Abstract
This study was to investigate the effects of intracerebral hemorrhage (ICH) and subsequent minimally invasive hematoma aspiration on the expression of apoptosis-related genes in rats. IV-collagenase was injected to the caudate nucleus of the rats to make ICH models. In the control group, 30 Sprague-Dawley (SD) rats were mock treated with saline instead of collagenase. Thirty SD rats with successful modeling were designated as the ICH group. Twenty-five SD rats with successful modeling and subsequent minimally invasive hematoma aspiration were designated as the therapy group. Expression of heat shock protein 70 (Hsp70), B-cell leukemia/lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) in the brain tissues was detected by immunohistochemical assays. The expression of Hsp70, Bcl-2 and Bax in the control group was very low, and significantly increased in the ICH group and the therapy group. At each indicated time point, Hsp70 expression in the therapy group was significantly lower than that of the ICH group, Bax expression in the therapy group was significantly lower than that of the ICH group and Bcl-2 expression in the therapy group was significantly higher than that of the ICH group. These results suggest that ICH led to increased expression of apoptosis-related genes in the brain tissues. Hematoma aspiration up-regulated ICH induced Bcl-2 expression while down-regulated ICH induced Hsp70 and Bax expression.
Keywords: Intracerebral hemorrhage, minimally invasive hematoma aspiration, Hsp70, Bcl-2, Bax
Introduction
Intracerebral hemorrhage (ICH) is a common disease of the nerve system with high incidence, mortality and morbidity rate. Secondary lesions such as neuron apoptosis could be the main cause of brain injuries after ICH [1]. Neuron apoptosis can be induced by the constituents of the hematoma and their degradation products such as the haemoglobin, bilirubin, ferric iron and carbon monoxide [2]. Reactive Oxygen Species (ROS) and lipid peroxide production after ICH can also lead to neuron apoptosis [3].
Nowadays, minimally invasive hematoma aspiration has aroused much attention. Aspiration treatment at the early stage of ICH could reduce the space-occupying effects and the direct chemical damage of the hematoma to the brain tissues, prevent the secondary cerebral edema and cerebral hernia and promote the neural functional recovery. ROS can lead to neuron apoptosis. Hematoma aspiration at 3 or 6 hours after ICH reduced ROS production and promoted ROS scavenge [4].
B-cell leukemia/lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) are two antagonistic factors and play important roles in apoptosis. Bax/Bax homodimers act as apoptosis inducers, while Bcl-2/Bax heterodimers act as apoptosis inhibitors [5,6]. Heat shock protein 70 (Hsp70) acts as the molecular chaperone [7] and shows protective effects on neurons [8-10]. Furthermore, it can interact with some apoptosis-related factors such as apoptotic protease activation factor-1 [11]. C-Jun N-terminal kinase (JNK) pathway might be one of the compartments inducing apoptosis in pulp cells, and Hsp70 might have an inhibitory role in the apoptosis of pulp cells during wound healing [12]. Hsp70 could also increase Bcl-2 expression and inhibit apoptosis [13,14]. Thus Hsp70 plays important roles in anti-apoptosis of neurons.
In this study, the expression kinetics of apoptosis-related genes in ICH rat models with or without subsequent minimally invasive hematoma aspiration were detected. ICH led to increased expression of apoptosis-related genes including Hsp70, Bax and Bcl-2 in the brain tissues of the rats. Hematoma aspiration up-regulated ICH induced Bcl-2 expression while down-regulated ICH induced Hsp70 and Bax expression.
Materials and methods
Reagents
Rabbit anti-Bax polyclonal antibody was purchased from Techgroup Biological Technology Co., Ltd. Rabbit anti-Bcl-2 and Hsp70 polyclonal antibodies and the Avidin/Biotin Complex (ABC) kit for immunohistochemical assays were purchased from Wuhan Boster Biological Engineering Co., Ltd.
Intracerebral hemorrhage
This study was approved by the Institutional Animal Care and Use Committee of Shandong University. The male Sprague-Dawley (SD) rats weighing 250 to 280 g were provided by Laboratory Animal Center of Shandong University of Traditional Chinese Medicine and kept under clean conditions. Thirty rats were in the control group, 30 rats were in the ICH group and 25 rats were in the therapy group.
