Abstract
Lung cancer is a common malignant tumor claiming the highest fatality worldwide for a long period of time. Unfortunately, most of the current treatment methods are still based on the characteristics of cancer cells in the primary lesion and the prognosis is often much poorer in patients with metastatic cancers. Amygdalin, a natural product of glycosides and lots of evidence shows that amygdalin can inhibit the proliferation of some kinds of cancer cells. In this study, we first obtained the highly metastatic NSCLC cell lines H1299/M and PA/M and further treated these cells with amygdalin. We found that the in vitro proliferability of H1299/M and PA/M was inhibited, but such inhibition required higher concentration of amygdalin. When lower concentration of amygdalin was used for the experiments, we observed that the in vitro invasive and migration capacities of H1299/M and PA/M were significantly inhibited. These results strongly suggested that amygdalin was likely to have anti-metastatic NSCLC effect. This study offers information of the role of amygdalin that may be useful as a therapeutic target in lung tumors.
Keywords: Lung cancer, amygdalin, invasion
Introduction
Metastasis is the main cause of mortality in patients with cancer, accounting for approximately 90% of the total number of deaths [12,20]. Lung cancer is a common malignant tumor claiming the highest fatality worldwide for a long period of time [13]. Lung cancer cells often metastases to the brain, bones, liver, adrenal glands and other organs [1]. These organs are important organs of the body in which cloning and growth of the cancer cells cause great harms and threaten human life. Currently, metastatic cancers are still treated with surgery, chemotherapy, radiation therapy, targeted therapy, biological therapy, combined therapies and other means, but these methods are difficult to effectively treat metastatic tumors. The main reason is that these metastatic cancer cells not only possess biological characteristics different from the cancer cells in the primary lesions but also manifest higher malignancy. Unfortunately, most of the current treatment methods are still based on the characteristics of cancer cells in the primary lesion [6]. Therefore, the prognosis is often much poorer in patients with metastatic cancers than those without metastasis. It is highly necessary to develop drugs that can inhibit and target at metastatic cancers.
Amygdalin, a natural product of glycosides, was originally isolated from the seeds of sweet almond. Subsequently, this natural product is also found in almonds, blackberries, apples and other plants [11]. In the 1970s, amygdalin was one of the most common non-conventional anti-cancer drugs in Western countries. According to the statistics, as of 1978, there are totally more than 70,000 patients with cancers in the United States have been treated with amygdalin [5]. However, the anti-tumor efficacy and mechanisms of action of amygdalin have remained controversial since then. Some recent studies provide positive evidence to resolve the controversy. The evidence shows that amygdalin can inhibit the proliferation of liver cancer [19], cervical cancer [3], bladder cancer [9] and other cancer cells and/or promote their apoptosis. Makarević et al [10] also found that amygdalin could inhibit the in vitro adhesion and invasion of bladder cancer cells. Accordingly, this study was designed to investigate the inhibition of amygdalin on metastatic non-small cell lung cancer (NSCLC) and preliminarily explores its molecular mechanisms of action. These studies can not only help us better understand the anti-tumor effects and mechanisms of amygdalin but also provide references for chemical modification of amygdalin and improvement of its anti-tumor activity.
Materials and methods
Cell culture and induction of highly metastatic cell line
The cells were maintained in RPMI-1640 containing 10% fetal bovine serum and cultured in an incubator under 37°C, 5% CO2 and saturated humidity condition. The cells were digested with 0.25% trypsin-EDTA for passaging. Cells in logarithmic growth phase were used in all experiments. In order to screen highly metastatic gastric cancer cell lines, H1299 and PA were cultured in the upper chamber of Transwell (Corning) coated with Matrigel (BD Biosciences). In 24 h, the cells migrating to the lower chamber were collected for cloning culture. The above procedures were repeated 10 times to screen out the highly metastatic cell lines H1299/M and PA/M.
