Abstract
Objectives: To evaluate the feasibility and efficacy of tumor enucleation (TE) for patients with small renal cell carcinoma (RCC) associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 RCC) by analyzing the pseudocapsule characteristics of Xp11.2 RCCs comparing with that of clear cell renal cell carcinoma (ccRCC). Methods: From June 2007 to February 2014, 22 patients with Xp11.2 RCC who were diagnosed by fluorescence in-situ hybridization polyclonal (FISH) assay and 32 patients with ccRCC treated in our institution were comparatively studied. 12 patients with ccRCC underwent radical nephrectomy (RN) and 20 received TE. Among 22 patients with Xp11.2 RCC, 19 were treated by RN and 3 by TE (1 by radiofrequency ablation assisted TE). Pseudocapsule and other clinicopathological characteristics of the two subtypes of RCC were compared. Survival of patients treated with different surgical methods was evaluated and compared. Results: Pseudocapsule incidence of Xp11.2 RCC (14/22, 63.6%) was lower than that of ccRCC (32/32, 100%, P<0.001). However, pseudocapsule integrity rate of Xp11.2 RCC (10/14, 71.4%) was comparable with that of ccRCC (23/32, 71.9%, P=1.000). The 5-year overall survival of patients with ccRCC treated with RN and TE was 86% and 81%, respectively (P=0.845). Three patients with small Xp11.2 RCC performed well after TE. Conclusions: Over half Xp11.2 RCC had pseudocapsules, whose integrity rate was comparable to that of ccRCC. Treatment effectives of TE and RN were comparable in ccRCC. A preliminary attempt to treat small Xp11.2 RCC with intact pseudocapsule by using TE produced a favorable treatment outcome.
Keywords: Carcinoma, renal cell, Xp11.2 translocation, pseudocapsule, tumor enucleation, treatment outcome
Introduction
Renal cell carcinoma (RCC) associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 RCC) was delineated as a distinct entity in the 2004 World Health Organization renal tumor classification [1]. This subtype of RCC primarily affects children and adolescents more than adults, accounts for 20%~40% of pediatric RCCs and 1%~1.6% of all renal tumors in adults [2]. Xp11.2 RCC in adults were reported more aggressive than other subtypes of RCC and associated with a poorer prognosis [3]. Radical nephrectomy (RN) is the primary treatment method for patients with Xp11.2 RCC [4,5].
With the progress in surgical technique, nephron sparing surgery (NSS) has been widely used now and regarded as the standard treatment for RCC [6]. Some large retrospective studies have confirmed that tumor enucleation (TE) is a safe and acceptable nephron-sparing treatment that provides excellent long-term local control and cancer-specific survival rates for early RCCs [7]. In particular, RCCs with intact pseudocapsules were regarded especially suitable for TE treatment [8].
Pseudocapsule of Xp11.2 RCC was described in previous literatures [9,10] and was commonly come across in our daily work. Since many patients with small Xp11.2 RCC were diagnosed at an early stage in our institution, we hypothesized that TE might be a rational alternative for such patients in order to preserve renal function. In this study, pseudocapsule characteristics of Xp11.2 RCC were analyzed and a preliminary attempt to treat early stage Xp11.2 RCC with intact pseudocapsule by using TE method was introduced. Since ccRCC is the most common subtype of RCC [11], its pseudocapsule features and the application of TE in ccRCC were also studied for a comparison purpose.
Material and methods
Patients and diagnosis
After receiving institutional review board approval we retrospectively gathered 22 patients with Xp11.2 RCC and 32 patients with ccRCC who underwent surgical resections in our institution from June 2007 to February 2014. Surgical specimens were routinely embedded in paraffin, cut into 4-µm sections and stained with haematoxylin and eosin. Both TFE3 immunohistochemical (IHC) staining and a TFE3 break-apart fluorescence in-situ hybridization polyclonal (FISH) assay were performed in the sections for detection of TFE3 rearrangements and to confirm the diagnosis of Xp11.2 RCC.
Pathological evaluation
The incidence and integrity rate of pseudocapsule in Xp11.2 RCC and ccRCC were evaluated and recorded by 2 pathologists (with 7 and 8 years’ experience in renal tumor pathology respectively), as well as surgical margins after TE. The TNM stage of each patient was determined according to the 7th American Joint Committee on Cancer (AJCC) staging criteria [12].
Surgical procedures
All tumors with a maximum diameter ≤7 cm [13] especially ≤4 cm [14,15], which was locally confined with a clear margin and without lymph node or distant metastasis on preoperative CT imaging were regarded as candidates for TE therapy in our institution, especially for those patients with anatomical or functional solitary kidney. After full communication between those candidates and surgeons, a choice between RN and TE was made by the patients.
