Abstract
The aim of this study is to determine the expression level of spindle assembly checkpoint (SAC) proteins-BubR1 and synuclein-gamma (SNCG) in human breast cancer tissues and to test whether there is a relationship between their expression levels and clinicopathologic parameters including respons to taxanes, tumor grade, estrogen receptor (ER) pozitivity, HER2 status, and overall survival (OS). We analyzed retrospectively paraffin-embedded tissue sections from 55 breast cancer patients whose clinical outcomes had been tracked after taxane treatment in neoadjuvan and metastatic setting. The expression status of BubR1 and SNCG was defined by immunohistochemistry (IHC) using the anti-BubR1 and anti-SNCG antibody. The BubR1 and SNCG was overexpressed in 38% and 62% of the study group, respectively. There was borderline significant correlation between low BubR1 expression and increased taxane sensitivity (P=0.05). In contrast, high SNCG expression was significantly associated with decreased taxane sensitivity (P=0.01). There was no association between the clinicopathologic parameters including histologic grade, ER positivity and HER2 status and the level of these proteins. However, triple negative tumors showed significantly more high BubR1 expression than those other molecular subtypes (P=0.04). Kaplan-Meier survival analysis failed to show a significant correlation between expression levels of BubR1 and SNCG and overall survival although patients with low levels of both proteins had a marginally longer survival time compared to those with high levels. In summary, our data suggest that both BubR1 and SNCG may be promising predictive marker rather than prognostic marker in patients with breast cancer.
Keywords: BubR1, synuclein gamma, predictive marker, prognostic marker, taxane, breast cancer
Introduction
Breast cancer is second leading cause of death in women from cancer [1]. In both adjuvant and metastatic setting the best affective chemotheropotic drugs are taxanes and antracyclines [2]. Due to the cumulative toxixity of antracyclines, the importance of taxenes has increased. However, one part of patient with breast cancer do not benefit from taxanes. The response rate to taxanes in metastatic tumors ranges from 30% to 50% [2]. For this reason, prediction of response or resistance to taxanes in breast cancer may be helpful to select those patients more likely to derive a clinical benefit.
The taxanes, paclitaxel and docetaxel are microtubule-stabilizing agents. They bind to β-tubulin and result in kinetic abnormalities with elevated microtubule formation in the dynamics of microtubules by increasing their polymerization and inhibiting their depolymerization. In metaphasa, defective sipinle formation induced by taxanes activates the mithotic checkpoint and causes cell cycle arrest, resulting in apoptosis [3]. Various molecules including microtubule-associated proteins (MAPs), spindle assembly checkpoint (SAC) proteins, β Tubulin, HER2, p53, BRCA1, CYP3A4, estrogen receptor (ER), BCL2, P-glycoprotein and Ki-67 have been examined for their ability to predict response to taxanes [3].
SAC monitors the attachment of spindle microtubules to the kinetochore of each sister chromatid for accurate chromosomal segregation in mitosis. In this regulation system, various SAC proteins play role including mitotic arrest deficient proteins (MAD 1-3), budding uninhibited by benzimidazoles proteins (BUB1-3), Bub1-related protein kinase (BubR1) and monopolar spindle 1 (Mps1) [3]. BubR1 is one of the several key proteins needed for correct SAC function suck as Mad2, centromere-associated protein-E (CENP-E), cell-division cycle protein 20 (Cdc20). It occur the mitotic checkpoint complex (MCC) together with Mad2,Bub3 and Cdc20, which delays anaphase onset by inhibiting the Anaphase Promoting Complex/cyclosome (APC/C) until all kinetochores attached to microtubules [4]. This regulatory system prevents aneuploidy by ensuring the segregation of only one copy of each pair of duplicated sister chromatids [3]. Moreover,BubR1 phosphorylates and stabilises p53 during mitosis. Therefore, BubR1 is implicated in both spindle assembly and DNA damage checkpoints [5].
Synuclein gamma are highly expressed in neuronal cells, Synuclein family consists of alpha-, beta- and gamma-synuclein. Especially, the role of alpha synuclein in neurodegenerative diseases has been well documented [6]. In addition there are evidences suggesting that SNCG increases metastasis and promotes genetic instability. In many different malignant diseases, its abnormal expression has been demostrated [7-10]. Generally, it rarely expressed in tumor-matched nonneoplastic adjacent tissues [11]. At the celluler level, had been demostrated that SNCG prevented the formation of MCC by inhibiting BubR1 activity, and resulted in an insufficiency of BubR1-related SAC function [12]. Considering that anti-mitotic drugs target microtubules, correct functioning of the BubR1 and SNCG would seem crucial for an appropriate drug response.