For induction of hemorrhage, each rat was anesthetized with pentobarbital (0.1 ml/100g, intraperitoneal injection) and placed in a stereotactic frame (Rui Wode Life Science and Technology Co., Ltd, Shenzhen, China). Collagenase-induced ICH in rats was produced according to the reports with minor modification [15,16]. Through a hole drilled in the skull, a microinjector (high-pigeon Ltd, Shanghai, China) was introduced into the caudate nucleus (0.1 mm anterior to the anterior fontanelle, 3.0 mm lateral right to the sagittal suture, depth 5.5 mm below the surface of the skull), and 2 μl of saline containing 0.4 U IV-collagenase (Soledad Biological Technology Co., Ltd, Beijing, China) was infused over 15 minutes. After the infusion, the needle was left in the place for 10 minutes and then removed. The bone hole was sealed with bone wax, the scalp wound was sutured. In the control group, 2 μl of saline without collagenase was infused into the caudate nucleus.
Six hours after the surgery, the Bederson score was used to monitor the neurologic functions of the rats: score of 0, no observable deficit; score of 1, rats with forelimb flexion when their tails were raised; score of 2, decreased resistance to lateral push (and forelimb flexion) without circling; score of 3, rats showed the same behavior as in score of 2, with circling. The criteria for successful modeling included: 1) Bederson score of ≥ 2, and 2) with ICH in the basal ganglia but not in the ventricles or subarachnoid space confirmed by magnetic resonance imaging.
Minimally invasive hematoma aspiration
In the therapy group, 6 hours after ICH, the rats were anesthetized again and placed in the stereotactic frame. With the same stereotactic coordinates, urokinase (5 μl, 200 IU/μl, Livzon Pharmaceutical Factory, Zhuhai, China) was injected by microinjector into the hematoma over 5 minutes. The microinjector was left in the place for 30 minutes and then removed. Then aspiration was performed slowly using our homemade device. The bone hole was sealed with bone wax, the scalp wound was sutured, and the rats were placed back in the feeding room. The rats were single caged with free access to food and water.
Cardiac perfusion fixation
In the ICH group and control group, 5 rats were sacrificed for sample collection at 6, 12, 24, 48, 72 and 168 hours after the surgery respectively. In the therapy group, 5 rats were sacrificed for sample collection at 12, 24, 48, 72 and 168 hours after ICH respectively. The rats were anesthetized with pentobarbital (0.1 ml/100 g, intraperitoneal injection). Normal saline and 4% formaldehyde solution were administered to perform cardiac perfusion fixation. Then the rats were decapitated. The brain tissues of the rats were fixed in 4% paraformaldehyde solution for at least 24 hours. The tissues around the hematoma of the rats in the ICH group and the therapy group and the tissues of the corresponding areas of the caudate nucleus of the rats in the control group with the size of 2 mm × 2 mm × 2 mm were removed and followed by dehydration with gradient ethanol and embedding with paraffin. The sections of the brain tissues were obtained for immunohistochemical assays.
Immunohistochemistry
Immunohistochemical assays of Hsp70, Bcl-2 and Bax expression were performed using anti- Hsp70, Bcl-2 and Bax antibodies respectively according to the manufactures’ instructions. The sections were observed under microscopy. For each section, five fields were randomly taken under a magnification of 40 ×. The positive cells and negative cells were counted in each field. The positive rate of each field was the percentage of the ratio of the number of positive cells to the number of total cells (the sum of positive and negative cells). The positive rate of each tissue section was expressed as the mean of the positive rate of the five fields.
Statistical analysis
All data were processed using SPSS18.0 statistical package. The measurement data were presented as means ± standard deviation. The Student’s t-test was performed to determine statistical significance of the differences. P-values of less than 0.05 were considered statistically significant.