MTS cell proliferation assay
Cells in logarithmic growth phase, 5×104 cells/ml, were seeded in 96-well microplates, 100 μ l/well, and cultured overnight to allow cell adhesion. Different concentrations of amygdalin were added to continue the culture for 48 h. After removing the medium, MTS was added in accordance with the instructions to continue the culture for 4 h. Finally, the OD was determined at 490 nm wavelength with a microplate reader to represent the number of cells. The inhibition rate of the drug on cells was calculated using the formula as follows: inhibition rate = (1- experimental group OD/control group OD)×100%.
In vitro invasion and migration assay
The in vitro invasibility was assessed using the CultreCoat® 96-Well BME-Coated Cell Invasion Optimization Assay Kit supplied by R & D Systems. The cells starving in serum-free medium for 16 h were seeded at 25000 cells/well in the upper chamber of Transwell, in the meantime, 2.5 and 5 mg/ml amygdalin was added to continue the culture for 48 h. Then, the number of invasion cells was analyzed in accordance with the kit instructions. The wound-healing assay was used to assess the in vitro migratability of cancer cells. Specifically, the cells were cultured in 6-well plate until single-layer confluence. After starving in serum-free medium overnight, the 200 μ L pipette tip was used to make scorings in the cell layer. Then, 2.5 and 5 mg/ml amygdalin was added to continue the culture for 48 h. The distance between the scorings was observed and measured.
Western blotting assay
Cells were treated with 2.5 and 5 mg/ml amygdalin for 48 h, collected and lysed to extract the proteins. The extracted proteins were separated in 12% SDS-PAGE and transferred onto PVDF membrane. The target proteins were determined with different antibodies (4°C, overnight). After washing off the primary antibodies, HRP-conjugated secondary antibody was added to incubate for 1 h. After several washes, ECL kit was used to develop the immunoreactive bands. Using β-actin as internal control, the intracellular expression level of MMP-2/9, CD44, integrin β1, integrin β4, ILK, FAK, p-FAK, E-cadherin and β-catenin, and the phosphorylation level of Akt and RICTOR were determined.
Real-time PCR assay
Cells were treated with 2.5 and 5 mg/ml amygdalin for 48 h. Then total RNA was extracted from various groups using Trizol method. Real-Time PCR kit was used to carry out reverse transcription; the obtained cDNA was used to determine the mRNA level of MMP-2/9, CD44, integrin β1, integrin β4, ILK, FAK, E-cadherin and β-catenin. Integrin β1 upstream primer sequence: 5’-CAA GCA GGG CCA AAT TGT GG -3’, downstream primer sequence: 5’-TGT CAT CTG GAG GGC AAC CC-3’; integrin β4 upstream primer sequence: 5’-GGC GCT GCA ACA CCC AGG CGG A-3’, downstream primer sequence: 5’-CTC TCC AGT GGC TCA AAC ACC T-3’; ILK upstream primer sequence: 5’-TTT GCA GTG CTT CTG TGG GAA-3’, downstream primer sequence: 5’-CTA CTT GTC CTG CAT CTT CTC-3’; E-cadherin upstream primer sequence: 5’-GTC ATC CAA CGG GAA TGC A-3’, downstream primer sequence: 5’-TGA TCG GTT ACC GTG ATC AAA A-3’; FAK upstream primer sequence: 5’-TGC AAG TAA GGA AAT ACA GTT TGG-3’, downstream primer sequence: 5’-CCA CAT ACA CAC ACC AAA CAT CAT CCA-3’; β-catenin upstream primer sequence: 5’-CCT ATG CAG GGG TGG TCA AC-3’, downstream primer sequence: 5’-CGA CCT GGA AAA CGC CAT CA-3’; MMP2 upstream primer sequence: 5’-TAC TGA GTG GCC GTG TTT GC-3’, downstream primer sequence: 5’-AGG GAG CAG AGA TTC GGA CTT-3’; MMP9 upstream primer sequence: 5’-GGG CTT AGA TCA TTC CTC AGT G-3’, downstream primer sequence: 5’-GCC ATT CAC GTC GTC CTT AT-3’; CD44 upstream primer sequence: 5’-ACC CCA ACT CCA TCT GTG C-3’, downstream primer sequence: 5’-TTC TGG ACA TAG CGG GTG-3’; GAPDH upstream primer sequence: 5’-CTTAGATTTGGTCGTATTGG-3’, downstream primer sequence 5’-GAAGATGGT-GATGGGATT-3’.