Previous studies have reported that tumor expression of BubR1 and SNCG can alter the sensitivity to Anthracycline-based agents [13], and neoadjuvant chemotherapy [14] in breast cancer, respectively. Their expression levels may be associated with survival [15-20]. In our study, We identified retrospectively the expression levels of BubR1 and SNCG proteins in paraffin-embedded breast cancer tissue samples obtained from patients with breast cancer and evaluated the role of BubR1 and SNCG expressions in predicting treatment response to taxanes, which has not been reported in the current medical literature. Survival and correlation with clinicophathologic parameters were also analyzed to determine the prognostic values of BubR1 and SNCG in these patients.
Materials and methods
Patient and tissue sapmles
Cases were selected retrospectively from records of Goztepe Medical Park Oncology Hospital between the years 2008-2014. The study has been approved by the Goztepe Medical Park Hospital Ethics Committee under the title ‘Retrospective analysis of tissue samples by immunohistochemistry (IHC)’. Eligibility criteria were as follows: (a) Responder and non responder patients who had taken AC (doxorubicin/cyclophosphamide) followed by taxanes in neoadjuvant setting for locale advanced breast cancer. (b) Non-responder patients who had taken combination of taxanes with cyclophosphamid and antracyclin or AC followed by taxanes in neoadjuvant setting. (c) Responder and non responder patients who had taken single agent taxane in metastatic setting (to exlude the effect of other chemotherapeutic on response as a bias source in patient selection in responder patients). (d) Non responder patients who had taken the combination of taxanes with any chemotherapeutic agent in metastatic setting. (e) Patients who had new biopsy for metastatic disease, if long time had elapsed from initial diagnosis to metstatic diseas. The taxanes had been administred as either weekly paklitaxel (80 mg/m2) or docetaxel every 3 weeks (75 mg/m2). A total of 55 patients who met egilibility criteria were stratified according to tretament responses to taxane into two groups as responders and non- responders. The responder group (patients with complet response, partial response and stable disease) and non responder group (progresive disease ) were defined according to the Respons Evaluation Criteria in Solid Tumor (RECIST).
Immunohistochemical evaluation
Formalin-fixed and paraffin-embedded tissue specimens of primary or metastatic breast cancer collected in the pathology department archive at Goztepe Medical Park Hospital were used for IHC staining. Formalin-fixed and paraffin-embedded tissue was cut (4-5 μm) and stained with hematoxylin and eosin. A representative slide of each case was selected for IHC studies. Sections 4-5 μm thick were placed on electrostatic-charged slides (X-traTM, Surgipath Medical Industries, Richmond, Illinois, USA) and dried at 60°C for at least two hours and stained with mouse monoclonal BubR1 antibody (GeneTex Clone 5D9, CA USA, overnight incubation at a dilution of 1:100) and gamma synuclein antibody (GeneTex Clone EP 1539Y 2 hours incubation at a dilution of 1:400), IHC staining was carried out according to standard streptavidin-biotin-peroxidase method. A section of tonsil and hippocampal tissue were used as a positive control for BubR1 and SNCG, respectively. Sections incubated without the primary antibody served as negative controls for both antibody. Expression of both proteins was mainly cytoplasmic, although some positive nuclei were also seen. For BubR1, cytoplasmic staining in tumor cells was scored on the following three-point scale: IHC score 0, no staining (Figure 1A); 1+, weak (Figure 1B); 2+, moderate (Figure 1C); 3+, strong expression, comparable to that of cells in the germinal centers of normal tonsil (Figure 1D). Tumors with 0 and 1+ staining intensity were considered BubR1 negative, and tumors with 2+ and 3+ staining were considered BubR1 positive [21]. For SNCG, positive cases were defined by the presence of any intensity of intracellular staining with red/brown color in breast cancer cells, since it is not expressed in normal or benign breast tissues (Figure 2B). Negative cases were defined by the absence of specific intracellular staining as seen in negative controls (Figure 2A).
Figure 1.