Results
Expression of Hsp70 in the brain tissues
To determine Hsp70 expression in the brain tissues of the rats, immunohistochemical assay was performed. In the control group, Hsp70 positively stained cells were rare (Figure 1A). In the ICH group and the therapy group, Hsp70 was broadly expressed in the cytoplasm of the cells which mainly located at the striatal region, cerebral cortex, subcortical region and hippocampus around hematoma (Figure 1B and 1C). The positive rates of Hsp70 expression were shown in Table 1. The expression of Hsp70 in the control group was significantly lower than that in the ICH group and the therapy group at each indicated time point (P < 0.05). In the ICH group, relatively weak Hsp70 expression could be detected at 6 hours after ICH. Seventy-two hours after ICH, Hsp70 expression was significantly higher than that of the other time points. One hundred and sixty-eight hours after ICH, Hsp70 expression was significantly reduced. We did not observe Hsp70 expression in the central region of hematoma. In the therapy group, Hsp70 expression could be detected at 12 hours and peaked at 72 hours after ICH, then decreased. At each indicated time point, Hsp70 expression in the therapy group was significantly lower than that of the ICH group (P < 0.05). These results indicate that ICH could lead to increased Hsp70 expression in the brain tissues of the rats and hematoma aspiration could down-regulate ICH induced Hsp70 expression.
Figure 1.
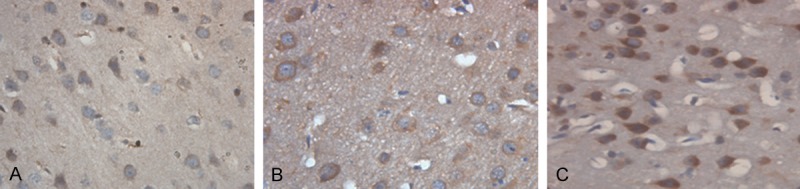
Hsp70 expression in the brain tissues of the rats (magnification × 400). Immunohistochemical assay was performed to detect the expression of Hsp70 in the brain tissues of the rats. Cells stained brown were Hsp70-positive. Representative immunohistochemical staining results of the control group (A), the ICH group (B) and the therapy group (C) 72 hours after ICH were shown. ICH, Intracerebral hemorrhage.
Table 1.
Positive rates of Hsp70 expression in brain tissues at indicated time points after ICH
| Group | n | 6 h | 12 h | 24 h | 48 h | 72 h | 168 h |
|---|---|---|---|---|---|---|---|
| Control group | 30 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.11 ± 0.01 | 0.05 ± 0.01 | 0.022 ± 0.01 | 0.02 ± 0.01 |
| ICH group | 30 | 0.034 ± 0.07* | 0.66 ± 0.07* | 0.77 ± 0.05* | 0.87 ± 0.04* | 0.93 ± 0.04* | 0.53 ± 0.04* |
| Therapy group | 25 | - | 0.50 ± 0.06*,# | 0.60 ± 0.05*,# | 0.72 ± 0.05*,# | 0.81 ± 0.04*,# | 0.45 ± 0.04*,# |
Note: ICH, intracerebral hemorrhage. The data represents means ± SD. Student’s t-test;
P < 0.05 vs. Control group;
P < 0.05 vs. ICH group.
Expression of Bax in the brain tissues
To determine Bax expression in the brain tissues of the rats, immunohistochemical assay was performed. In the control group, Bax positively stained cells were rare (Figure 2A). In the ICH group and the therapy group, Bax was broadly expressed in the cytoplasm of the cells which mainly located at the striatal region, cerebral cortex, subcortical region and hippocampus around hematoma (Figure 2B and 2C). The positive rates of Bax expression were shown in Table 2. The expression of Bax in the control group was significantly lower than that in the ICH group and the therapy group at each indicated time point (P < 0.05). In the ICH group, relatively strong Bax expression could be detected at 6 hours after ICH. Bax expression peaked at both 12 and 72 hours after ICH with significantly higher levels than those of the other time points (P < 0.05). One hundred and sixty-eight hours after ICH, Bax expression was significantly reduced. In the therapy group, Bax expression was strong at 12 hours, decreased at 24 hours, increased again with the second peak at 72 hours and significantly reduced at 168 hours after ICH. Bax expression at both 12 and 72 hours after ICH was significantly higher than that of the other time points (P < 0.05), while there was no significantly difference in Bax expression between these two time points. Bax expression in the therapy group was significantly lower than that of the ICH group at each indicated time point. These results indicate that ICH could lead to increased Bax expression in the brain tissues of the rats and hematoma aspiration could down-regulate ICH induced Bax expression.