Statistical analysis
The experimental data were expressed as mean ± standard deviation. SPSS13.0 software was used for analysis. One-way ANOVA was used for comparison. P<0.05 indicated that the difference had statistical significance.
Results
Potential of proliferation, migration, invasion of H1299, H1299/M, PA and PA/M in vitro
After the in vitro screening, the highly metastatic cell lines H1299/M and PA/M were successfully obtained. As shown in Figure 1A-E, both the in vitro invasion assay and wound-healing assay showed that the invasibility of H1299/M and PA/M were significantly higher than the parent cell lines H1299 and PA.MTS cell proliferation assay showed that high-concentration amygdalin inhibited the in vitro proliferation of H1299/M and PA/M cells; its IC50 was 12.2 mg/ml. The inhibition rate of 2.5 and 5 mg/ml amygdalin on cell proliferation was 15.6% and 25.1%, respectively, exhibiting a weaker inhibitory effect (Figure 1F). Therefore, these two drug concentrations were selected for the subsequent invasion and migration experiments.
Figure 1.
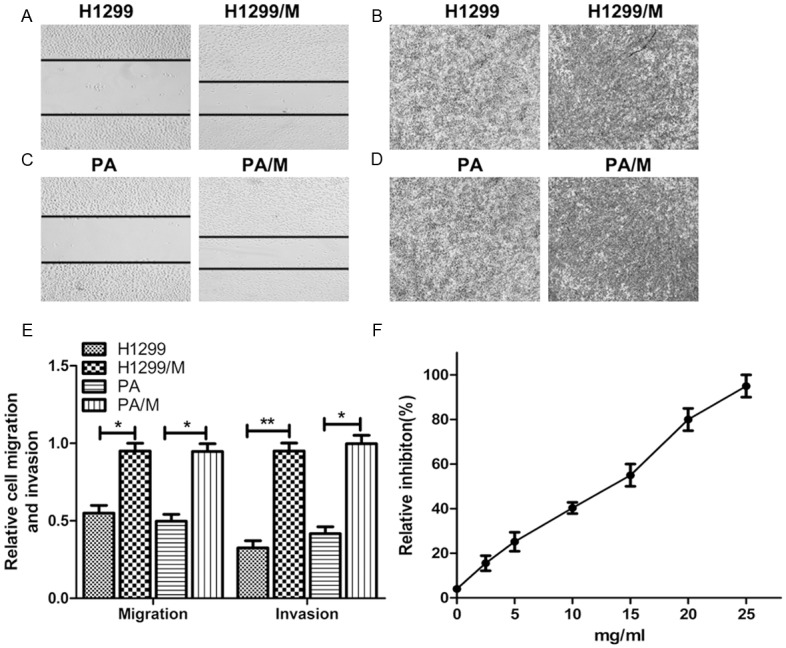
Enhanced metastatic ability of H1299/M and PA/M cell lines. A. Representative images of wound-healing assay for H1299/M cell migration. B. Representative images of transwell assay for H1299/M cell invasion. C. Representative images of wound-healing assay for PA/M cell migration. D. Representative images of transwell assay for PA/M cell invasion. E. The relative metastasis of H1299/M and PA/M. *P<0.05; **P<0.01, compared with the control. F. Effect of amygdalin on the proliferation of H1299/M cells as assessed by MTT assay.