Among 32 patients with ccRCC, 12 underwent RN and 20 received TE. Among 22 patients with Xp11.2 RCC, 19 were treated by RN and 3 by TE (1 by radiofrequency ablation assisted TE). All surgical procedures were performed according to a standard protocol [15] and no perioperative mortality was recorded.
Follow-up
All the patients were followed up regularly after discharge. Contrast enhanced ultrasonography or computed tomography (CT) was performed every 3 months during the first year, every 6 months during the following four years, and annually after five years. Recurrence time and patterns, death time and causes of involved cases were recorded.
Statistical analysis
The incidence and integrity rate of pseudocapsule in Xp11.2 RCC and ccRCC were compared with chi-square test. Other clinicopathological characteristics of patients with those two subtypes of RCC treated with RN and TE were also compared with the student t test or chi-square test. Survival rates of patients with ccRCC and Xp11.2 RCC treated with RN and TE were calculated by Kaplan-Meier method and compared by the log-rank test. All the statistical analysis was performed on SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
All the diagnosis of Xp11.2 RCC was confirmed by IHC and TFE3 break-apart FISH, as shown in Figure 1. Comparison of clinicopathological characteristics of Xp11.2 RCC and ccRCC was shown in Table 1. Patients with Xp11.2 RCC were significantly younger with more gross hematuria than those with ccRCC (P<0.001). Xp11.2 RCCs were significantly larger than ccRCCs (P=0.002). The incidence and integrity pseudocapsule in Xp11.2 RCC and ccRCC were recorded, as shown in Figure 2. Pseudocapsule incidence of Xp11.2 RCC (14/22, 63.6%) was significantly lower than that of ccRCC (32/32, 100%, P<0.001). Pseudocapsule incidence of smaller Xp11.2 RCC (diameter ≤7 cm) was significantly higher than that of larger ones (76.5% vs. 20.0%, P=0.039). Pseudocapsule integrity rate of Xp11.2 RCC (10/14, 71.4%) was comparable with that of ccRCC (23/32, 71.9%, P=1.000). All penetrations of the pseudocapsule occurred on the parenchymal side.
Figure 1.
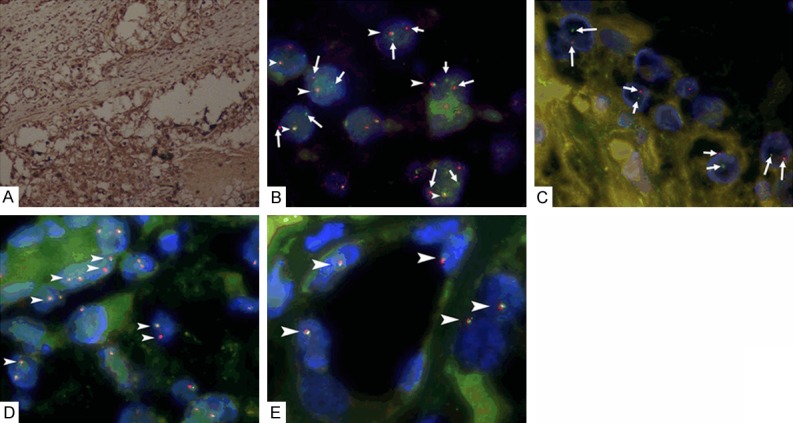
A. Immunohistochemical staining demonstrates strong TFE3 nuclear staining of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 RCC) (×100); B. Fluorescence in situ hybridization (FISH) assay for Xp11.2 RCC shows separated red and blue signals(arrows) as well as a fusion signal (arrowheads) in each tumor cell nucleus of Xp11.2 RCC in a female patient in one X chromosome, indicating the translocation of one chromosome X and a normal another (×1000); C. FISH assay shows separated red and green signals (arrows) in each tumor cell nucleus of Xp11.2 RCC in a male patient, indicating the translocation of chromosome X (×1000); D. FISH assay shows two fusion signals (arrowheads) in each tumor cell nucleus of clear cell renal cell carcinoma (CCRCC) in a female patient (×1000); E. FISH assay shows a fusion signal (arrowheads) in each tumor cell nucleus of CCRCC in a male patient (×1000).
Table 1.