Figure 2.
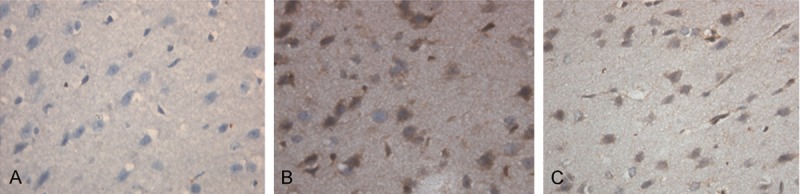
Bax expression in the brain tissues of the rats (magnification × 400). Immunohistochemical assay was performed to detect the expression of Bax in the brain tissues of the rats. Cells stained brown were Bax-positive. Representative immunohistochemical staining results of the control group (A), the ICH group (B) and the therapy group (C) 24 hours after ICH were shown. ICH, Intracerebral hemorrhage.
Table 2.
Positive rates of Bax expression in brain tissues at indicated time points after ICH
| Group | n | 6 h | 12 h | 24 h | 48 h | 72 h | 168 h |
|---|---|---|---|---|---|---|---|
| Control group | 30 | 0.01 ± 0.007 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.14 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 |
| ICH group | 30 | 0.57 ± 0.06* | 0.90 ± 0.04* | 0.66 ± 0.04* | 0.79 ± 0.03* | 0.89 ± 0.03* | 0.47 ± 0.02* |
| Therapy group | 25 | - | 0.83 ± 0.03*,# | 0.57 ± 0.01*,# | 0.65 ± 0.06*,# | 0.81 ± 0.04*,# | 0.39 ± 0.03*,# |
Note: ICH, intracerebral hemorrhage. The data represents means ± SD. Student’s t-test;
P < 0.05 vs. Control group;
P < 0.05 vs. ICH group.
Expression of Bcl-2 in the brain tissues
To determine Bcl-2 expression in the brain tissues of the rats, immunohistochemical assay was performed. In the control group, Bcl-2 positively stained cells were rare (Figure 3A). In the ICH group and the therapy group, Bcl-2 was broadly expressed in both the cytoplasm and the nucleus of the cells which mainly located at the striatal region, cerebral cortex, subcortical region and hippocampus around hematoma (Figure 3B and 3C). The positive rates of Bcl-2 expression were shown in Table 3. The expression of Bcl-2 in the control group was significantly lower than that in the ICH group and the therapy group at each indicated time point (P < 0.05). In each group, Bcl-2 expression peaked at 24 hours after ICH with significantly higher levels than those of the other time points (P < 0.05), maintained at 48 hours then decreased at 72 hours after ICH. Bcl-2 expression in the therapy group was significantly lower than that of the ICH group at each indicated time point. These results indicate that ICH could lead to increased Bcl-2 expression in the brain tissues of the rats and hematoma aspiration could up-regulate ICH induced Bcl-2 expression.
Figure 3.
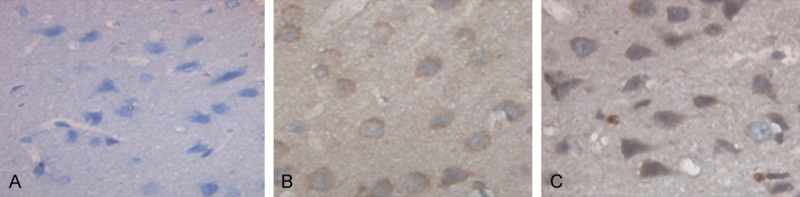
Bcl-2 expression in the brain tissues of the rats (magnification × 400). Immunohistochemical assay was performed to detect the expression of Bcl-2 in the brain tissues of the rats. Cells stained brown were Bcl-2-positive. Representative immunohistochemical staining results of the control group (A), the ICH group (B) and the therapy group (C) 12 hours after ICH were shown. ICH, Intracerebral hemorrhage.
Table 3.