Low-concentration amygdalin inhibits the in vitro invasion and migration
As described above, the inhibitor concentrations used for the in vitro invasion and migration assays were 2.5 and 5 mg/ml, respectively. Within the experimental time, selecting these drug concentrations would not significantly inhibited cell growth. In 48 h, the number of invasive cells in inhibitor-treated group significantly reduced. The wound-healing assay showed that the gap between the scorings was smaller in inhibitor-treated group than in control group in 48 h (Figure 2).
Figure 2.
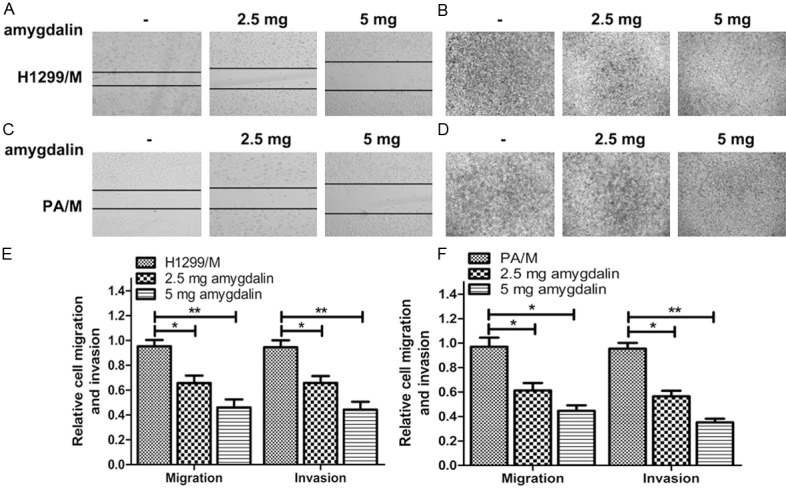
Effects of amygdalin on the metastatic capacities of H1299/M and PA/M cell lines. A. Representative wound-healing assay images for H1299/M cell migration pretreatment with different amygdalin concentration. B. Representative transwell assay images for H1299/M cell invasion pretreatment with different amygdalin concentration. C. Representative wound-healing assay images for PA/M cell migration pretreatment with different amygdalin concentration. D. Representative transwell assay images for PA/M cell invasion pretreatment with different amygdalin concentration. E/F. The relative cells invasion and migration of H1299/M and PA/M cells treatment with different amygdalin concentration. *P<0.05; **P<0.01, compared with the control.
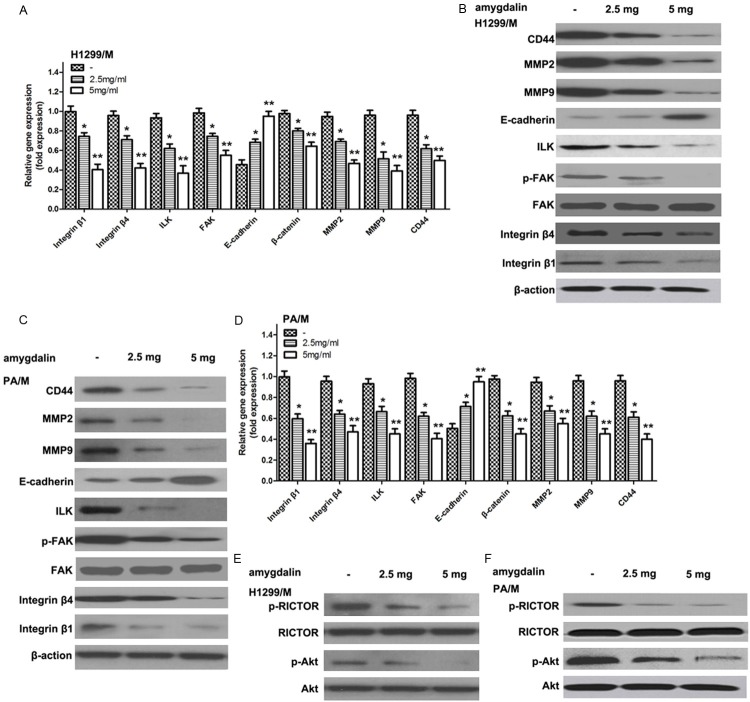
Low-concentration amygdalin down-regulates the expression of cell integrin β1, integrin β4, ILK, FAK, p-FAK and β-catenin while up-regulates the expression of E-cadherin
After the cells have been treated with 5 mg/ml amygdalin for 48 h, the relevant protein expression was determined. WB assay result showed that the expression levels of integrin β1, integrin β4, ILK, FAK, and β-catenin, which were proteins with metastasis-promoting action, in highly metastatic cancer cells significantly decreased after inhibitor treatment when compared with control group. Among them, the down-regulation of amygdalin affected FAK phosphorylation (p-FAK) more than FAK expression. In contrast, the expression of E-cadherin protein capable of inhibiting metastasis was significantly up-regulated. See details in Figure 3B and 3C. Real-time-PCR study showed that the regulation of all these protein expressions occurred at transcriptional level as shown in Figure 3A and 3D.