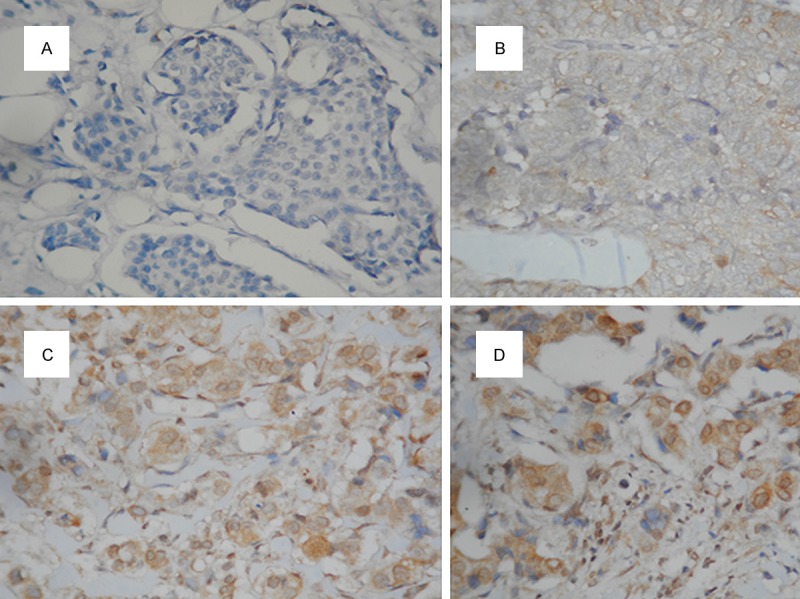
Stained by immunohistochemistry using the primary antibody againts BubR1 (×200); A. No staining; B. Weak (1+); C. Moderate (2+); D. Strong (3+) expression.
Figure 2.
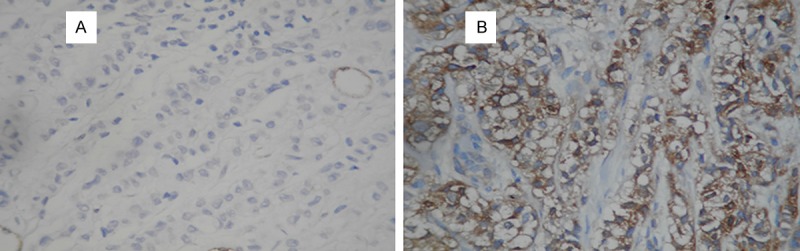
Stained by immunohistochemistry using the primary antibody againts SNCG (×200); A. Negative; B. Positive expression.
Statistical analysis
Each clinicopathological variable was compared between the BubR1 and SNCG-positive and -negative expression groups, and evaluated with χ2 test. Overall survival (OS) time was calculated using the Kaplan-Meier method as the duration from the date of diagnosis to the date of death or last control. Diferences in survival among the groups were compared using the log-rank test. P < 0.05 (two-tailed) was considered statistically significant. Statistical analysis was performed using SPSS, version 15.
Results
The BubR1 and SNCG expression in breast cancer and their relationship to the clinic effectiveness of taxanes
21 tumors (38%) and 34 tumors (62%) showed positive expression for BubR1 and SNCG, respectively. 40% of all patients (22/55) were resistant to taxanes. 76% of BubR1 positive tumors (16/21) had taxane responsive disease, compared with 50% of BubR1 negative tumors (17/34) (P=0.05). Despite statistically borderline correlation, tumors with high BubR1 expression were more sensitive the taxane than those low expression. In contrast, 53% of SNCG positive (18/34) tumors was resistant to taxane, compared with 19% of SNCG negative tomors (4/21) (P=0.01). There was significan differance between the SNCG negative and positive group with respect to clinic effectiveness of taxanes. The association between the response to taxane and the expressions of these proteins is summarized in Tables 1 and 2.
Table 1.
Associations between clinicopathological variables and the expressions of BubR1
| Variable | n | BubR1 expression | P | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Responder | 33 | 16 | 17 | 0.05* |
| Non responder | 22 | 5 | 17 | |
| Triple negative | 13 | 8 | 5 | 0.04** |
| Other subtypes | 42 | 13 | 29 | |
| ER positive | 35 | 12 | 23 | 0.3 |
| ER negative | 19 | 9 | 10 | |
| HER2 positive | 11 | 3 | 8 | 0.4 |
| HER2 negative | 43 | 18 | 25 | |
| Grade 1-2 | 32 | 20 | 12 | 0.7 |
| Grade 3 | 20 | 12 | 8 | |
P=0.05;
P < 0.05.