Positive rates of Bcl-2 expression in brain tissues at indicated time points after ICH
| Group | n | 6 h | 12 h | 24 h | 48 h | 72 h | 168 h |
|---|---|---|---|---|---|---|---|
| Control group | 30 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.11 ± 0.01 | 0.05 ± 0.01 | 0.022 ± 0.01 | 0.02 ± 0.01 |
| ICH group | 30 | 0.34 ± 0.07* | 0.66 ± 0.07* | 0.95 ± 0.04* | 0.87 ± 0.04* | 0.77 ± 0.05* | 0.53 ± 0.04* |
| Therapy group | 25 | - | 0.76 ± 0.02*,# | 0.98 ± 0.02*,# | 0.90 ± 0.05*,# | 0.80 ± 0.03*,# | 0.65 ± 0.05*,# |
Note: ICH, intracerebral hemorrhage. The data represents means ± SD. Student’s t-test;
P < 0.05 vs. Control group;
P < 0.05 vs. ICH group.
Discussion
Neuron apoptosis is one of the major causes of brain injuries after ICH. It is important to detect the expression kinetics of apoptosis-related genes in the brain tissues after ICH and investigate the effects of different strategies for ICH therapy on the expression of the apoptosis-related genes. In this work, the effects of ICH and subsequent minimally invasive hematoma aspiration on the expression of apoptosis-related genes including Hsp70, Bax and Bcl-2 were studied with collagenase-induced ICH rat models.
Hsp70 expression in the normal brain tissues is very low. Under stress, the levels of many proteins decrease, while Hsp70 expression increased [17,18]. In this study, Hsp70 expression in the control group was very low and significantly increased in the ICH group and the therapy group. Hsp70 can inhibit apoptosis through different mechanisms [19-21]. Apoptosis was observed in surgical specimens obtained from ICH patients within 1 day, 2 days, and 5 days after the onset of symptoms [22]. Apoptotic neurons and astrocytes could be observed from 4 hours to 4 weeks after ICH induction in rats [23]. It has been accepted that apoptosis occurred at 24 to 72 hours after ICH [24]. The reversible recovery of brain tissues around hematoma might be expected within 72 hours after ICH [25]. Interestingly, we also found Hsp70 expression peaked at 72 hours after ICH. It seems that there might be a correlation between Hsp70 expression and apoptosis after ICH.
There were closed correlations among Bax expression, Bcl-2 expression and the status of neuron apoptosis after ICH. Bax expression increased fasters and peaked earlier than that of Bcl-2. Their expression positively correlated with each other within 48 hours after ICH, while negatively correlated after that. The number of apoptotic neurons positively correlated with Bax expression. They peaked at 48 hours and significantly decreased at 96 hours after ICH. Accordingly, the number of apoptotic neurons positively correlated with Bcl-2 expression within 48 hours after ICH, while negatively correlated with Bcl-2 expression after 48 hours [26]. In this study, we also found that Bcl-2 expression peaked at 24 hours, maintained at 48 hours then decreased at 72 hours after ICH. Bcl-2 expression peaked later than Bax expression. Interestingly, Bax expression reached a second peak at 72 hours after ICH. This might be because the inflammatory response around hematoma was the most severe from 48 to 72 hours after ICH, thus leading to increased neuron apoptosis [27].
Both Bcl-2 and Hsp70 could inhibit apoptosis. In this study, Bcl-2 expression peaked at 24 hours, maintained at 48 hours after ICH then decreased. Hsp70 expression peaked at 72 hours after ICH. Bcl-2 and Hsp70 may have synergistic effects on apoptosis inhibition. It has been reported that Hsp70 could regulate Bcl-2 and Bax expression and protect neurons. However, it is not known whether this directly correlates with the survival of the neurons [28]. Inhibition of apoptosis is a potential strategy for ICH therapy. A2A receptor agonist (CGS 21680) administration directly into the striatum immediately prior to the induction of ICH reduced parenchymal neutrophil infiltration and apoptotic cells within and bordering the hematoma [29]. The density of apoptotic cells at 24 and 48 hours after hemorrhage was significantly reduced by treatment with the broad-spectrum caspase inhibitor zVADfmk [23]. Injection of blood plus hirudin (thrombin inhibitor) from 24 to 72 hours after ICH reduced edema and apoptosis of neurons [30]. Our results suggest that induction of Hsp70 expression to inhibit apoptosis might be a new strategy for ICH therapy which deserves further investigation.