Comparison of clinicopathological characteristics of Xp11.2 RCC and CCRCC
| Xp11.2 RCC (n=22) | CCRCC (n=32) | P | |
|---|---|---|---|
| Gender (male/female) | 9/13 | 22/10 | 0.042 |
| Age: mean ± SE (range, years) | 25.8±2.5 (3~51) | 60.2±2.2 (40~81) | <0.001 |
| Gross hematuria incidence | 9/22 (40.9%) | 0/32 (0%) | <0.001 |
| Tumor diameter: mean ± SE (range, cm) | 5.28±0.45 (3.0~10.0) | 3.80±0.23 (2.0~6.2) | 0.002 |
| Pseudocapsule incidence (percentage) | 14/22 (63.6%) | 32/32 (100.0%) | <0.001 |
| Pseudocapsule integrity rate (percentage) | 10/14 (71.4%) | 23/32 (71.9%) | 1.000 |
| Numbers of small/large tumors (threshold: 7 cm) | 17/5 | 31/1 | 0.036 |
| Age of small tumors: Mean ± SE (range, years) | 25.5±2.9 (3~51) | 59.7±2.2 (40~81) | <0.001 |
| Diameter of smaller tumors (Mean ± SE, cm) | 4.27±0.24 | 3.72±0.22 | 0.118 |
| Diameter of larger tumors (Mean ± SE, cm) | 8.72±0.33 | -- | -- |
| Pseudocapsule incidence of small tumors (%) | 13/17 (76.5%) | 32/32 (100%) | 0.011 |
| Pseudocapsule incidence of large tumors (%) | 1/5 (20.0%) | -- | -- |
| Pseudocapsule integrity rate of small tumors (%) | 9/13 (69.2%) | 23/32 (71.9%) | 0.711 |
| Pseudocapsule integrity rate of large tumors (%) | 1/1 (100%) | -- | -- |
| Pseudocapsule penetration information | 3 cases ≥4 cm 1 case =3.9 cm | 8 cases ≥4 cm 1 case =3.5 cm | 1.000 |
Xp11.2 RCC, renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion; CCRCC, clear cell renal cell carcinoma; SE, standard error.
Figure 2.
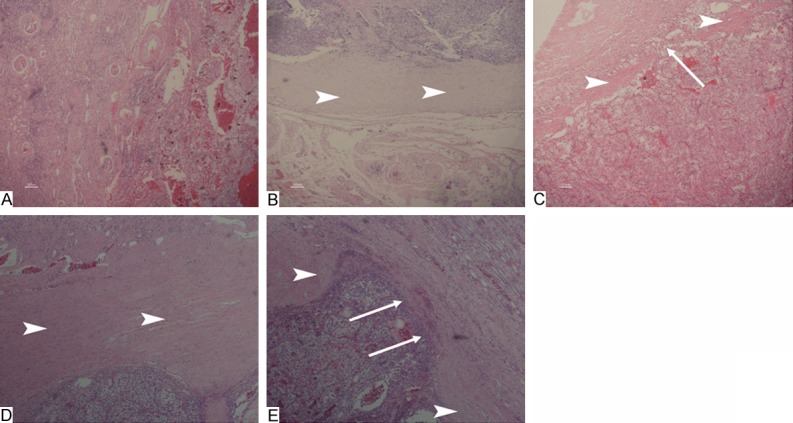
A. No pseudocapsule can be detected in a renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 RCC) (H&E, ×40). B. Pseudocapsule intact and free from invasion is found in an Xp11.2 RCC (H&E, ×40); C. Pseudocapsule with signs of neoplastic penetration on parenchymal kidney side is found in an Xp11.2 RCC (H&E, ×40); D. Pseudocapsule intact and free from invasion is found in clear cell renal cell carcinoma (CCRCC) (H&E, ×40); E. Pseudocapsule invasion with signs of neoplastic penetration on parenchymal kidney side is found is found in CCRCC (H&E, ×40). Arrowheads indicate pseudocapsule. Arrows indicate neoplastic penetration.
Clinicopathologic characteristics of ccRCC and Xp11.2 RCC treated with different surgical methods were shown in Table 2. The overall survival curves of ccRCC and Xp11.2 RCC treated by RN and TE were shown in Figure 3. The 5-year overall survival of patients with ccRCC treated with RN and TE was 86% and 81%, respectively (P=0.845). The 5-year overall survival of patients with Xp11.2 RCC treated with RN was 65%. Detailed information of 3 patients with Xp11.2 RCC treated by TE was shown in Table 3. In the 3 cases, pseudocapsule was intact and surgical margins after TE were negative.
Table 2.