Table 2.
Associations between clinicopathological variables and the expressions of SNCG
| Variable | n | SNCG expression | P | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Responder | 33 | 16 | 17 | 0.01* |
| Non responder | 22 | 18 | 4 | |
| Triple negative | 13 | 8 | 5 | 0.9 |
| Other subtypes | 42 | 26 | 16 | |
| ER positive | 35 | 20 | 15 | 0.4 |
| ER negative | 19 | 13 | 6 | |
| HER2 positive | 11 | 7 | 4 | 0.8 |
| HER2 negative | 43 | 26 | 17 | |
| Grade 1-2 | 32 | 18 | 14 | 0.7 |
| Grade 3 | 20 | 14 | 6 | |
P< 0.05.
Association of BubR1 and SNCG expression with clinical parameters
Three tumors whose diagnosis are available only from metastatic sites were not evaluated for histologic grade. There was no significan association between clinical parameters such as tumor grade, ER positivity, HER2 status and expression levels of BubR1 and SNCG proteins. However, 61% of triple negative tumors (8/13) were BubR1 positive, compared with 31% of other molecular subtypes (13/42) (P=0.04). There was significant association between triple negative tumors and level of BubR1 expression (Tables 1 and 2).
Association of BubR1 expresssion with survival
After 15 patients with short term follow up taking neoadjuvant treatment were exluded, the remaining 40 metastatic patients were evaluated for OS. 18 of 40 patients died in the duration from the date of diagnosis to last control. The longest survival 117 mounts. Median survival time in patient with positive and negative BubR1 expression was 36 and 71 mounts, respectively (Log-rank test, P=0.2, Kaplan Meier curve; Figure 3A). Median survival time in patient with positive and negative SNCG expression was 32 and 58 mounts, respectively (Log-rank test, P=0.3, Kaplan Meier curve; Figure 3B). There was no significant association between the BubR1 and SNCG-positive and -negative groups with respect to OS.
Figure 3.
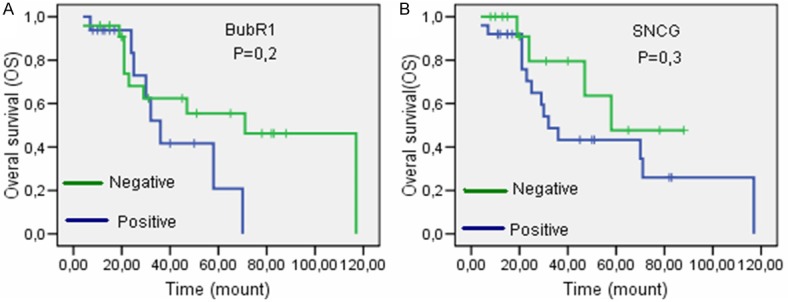
Overall survival in (A) BubR1 and (B) SNCG positive and negative patients. Despite no significant correlation, patients with low BubR1 and SNCG expression had a longer survival time.
Discussion
The association of SAC proteins with response to taxanes and clinical significance of their overexpression have been recently investigated [22-24]. In this study, we focused on whether BubR1 and SNCG expressions in breast cancer tissues were predictive marker of taxane sensitivity. The contradictory results had been reported for BubR1 in various studies performed in cancer cell lines. While two study reported that low level expression of BubR1 was associated with increased sensitivity to taxanes [22,25], conversely, other two study reported that low BubR1 expression was associated with decreased sensitivity to taxanes [24,26]. However, so far there have been no clinical study performed to confirm these preclinical observations except for our study in prostate cancer. Previously, we reported that high BubR1 expression was not associated with respons to docataxel, but significantly associated with OS in patients with prostate cancer [19]. Here we showed that tumors with high BubR1 expression were more sensitive to taxane than those low expression (borderline significance P=0.05).