It has been accepted that the best time for surgery is between 6-24 hours after ICH. The hemorrhage does not necessarily stop within 6 hours after ICH and minimally invasive hematoma aspiration could lead to increased bleeding and increased mortality. If the aspiration is performed later than 24 hours after ICH, the hematoma could lead to intracranial hypertension, increased necrosis of brain cells because of the space-occupying effects of the hematoma, increased mortality and bleeding again with repeated washing [31,32]. Therefore, we performed hematoma aspiration within 6 hours after ICH to reduce the space-occupying effects and the secondary lesions, thus reducing neurological damage and promoting neural functional recovery. In this study, Hsp70 and Bax expression was significantly decreased in the therapy group compared with the ICH group, indicating decreased stress and neurological damage in the therapy group. Accordingly, anti-apoptotic Bcl-2 expression was significantly increased in the therapy group compared with the ICH group. In conclusion, ICH led to increased expression of apoptosis-related genes in the brain tissues of the rats. Hematoma aspiration up-regulated ICH induced Bcl-2 expression and down-regulated ICH induced Hsp70 and Bax expression.
Acknowledgements
This work was supported by the Science and Technology Planning Project of Shandong Province (No. 2011GGH21821) and Shandong Provincial Medical and Health Science and Technological Program of China (No. 2013WSB02001).
Disclosure of conflict of interest
None.
References
- 1.Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH. Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Early Brain Injury or Cerebral Vasospasm. Acta Neurochir Suppl. 2011;110:43–48. doi: 10.1007/978-3-7091-0353-1_8. [DOI] [PubMed] [Google Scholar]
- 2.Hu SC, Zhong XJ. The mechanism of nerve cell apoptosis and therapeutic strategy after intracerebral hemorrhage. Guo Wai Yi Xue (Nao Xue Guan Ji Bing Fen Ce) 2004;12:524–526. [Google Scholar]
- 3.Nishio A, Hara M, Otsuka Y, Uno T, Murata T. Endovaseular treatment of posterior cerebral aneurysm associated with Moyamoya disease. J Neuroradiol. 2004;31:60–62. doi: 10.1016/s0150-9861(04)96879-4. [DOI] [PubMed] [Google Scholar]
- 4.Mu F, Li XG, Feng J. Minimally invasive aspiration for intracerebral hematoma in rats at different operative timings. Clin J Cerebrovasc Dis. 2009;6:359–362. [Google Scholar]
- 5.Oltvai ZN, Milliman CL, Koesmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed all death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 6.Johnsen SP, Pedersen L, Friis S, Blot WJ, McLaughlin JK, Olsen JH, Sørensen HT. Nonaspirin nonsteroidal anti-inflammatory drugs and risk of hospitalization for intracerebral hemorrhage: a population based case control study. Stroke. 2003;34:387–391. doi: 10.1161/01.str.0000054057.11892.5b. [DOI] [PubMed] [Google Scholar]
- 7.Wynn RM, Davie JR, Cox RP, Chuang DT. Molecular chaperones: heat-shock proteins, foldases, and matchmakers. J Lab Clin Med. 1994;124:31–36. [PubMed] [Google Scholar]
- 8.Sato K, Saito H, Matsuki N. HSP70 is essential to the neuro protective effect of heat-shock. Brain Res. 1996;740:117–123. doi: 10.1016/s0006-8993(96)00846-3. [DOI] [PubMed] [Google Scholar]
- 9.Sashchenko LP, Dukhanina EA, Yashin DV. Peptidoglycan recognition protein tag 7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J Biol Chem. 2004;279:2117–2124. doi: 10.1074/jbc.M307513200. [DOI] [PubMed] [Google Scholar]
- 10.Khawaja X, Xu J, Liang JJ, Barrett JE. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: Implications for depressive disorders and future therapies. J Neurosci Res. 2004;75:451–460. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- 11.Cao JH, Deng SX. Heat shock protein 70 and brain injury. J Trauma Surg. 2008;10:287–289. [Google Scholar]