Figure 3.
Effects of amygdalin on cell signaling pathway in H1299/M and PA/M cell lines. A/D. The mRNA expressions of integrin β1, integrin β4, ILK, FAK, β-catenin, MMP2, MMP9, CD44 and E-cadherin in H1299/M and PA/M cells pretreatment with amygdalin were analyzed by real-time PCR. Real-time PCR reactions were performed on templates of cDNA from H1299/M and PA/M cell lines using a set of primers as in Materials and methods. *P<0.05; ** P<0.01, compared with the control. B/C. The protein expressions of integrin β1, integrin β4, ILK, FAK, p-FAK, MMP2, MMP9, CD44 and E-cadherin in H1299/M and PA/M cells pretreatment with amygdalin were analyzed by Western blot. E/F. The p-Akt and p-RICTOR protein expressions in H1299/M and PA/M cells were analyzed by Western blot.
Low-concentration amygdalin inhibits the phosphorylation Akt and RICTOR
Western blot results showed that amygdalin had no significant impact on Akt and RICTOR expression. The determination of Akt and RICTOR phosphorylation level showed that amygdalin significantly reduced the phosphorylation level of these two proteins in highly metastatic cells, suggesting that amygdalin was able to regulate the activity of Akt and RICTOR signaling pathways (Figure 3E and 3F).
Discussion
Although studies have shown that amygdalin has anti-tumor proliferation and metastasis effects, studies of the effect of amygdalin on lung cancer invasion and metastasis are few. In this study, we first obtained the highly metastatic NSCLC cell lines H1299/M and PA/M and further treated these cells with amygdalin. We found that the in vitro proliferability of H1299/M and PA/M was inhibited, but such inhibition required higher concentration of amygdalin. When lower concentration of amygdalin was used for the experiments, we observed that the in vitro invasibility (infiltrating Matrigel) and migrationality of H1299/M and PA/M were significantly inhibited. These results strongly suggested that amygdalin was likely to have anti-metastatic NSCLC effect.