BubR1 was reported to be upregulated in various cancers suck as lung, breast, colon, esophagus, stomach, kidney, bladder, ovary, thyroid and liver [27-34]. In a number of these studies, its overexpression has been related to chromosomal instability, DNA aneuploidy, more advanced pathologic stage and a higher histologic grade. In our study, 38% of patients with breast cancer had BubR1 positive tumors. This proportion was slighty greater compared with those of previous two reports in breast cancer (25% and 32%) [16,21]. There were no correlation with tumor grade, ER positivity and HER2 status. Hovever, triple negative tumors had significantly more high BubR1 expression than those other molecular subtypes.
In a current study Kung et al demostrated the elevated CENP-E expression in the basal like molecular subtype relative to other subtypes and reported that CENP-E inhibition by PF-2771 selectively inhibits proliferation of basal breast cancer cell lines [35]. BubR1 kinase activity is essential the mitotic checkpoint and is directly stimulated by CENP-E binding to it [36]. Considering that the interaction between CENP-E and BubR1 is curicial for correct mitotic checkpoint fonction, our findings have supported that like CENP-E, BubR1 may be also likeley to a important target for triple negative breast cancer. Maciejczyk et al. analyzed the expression of BubR1 in 98 stage II breast cancer patients with a median follow-up of 15 years. They reported that Elevated BubR1 expression was associated with precense of metastasis to lympf node and poor survival in early stage breast cancer patients but without any asssociation of BubR1 with the histological tumor grade, estrogen ve progesterone receptor positivity, HER2 status and tumor stage [18]. Du et al reported that high BubR1 expression was associated with high Ki67 labeling index and high histological grade [37].
There are several contradictory results between high BubR1 expression and survival in literature. Ovarian and breast cancer studies showed that patients with high BubR1 expression had significantly shorter recurrence-free survival (RFS) rates [17,18]. Conversely, patients with low BubR1 expression showed shorter survival in colorectal cancer [20], and oral squamose cell cancer study showed no correlation between BubR1 and survival [21]. We found that despit no statisticaly significant corelation, patients with low BubR1 expression showed longer overall survival period, compared with those of high BubR1 expression. Our results related to triple negative tumors and survival may reflect some limitation due to the small size of the semple and presence of censored data.
SNCG was first named Breast cancer-specific gene 1 (BCSG1) as it is highly expressed in advanced breast cancer. Then, its overexpression was showed in many other solid tumors including, gastric, lung, pancreatic, colon and ovarian cancer [38-40]. At the cellular level, SNCG increases metastasis and promotes genetic instability. At the molecular level, SNCG functions like a heat-shock protein (Hsp)-based multiprotein chaperone complex for stimulation of ER signalling. It increases the ligand-binding capacity of ER [41]. Moreover, SNCG protects the function of HER2 by preventing disruption of Hsp90 [42]. Wu et al confirmed the preclinical observation mentioned above by showing that patients whose tumors expressed SNCG had a significantly shorter DFS and a high probability of death when compared with those whose tumors did not express SNCG [15]. Moreover, they reported that there was no significant correlation between the BubR1 expression and clinicopathologic parameters including ER, PR and HER2 status except for lenf nodu involvement and stage. In contrast, Martin et al reported that high SNCG expression was associated with tumour grade but not with survival of patients with breast cancer [11].
At the cellular level, a number of study showed that overexpressed SNCG resulted in resistance to microtubilising drugs [12,43-45]. However, there is no clinic study performed to confirm these preclinic observations. In our study we confirmed the findings at the cellular level by showing the strong association between the high SNCG expression and the resistanse to taxane (P=0.01). But we did not find any association with clinicopathologic paremeters includin tumor grade, ER, HER2, molecular subtypes and survival. Our results related to clinic parameters and survival may reflect some limitations related to the small size of the sample, heterogeneity in the distribution of cases for ER, PR, HER2 and molecular subtypes, and to the presence of censored data.
In summary, BubR1 and SNCG was overexpressed in about 38% and 62% of patients with breast cancer, respectively. Low BubR1 and high SNCG espression were associated with decreased taxane sensitivity. Triple negative tumors showed more high BubR1 expression than other molecular subtypes. Despit no ststistical significance, patients with high BubR1 and SNCG expression tended to have shorter overall survival period. In this clinicopathological study, our findings confirmed the preclinical results that low BubR1 and high SNCG expression was associaated with decreased taxane sensitivity. Despite the relatively limited number of cases, our data imply that both BubR1 and SNCG may be promising predictive marker rather than prognostic marker in patients with breast cancer.