- 12.Kitamura C, Ogawa Y, Nishihara T, Morotomi T, Terashita M. Transient colocalization 0f c-Jun N-terminal kinase and c-Jun with heat shock protein 70 in pulp cell during apoptosis. J Dent Res. 2003;82:91–95. doi: 10.1177/154405910308200203. [DOI] [PubMed] [Google Scholar]
- 13.Samali A, Cotter TC. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Liu L, Xing D, Chen WR. Inhibition of the JNK/Bim pathway by Hsp70 prevents Bax activation in UV-induced apoptosis. FEBS Lett. 2010;584:4672–4678. doi: 10.1016/j.febslet.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 16.Xinmin Bao XM, Shu SY. The stereo taxic atlas of the rar brain. Atlas: 1991. pp. 3–138. [Google Scholar]
- 17.Liu YT, Wang GY, Niu XY. The relative research of the relationship between the expression of peripheral blood heat shock protein-70 and defection of neural function in rats following focal cerebral ischemia and reperfusion. Zhong Guo Xian Dai Yao Wu Ying Yong Za Zhi. 2008;2:90–91. [Google Scholar]
- 18.Jin XF, Wang R, Xiao CF. Serum and lymphocyte levels of heat shock protein 70 in aging: a study in the normal Chinese population. Cell Stress Chaperones. 2004;9:69–75. doi: 10.1379/477.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dapunt OE, Raji MR, Jeschkeit S, Dhein S, Kuhn-Régnier F, Südkamp M, Fischer JH, Mehlhorn U. Intracoronary shunt insertion prevents myocardial stunning in a juvenile porcine MIDCAB model absent of coronary artery disease. Eur J Cardiothorac Surg. 1999;15:173–178. doi: 10.1016/s1010-7940(98)00290-5. [DOI] [PubMed] [Google Scholar]
- 20.Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. HSP70 prevents activation of stress kinase, A novel pathway of cellular the motolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- 21.Tan HM, Wu WK. Role of heat shock protein in regulation of JNK and apoptosis. Zhong Guo Bing Li Sheng Li Za Zhi. 2001;17:477–480. [Google Scholar]
- 22.Quresbi AI. Suri MF. Ostrow PT. Apoptosis as a form of cell deam in intracerebral hemorhage. Neurosurgery. 2003;52:1041–1047. [PubMed] [Google Scholar]
- 23.Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, Lo EH. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats:time course of inflammation and cell death. NeuroSci lett. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- 25.Cao G, Minami M, Pei W, Yan C, Chen D, O’Horo C, Graham SH, Chen J. 1ntracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab. 2001;21:321–333. doi: 10.1097/00004647-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Shi YT, Zhao Y, Zhang XD. Experimental study of expression of Bax and Bcl-2 in perifocal tissue of intracerebral hemorrhage and neuronal apoptosis. Zhong Guo Shi Yong Shen Jing Ji Bing Za Zhi. 2006;9:4–5. [Google Scholar]
- 27.Guo FQ, Li XJ, Chen LY, Yang H, Dai HY, Wei YS, Huang YL, Yang YS, Sun HB, Xu YC, Yang ZL. Study of relationship between inflammatory response and apoptosis in perihematoma region in patients with intracerebral hemorrhage. Zhong Guo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:290–293. [PubMed] [Google Scholar]
- 28.Nagashima M, Fujikawa C, Mawatari K, Mori Y, Kato S. HSP70, the earliest-induced gene in the zebrafish retina during optic nerve regeneration: its role in cell survival. Neurochem Int. 2011;58:888–95. doi: 10.1016/j.neuint.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Mayne M, Fotheringham J, Yan HJ, Power C, Del Bigio MR, Peeling J, Geiger JD. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following Intracerebral hemorhage. Ann Neurol. 2001;49:727–735. doi: 10.1002/ana.1010. [DOI] [PubMed] [Google Scholar]
- 30.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Yang B, Yao KH. Basic research and clinical application of aspiration and lysis drainage on hypertensive cerebral hemorrhage patients. Zhong Guo Wei Qin Xi Shen Jing Wai Ke Za Zhi. 2001;6:185–186. [Google Scholar]
- 32.Huang QQ, Huang CB, Liu CQ. Effects of minimally invasive hematoma aspiration on hypertensive cerebral hemorrhage. J Clin Res. 2011;28:775–776. [Google Scholar]