The interaction between tumors and extracellular matrix is a key part for successful metastasis. Under normal circumstances, the cells interact with the extracellular matrix or the surrounding cells through membrane-associated proteins such as integrin and E-cadherin to immobilize the cells in a particular environment. Such interaction is a dynamic process to ensure cell movement in response to the normal physiological needs, but such movement is strictly controlled under normal physiological conditions. In contrast, the tumor cells often spontaneously acquire the moving ability and break away from normal physiological regulation. Integrin is a protein containing multi-subunits including α and β-subunits, also known as heterodimer protein. Wherein, the two subtypes, namely β1 and β4, are often related to tumor metastasis [4,14,16] . We observed in Western blotting and real-time-PCR assays that amygdalin was able to down-regulate β1 and β4 expression level in H1299/M and PA/M cells, which was consistent with the results that amygdalin inhibit in vitro metastasis of H1299/M and PA/M cells. E-cadherin is another type of protein mediating intercellular interactions. In contrast with integrin β1 and β4, E-cadherin plays a negative role in tumor cell migration. Because the loss of E-cadherin function causes the cancer cells to break free from constrains of the surrounding cells, making it easier to penetrate through the basal membrane and invade the surrounding tissues. Clinically, the loss of its function or under-expression is generally related to cancer progression and metastasis [15]. Both Western blotting and real-time-PCR assay results showed that the intracellular E-cadherin expression level increased after treatment with amygdalin. In addition, the functions of integrin and E-cadherin should not be simply regarded as a protein used to connect the extracellular matrix with the surround cells because they also transmit signals into the cells. Integrin β1 and β4 can activate FAK to further release β-catenin; the latter can migrate into the nucleus to mediate the expression of tumor growth and migration-related proteins [18,20]. Integrin β1 can activate ILK to initiate the downstream signaling pathways such as Akt-mTOR pathway and mediate cell proliferation, adhesion and metastasis functions [8,17]. Our study showed that ILK, FAK (especially p-FAK) and β-catenin levels decreased significantly with the down-regulation of integrin β1 and β4 expression. RICTOR as a subunit of mTOR plays an important role in the Akt-mTOR signaling pathway; its phosphorylation level is positively regulated by Akt [2,7]. After having observed that amygdalin was able to inhibit ILK expression level, we further found that the phosphorylation level of Akt and RICTOR significantly decreased although their expression levels were not significantly affected. These results further confirmed that amygdalin was able to inhibit tumor cell metastasis by regulating integrin expression and its downstream signaling pathways.
In summary, this study showed that amygdalin inhibited the in vitro proliferation, invasion and migration of NSCLC. The mechanisms may be related to the more extensive regulation of gene expression by regulating integrin and E-cadherin expression, and the downstream key signaling pathways such as Akt-mTOR.
Disclosure of conflict of interest
None.
References
- 1.Cai Y, Wang JY, Liu H. Clinical observation of whole brain radiotherapy concomitant with targeted therapy for brain metastasis in non-small cell lung cancer patients with chemotherapy failure. Asian Pac J Cancer Prev. 2013;14:5699–5703. doi: 10.7314/apjcp.2013.14.10.5699. [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Ma J, Wang F, Hu J, Cui A, Wei C, Yang Q, Li F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol. 2013;35:43–51. doi: 10.3109/08923973.2012.738688. [DOI] [PubMed] [Google Scholar]
- 4.Hashiba K, Sano M, Nio-Kobayashi J, Hojo T, Skarzynski DJ, Okuda K. Galectin-3 contributes to luteolysis by binding to Beta 1 integrin in the bovine corpus luteum. Biol Reprod. 2014;91:2. doi: 10.1095/biolreprod.114.119057. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor S. Safety of studies analysing clinical benefit of amygdalin. Immunopharmacol Immunotoxicol. 2014;36:87. doi: 10.3109/08923973.2013.861846. [DOI] [PubMed] [Google Scholar]
