the ER membrane will lead to the response of UPR. PERK, IRE-1, ATF6 on the ER membrane respectively dissociate with GRP78 which then will be activated by the three protein mentioned above. Then, after been activated, the signal channel made of CHOP, caspase-12 and JNK will accelerate cell apoptosis. After detecting the expression of the target gene (CHOP, caspase-4, IRE1, XBP-1) on RNA and Protein level by Real-time PCR and Western blotting in experiments, we have found that the expression have increased more or less when compared with the control group which further proves that the signal channel of ERS participates in the experimental process.
Our experiments suggest that bevacizumab will probably induce A549 cell apoptosis by exacerbating the reaction of ERS which thus have made it clear that bevacizumab can induce A549 cell apoptosis through the mechanism of ERS. The results of our studies offer the reference of how to screen the treatment objects suffered from lung tumor and how to carry out the individualized treatment and thus further improve the treatment effects to the medical workers.
Although we have confirmed that bevacizumab can induce A549 cell apoptosis and that apoptosis signal channels can be activated by the induction of ERS, whether other signal channels have participated in inducing A549 cell apoptosis in our experiments or not awaits the results of further studies.
Acknowledgements
This study was supported by grant from Natural Science Foundation of China (No. 81070035). We wish to thank all the staffs from Institute of Respiratory Disease of Hunan Province Geriatric Hospital for help with this study.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Zhang S, Zou X, Zhao P, He J. Lung cancer incidence and mortality in China, 2009. Thorac Cancer. 2013;4:102–108. doi: 10.1111/1759-7714.12025. [DOI] [PubMed] [Google Scholar]
- 2.Shin JY, Woo SJ, Ahn J, Park KH. Anti-VEGF-refractory exudative age-related macular degeneration: differential response according to features on optical coherence tomography. Korean J Ophthalmol. 2013;27:425–432. doi: 10.3341/kjo.2013.27.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gostino RB. Changing end points in breast-cancer drug approval: the Avastin story. N Engl J Med. 2011;365:e2. doi: 10.1056/NEJMp1106984. [DOI] [PubMed] [Google Scholar]
- 4.Sekeres MA. The avastin story. N Engl J Med. 2011;365:1454–1455. doi: 10.1056/NEJMc1109550. [DOI] [PubMed] [Google Scholar]
- 5.Zeng L, Xiao Q, Chen M, Margariti A, Martin D, Ivetic A, Xu H, Mason J, Wang W, Cockerill G, Mori K, Li JY, Chien S, Hu Y, Xu Q. Vascular endothelial cell growth-activated XBP1 splicing in endothelial cells is crucial for angiogenesis. Circulation. 2013;127:1712–1722. doi: 10.1161/CIRCULATIONAHA.112.001337. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Jia L, Kuang Z, Wu T, Hong Y, Chen X, Leung WK, Xia J, Cheng B. The in vitro and in vivo antitumor effects of clotrimazole on oral squamous cell carcinoma. PLoS One. 2014;9:e98885. doi: 10.1371/journal.pone.0098885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J, Wang Y, Peng J, Guo L, Hu J, Cao M, Zhang H, Wang Z, Li X, Yang S, Yang H, Liang G. A synthetic compound, 1,5-bis (2-methoxyphenyl) penta-1,4-dien-3-one (b63), induces apoptosis and activates endoplasmic reticulum stress in non-small cell lung cancer cells. Int J Cancer. 2012;131:1455–1465. doi: 10.1002/ijc.27406. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhu C, Li X, Zhang Z, Yuan Y, Ni Y, Liu T, Deng S, Zhao J, Wang Y. Asterosaponin 1 induces endoplasmic reticulum stress-associated apoptosis in A549 human lung cancer cells. Oncol Rep. 2011;26:919–924. doi: 10.3892/or.2011.1358. [DOI] [PubMed] [Google Scholar]
- 9.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Rahman S, Ayaub EA, Dickhout JG, Ask K. Protein misfolding and endoplasmic reticulum stress in chronic lung disease. Chest. 2013;143:1099–1105. doi: 10.1378/chest.12-2133. [DOI] [PubMed] [Google Scholar]
- 11.Zhu GY, Wong BC, Lu A, Bian ZX, Zhang G, Chen HB, Wong YF, Fong WF, Yang Z. Alkyl phenols from the roots of ardisia brevicaulis induce g1 arrest and apoptosis through endoplasmic reticulum stress pathway in human non-small-cell lung cancer cells. Chem Pharm Bull. 2012;60:1029–1036. doi: 10.1248/cpb.c12-00302. [DOI] [PubMed] [Google Scholar]
- 12.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Su L, Zhong N, Hao X, Zhong D, Singhal S, Liu X. Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells. Autophagy. 2013;9:1057–1068. doi: 10.4161/auto.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi AY, Choi JH, Yoon H, Hwang KY, Noh MH, Choe W, Yoon KS, Ha J, Yeo EJ, Kang I. Luteolin induces apoptosis through endoplasmic reticulum stress and mitochondrial dysfunction in Neuro-2a mouse neuroblastoma cells. Eur J Pharmacol. 2011;668:115–126. doi: 10.1016/j.ejphar.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 15.Moriya S, Miyazawa K, Kawaguchi T, Che XF, Tomoda A. Involvement of endoplasmic reticulum stress-mediated CHOP (GADD153) induction in the cytotoxicity of 2-aminophenoxazine-3-one in cancer cells. Int J Oncol. 2011;39:981–988. doi: 10.3892/ijo.2011.1072. [DOI] [PubMed] [Google Scholar]