Disclosure of conflict of interest
None.
References
- 1.Autier P, Boniol M, La Vecchia C, Vatten L, Gavin A, Héry C, Heanue M. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341:3620. doi: 10.1136/bmj.c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paridaens R, Biganzoli R, Bruning P, Klijn JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A, Sylvester R, Awada A, Wildiers J, Piccart M. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J. Clin. Oncol. 2000;18:724–33. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- 3.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemo resistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Rieder C, Cole R, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha GH, Baek KH, Kim HS, Jeong SJ, Kim CM, McKeon F, Lee CW. p53 Activation in Response to Mitotic Spindle Damage Requires Signaling via BubR1-Mediated Phosphorylation. Cancer Res. 2007;67:7155–64. doi: 10.1158/0008-5472.CAN-06-3392. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Shou C, Meng L, Jiang B, Dong B, Yao L, Xie Y, Zhang J, Chen Y, Budman DR, Shi YE. Neuronal protein synuclein gamma predicts poor clinical outcome in breast cancer. Int J Cancer. 2007;121:1296–305. doi: 10.1002/ijc.22763. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C, Wang L, Zhao W, Jiang JD, Liu J. Loss of epigenetic control of synuclein-gamma gene as a molecular indicator of metastasis in a wide range of human cancers. Cancer Res. 2005;65:7635–43. doi: 10.1158/0008-5472.CAN-05-1089. [DOI] [PubMed] [Google Scholar]
- 9.Hibi T, Mori T, Fukuma M, Yamazaki K, Hashiguchi A, Yamada T, Tanabe M, Aiura K, Kawakami T, Ogiwara A, Kosuge T, Kitajima M, Kitagawa Y, Sakamoto M. Synuclein-gamma is closely involved in perineural invasion and distant metastasis in mouse models and is a novel prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:2864–71. doi: 10.1158/1078-0432.CCR-08-2946. [DOI] [PubMed] [Google Scholar]
- 10.Fung KM, Rorke LB, Giasson B, Lee VM, Trojanowski JQ. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106:167–75. doi: 10.1007/s00401-003-0718-x. [DOI] [PubMed] [Google Scholar]
- 11.Martin TA, Gomez K, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Expression of breast cancer specific gene-1 (BCSG-1/gamma-synuclein) is associated with tumour grade but not with clinical outcome of patients with breast cancer. Oncol Rep. 2006;16:207–12. [PubMed] [Google Scholar]
- 12.Miao S, Wu K, Zhang B. Synuclein γ compromises spindle assembly checkpoint and renders resistance to antimicrotubule drugs. Mol Cancer Ther. 2014;13:699–713. doi: 10.1158/1535-7163.MCT-13-0671. [DOI] [PubMed] [Google Scholar]
- 13.Munro A, Cameron D, Thomas J, Twelves C, Bartlett J. BUBR1 and MAD2: novel markers for predicting benefit from adjuvant anthracyclines? abstracts: thirty-second annual ctrc-aacr san antonio breast cancer symposium 2009; San Antonio. J Cancer Res. 2009;69:2124. [Google Scholar]
- 14.Wan F, Dong L, Zhang F, Wang Y, Chen F, Ni S, Chen Y, Long J. Clinical study of the relationship between γ-synuclein and the response of neoadjuvant chemotherapy in breast cancer. J Int Med Res. 2013;41:743–53. doi: 10.1177/0300060513484434. [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Quan Z, Weng Z, Li F, Zhang Y, Yao X, Chen Y, Budman D, Goldberg ID, Shi YE. Expression of neuronal protein synuclein gamma gene as a novel marker for breast cancer prognosis. Breast Cancer Res Treat. 2007;101:259–67. doi: 10.1007/s10549-006-9296-7. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Shou C, Meng L Jiang B, Dong B, Yao L, Xie Y, Zhang J, Chen Y, Budman DR, Shi YE. Neuronal protein synucleinc γ predicts poor clinical outcome in breast cancer. Int J Cancer. 2007;121:1296–1305. doi: 10.1002/ijc.22763. [DOI] [PubMed] [Google Scholar]