- 6.Ku MJ, Park JW, Ryu BJ, Son YJ, Kim SH, Lee SY. CK2 inhibitor CX4945 induces sequential inactivation of proteins in the signaling pathways related with cell migration and suppresses metastasis of A549 human lung cancer cells. Bioorg Med Chem Lett. 2013;23:5609–5613. doi: 10.1016/j.bmcl.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Lamming DW, Mihaylova MM, Katajisto P, Baar EL, Yilmaz OH, Hutchins A, Gultekin Y, Gaither R, Sabatini DM. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014;13:911–917. doi: 10.1111/acel.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JC, Zhu HY, Chen TX, Zou LY, Wang XY, Zhao HC, Xu J. Roles of mTOR and p-mTOR in gastrointestinal stromal tumors. Asian Pac J Cancer Prev. 2013;10:5925–8. doi: 10.7314/apjcp.2013.14.10.5925. [DOI] [PubMed] [Google Scholar]
- 9.Makarevic J, Rutz J, Juengel E, Kaulfuss S, Reiter M, Tsaur I, Bartsch G, Haferkamp A, Blaheta RA. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS One. 2014;9:e105590. doi: 10.1371/journal.pone.0105590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makarevic J, Rutz J, Juengel E, Kaulfuss S, Tsaur I, Nelson K, Pfitzenmaier J, Haferkamp A, Blaheta RA. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS One. 2014;9:e110244. doi: 10.1371/journal.pone.0110244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirmiranpour H, Khaghani S, Zandieh A, Khalilzadeh OO, Gerayesh-Nejad S, Morteza A, Esteghamati A. Amygdalin inhibits angiogenesis in the cultured endothelial cells of diabetic rats. Indian J Pathol Microbiol. 2012;55:211–214. doi: 10.4103/0377-4929.97874. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki J, Hirota S, Abe T. Metastasis of lung cancer to the gastrointestinal tract, presenting with a volcano-like ulcerated mass. Dig Endosc. 2015;27:397–8. doi: 10.1111/den.12412. [DOI] [PubMed] [Google Scholar]
- 13.Na SS, Aldonza MB, Sung HJ, Kim YI, Son YS, Cho S, Cho JY. Stanniocalcin-2 (STC2): a potential lung cancer biomarker promotes lung cancer metastasis and progression. Biochim biophys Acta. 2015;1854:668–76. doi: 10.1016/j.bbapap.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Nunez MA, de Matos FR, Freitas Rde A, Galvao HC. Immunohistochemical study of integrin alpha(5)beta(1), fibronectin, and Bcl-2 in normal oral mucosa, inflammatory fibroepithelial hyperplasia, oral epithelial dysplasia, and oral squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2013;21:354–361. doi: 10.1097/PAI.0b013e318266c39d. [DOI] [PubMed] [Google Scholar]
- 15.Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu N, Lan F, Liu Y, Yan S, Dong X, Cai L. Prognostic values of osteopontin-c, E-cadherin and beta-catenin in breast cancer. Cancer Epidemiol. 2013;37:985–992. doi: 10.1016/j.canep.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Solano-Lopez G, Concha-Garzon MJ, Sanchez-Perez J, Hirako Y, Li X, Ishii N, Hashimoto T, Dauden E. Pure ocular mucous membrane pemphigoid reactive with both integrin beta-4 and BP180 C-terminal domain. Br J Dermatol. 2015;172:542–4. doi: 10.1111/bjd.13290. [DOI] [PubMed] [Google Scholar]
- 17.Watabe H, Furuhama T, Tani-Ishii N, Mikuni-Takagaki Y. Mechanotransduction activates alpha(5)beta(1) integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell Res. 2011;317:2642–2649. doi: 10.1016/j.yexcr.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Xia H, Seeman J, Hong J, Hergert P, Bodem V, Jessurun J, Smith K, Nho R, Kahm J, Gaillard P, Henke C. Low alpha(2)beta(1) integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the beta-catenin pathway. Am J Pathol. 2012;181:222–233. doi: 10.1016/j.ajpath.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Li X, Rong J. Amygdalin isolated from Semen Persicae (Tao Ren) extracts induces the expression of follistatin in HepG2 and C2C12 cell lines. Chin Med. 2014;9:23. doi: 10.1186/1749-8546-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Zhang L, Zhang GD, Wang HO, Liu MY, Jiang Y, Qi LS, Li Q, Yang P. Potential mechanisms of benzyl isothiocyanate suppression of invasion and angiogenesis by the U87MG human glioma cell line. Asian Pac J Cancer Prev. 2014;19:8225–8. doi: 10.7314/apjcp.2014.15.19.8225. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Zhu L, Lu L, Zhang L, Zhang G, Wang Q, Yang P. Role and mechanism of the alkylglycerone phosphate synthase in suppressing the invasion potential of human glioma and hepatic carcinoma cells in vitro. Oncol Rep. 2014;1:431–6. doi: 10.3892/or.2014.3189. [DOI] [PubMed] [Google Scholar]