- 17.Lee YK, Choi E, Kim MA, Park PG, Park NH, Lee H. BubR1 as a prognostic marker for recurrence-free survival rates in epithelial ovarian cancers. Br J Cancer. 2009;101:504–10. doi: 10.1038/sj.bjc.6605161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maciejczyk A, Szelachowska J, Czapiga B. Elevated bubr1 expression is associated with poor survival in early breast cancer patients: 15-year follow-up analysis. J Histochem Cytochem. 2013;61:330–9. doi: 10.1369/0022155413480148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirak Y, Sarsik B, Cakar B, Sen S, Simsir A, Uslu R. Predictive and prognostic values of Tau and BubR1 protein in prostate cancer their relationship to the gleason score. Med Oncol. 2013;30:526–7. doi: 10.1007/s12032-013-0526-7. [DOI] [PubMed] [Google Scholar]
- 20.Shichiri M, Yoshinaga K, Hisatomi H, Sugihara K, Hirata Y. Genetic and epigenetic inactivation of mitotic checkpoint genes hBUB1 and hBUBR1 and their relationship to survival. Cancer Res. 2002;62:13–7. [PubMed] [Google Scholar]
- 21.Rizzardi C, Torelli L, Barresi E, Schneider M, Canzonieri V, Biasotto M, Di Lenarda R, Melato M. Bubr1 expression in oral squamous cell carcinoma and its relationship to tumor stage and survival. Head Neck. 2011;33:727–33. doi: 10.1002/hed.21532. [DOI] [PubMed] [Google Scholar]
- 22.Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–8. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- 23.Fang Y, Liu T, Wang X, Yang YM, Deng H, Kunicki J, Traganos F, Darzynkiewicz Z, Lu L, Dai W. BubR1 is involved in regulation of DNA damage responses. Oncogene. 2006;25:3598–605. doi: 10.1038/sj.onc.1209392. [DOI] [PubMed] [Google Scholar]
- 24.Lee EA, Keutmann MK, Dowling ML, Harris E, Chan G, Kao GD. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther. 2004;3:661–9. [PubMed] [Google Scholar]
- 25.Fu Y, Ye D, Chen H, Lu W, Ye F, Xie X. Weakened spindle checkpoint with reduced BubR1 expression in paclitaxel-resistant ovarian carcinoma cell line SKOV3-TR30. Gynecol Oncol. 2007;105:66–73. doi: 10.1016/j.ygyno.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Mohri Y, Ohi M, Yokoe T. Mitotic checkpoint genes, hsMAD2 and BubR1, in oesophageal squamous cancer cells and their associationwith 5-fluorouracil and cisplatin-based radiochemotherapy. Clin Oncol (R Coll Radiol) 2008;20:639–46. doi: 10.1016/j.clon.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Seike M, Gemma A, Hosoya Y, Hosomi Y, Okano T, Kurimoto F, Uematsu K, Takenaka K, Yoshimura A, Shibuya M, Ui-Tei K, Kudoh S. The promoter region of the human BUBR1 gene and its expression analysis in lung cancer. Lung Cancer. 2002;38:229–234. doi: 10.1016/s0169-5002(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 28.Yuan B, Xu Y, Woo JH, Wang Y, Bae YK, Yoon DS, Wersto RP, Tully E, Wilsbach K, Gabrielson E. Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin Cancer Res. 2006;12:405–410. doi: 10.1158/1078-0432.CCR-05-0903. [DOI] [PubMed] [Google Scholar]
- 29.Burum-Auensen E, De Angelis PM, Schjølberg AR, Røislien J, Mjaland O, Clausen OP. Reduced level of the spindle checkpoint protein BUB1B associated with aneuploidy in colorectal cancers. Cell Prolif. 2008;41:645–659. doi: 10.1111/j.1365-2184.2008.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando K, Kakeji Y, Kitao H, Iimori M, Zhao Y, Yoshida R, Oki E, Yoshinaga K, Matumoto T, Morita M, Sakaguchi Y, Maehara Y. High expression of BUBR1 is one of the factors for inducing DNA aneuploidy and progression in gastric cancer. Cancer Sci. 2010;101:639–645. doi: 10.1111/j.1349-7006.2009.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto M, Vieira J, Ribeiro FR, Soares MJ, Henrique R, Oliveira J, Jerónimo C, Teixeira MR. Overexpression of the mitotic checkpoint genes BUB1 and BUBR1 is associated with genomic complexity in clear cell kidney carcinomas. Cell Oncol. 2008;30:389–395. doi: 10.3233/CLO-2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Matsuyama H, Chochi Y, Okuda M, Kawauchi S, Inoue R, Furuya T, Oga A, Naito K, Sasaki K. Overexpression of BUBR1 is associated with chromosomal instability in bladder cancer. Cancer Genet Cytogenet. 2007;174:42–47. doi: 10.1016/j.cancergencyto.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Wada N, Yoshida A, Miyagi Y, Yamamoto T, Nakayama H, Suganuma N, Matsuzu K, Masudo K, Hirakawa S, Rino Y, Masuda M, Imada T. Overexpression of the mitotic spindle assembly checkpoint genes hBUB1, hBUBR1 andhMAD2 in thyroid carcinomas with aggressive nature. Anticancer Res. 2008;28:139–144. [PubMed] [Google Scholar]
- 34.Liu AW, Cai J, Zhao XL, Xu AM, Fu HQ, Nian H, Zhang SH. The clinicopathological significance of BUBR1 overexpression in hepatocellular carcinoma. J Clin Pathol. 2009;62:1003–1008. doi: 10.1136/jcp.2009.066944. [DOI] [PubMed] [Google Scholar]
- 35.Kung PP, Martinez R, Zhu Z. Chemogenetic evaluation of the mitotic kinesin CENP-E reveals a critical role in triple-negative breast cancer. Mol Cancer Ther. 2014;13:2104–15. doi: 10.1158/1535-7163.MCT-14-0083-T. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Kim C, Ahmad S, Zhang J, Mao Y. CENP-E–dependent BubR1 autophosphorylation enhances chromosome alignment and the mitotic checkpoint. J Cell Biol. 2012;198:205–17. doi: 10.1083/jcb.201202152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du J, Du Q, Zhang Y, Sajdik C, Ruan Y, Tian XX, Fang WG. Expression of cell-cycle regulatory proteins BUBR1, MAD2, Aurora A, cyclin A and cyclin E in invasive ductal breast carcinomas. Histol Histopathol. 2011;26:761–8. doi: 10.14670/HH-26.761. [DOI] [PubMed] [Google Scholar]
- 38.Bruening W, Giasson BI, Klein-Szanto AJ, Lee VM, Trojanowski JQ, Godwin AK. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000;88:2154–63. [PubMed] [Google Scholar]
- 39.Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C, Wang L, Zhao W, Jiang JD, Liu J. Loss of epigenetic control of synuclein-gamma gene as a molecular indicator of metastasis in a wide range of human cancers. Cancer Res. 2005;65:7635–43. doi: 10.1158/0008-5472.CAN-05-1089. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Sclabas GM, Peng B, Hess KR, Abbruzzese JL, Evans DB, Chiao PJ. Overexpression of synuclein-gamma in pancreatic adenocarcinoma. Cancer. 2004;101:58–65. doi: 10.1002/cncr.20321. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Liu YE, Goldberg ID, Shi YE. Gamma synuclein, a novel heat-shock protein-associated chaperone, stimulates liganddependent estrogen receptor alpha signaling and mammary tumorigenesis. Cancer Res. 2004;64:4539–4546. doi: 10.1158/0008-5472.CAN-03-3650. [DOI] [PubMed] [Google Scholar]
- 42.Shao Y, Wang B, Shi D, Miao S, Manivel P, Krishna R, Chen Y, Eric Shi Y. Synuclein gamma protects HER2 and renders resistance to Hsp90 disruption. Mol Oncol. 2014;8:1521–31. doi: 10.1016/j.molonc.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng SX, Zhang S, Zhang H, Song DQ, Wang YP, Li YH, You XF, Wang YM, Jiang JD. Overexpression of synuclein-gamma confers resistance to antimicrotubule drugs against human hepatoma cells. Yao Xue Xue Bao. 2010;45:724–9. [PubMed] [Google Scholar]
- 44.Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J Biol Chem. 2002;277:35050–60. doi: 10.1074/jbc.M201650200. [DOI] [PubMed] [Google Scholar]
- 45.Swanton C, Tomlinson I, Downward J. Chromosomal instability, colorectal cancer and taxane resistance. Cell Cycle. 2006;5:818–23. doi: 10.4161/cc.5.8.2682. [DOI] [PubMed] [Google Scholar]