Clinicopathological characteristics and survival of patients with CCRCC and Xp11.2 RCC treated with different surgical methods
| CCRCC (n=32) | Xp11.2 RCC (n=22) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| RN group (n=12) | TE group (n=20) | P | RN group (n=19) | RN in small group (n=14) | Pa | |
| Age: mean ± SE (range, years) | 70.2±2.8 (51~81) | 54.2±2.1 (40~69) | <0.001 | 24.1±2.5 (3~40) | 23.1±2.9 (3~40) | <0.001 |
| Tumor diameter: mean ± SE (range, cm) | 4.78±0.28 (3.2~6.2) | 3.22±0.24 (2.2~5.5) | <0.001 | 5.50±0.50 (3~10) | 4.35±0.27 (3~6) | 0.215 |
| Stage (I/II/III/IV) | 9/0/1/2 | 18/0/0/2 | 0.395 | 11/1/4/3 | 11/0/2/1 | 0.911 |
| Pseudocapsule incidence (%) | 12/12 (100%) | 20/20 (100%) | 1.000 | 11/19 (57.9%) | 10/14 (71.4%) | 0.012 |
| Pseudocapsule integrity rate (%) | 5/12 (41.7%) | 18/20 (90%) | 0.006 | 7/11 (63.6%) | 6/10 (60%) | 0.292 |
| Median follow-up time (range, months) | 39.5 (14~70) | 36.5 (7~71) | 0.795 | 39 (5~85) | 45.5 (5~85) | 0.820 |
| 3-year progression-free survival rate (%) | 77% | 85% | 0.733 | 86% | 100% | 0.931 |
| 5-year progression-free survival rate (%) | 77% | 66% | 0.733 | 72% | 82% | 0.931 |
| 3-year overall survival rate (%) | 86% | 92% | 0.845 | 84% | 100% | 0.567 |
| 5-year overall survival rate (%) | 86% | 81% | 0.845 | 65% | 71% | 0.567 |
Xp11.2 RCC, renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion; CCRCC, clear cell renal cell carcinoma; RN, radical nephrectomy; TE, tumor enucleation; SE, standard error.
P value concerning comparison between 19 Xp11.2 RCC and 12 CCRCC treated by RN.
Figure 3.

Overall survival curves of patients with clear cell renal cell carcinoma (CCRCC) and renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 RCC) treated by radical nephrectomy (RN) and tumor enucleation (TE).
Table 3.
Clinical information of patients with Xp11.2 RCC treated by tumor enucleation (TE)
| Case | Gender | Age (years) | Tumor site | Greatest diameter (cm) | Stage | Operative method | Follow up (months) | outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 30 | Right | 3.2 | T1aM0N0 | Radiofrequency ablation assisted enucleation | 44 | without recurrence |
| 2 | Male | 29 | Left | 3.5 | T1aM0N0 | TE | 11 | without recurrence |
| 3 | Female | 51 | Right | 5.0 | T1bM0N0 | TE | 34 | without recurrence |
Discussion
NSS has been widely used and provided effective local control and a similar disease specific survival rate as RN in treating RCCs smaller than 4 cm [14,16]. With renal function preserved, NSS could achieve equivalent local tumor control to RN even in RCCs between 4 and 7 cm [11]. TE is a nephron-sparing procedure in which the tumor is excised by blunt dissection following the natural plane between the pseudocapsule and the renal parenchyma without removing a visible rim of renal parenchyma [15]. The technical feasibility and oncologic safety of TE for a renal neoplasm depend on the possibility of obtaining negative surgical margins confirmed at the postoperative pathologic examination, which is influenced by the presence of an intact fibrous pseudocapsule around the tumor [17]. In three cases of Xp11.2 RCC treated by TE, the surgical margins were negative, possibly due to intact pseudocapsule existence.
It was reported that pseudocapsule existed in almost all RCCs ≤7 cm [18-20]. In our study, pseudocapsule incidence of ccRCC was as high as 100%. Although lower than that of ccRCC, pseudocapsule incidence of Xp11.2 RCC also reached 63.6%. Altinok et al. [10] reported that 75% cases of Xp11.2 RCC had a thick fibrous pseudocapsule and Dehner et al. [21] reported that the incidence in children patients was 73%. Possible reasons for those differences may lie in tumor diameters. Furthermore, in our study, pseudocapsule incidence of Xp11.2 RCCs ≤7 cm was 76.5% while only 20% of those >7 cm showed pseudocapsules.
Pseudocapsule integrity rate of Xp11.2 RCC (71.4%) was comparable with that of ccRCC (71.9%). Three of 4 Xp11.2 RCCs with pseudocapsule penetrations had a maximum diameter larger than 4 cm. Rosenthal et al. [22] noted that 80% of RCCs <7 cm had intact pseudocapsules while only 23.5% of larger tumors had continuous fibrotic capsule. Pseudocapsule invasion was more frequently detected in large (>6 cm) and poor differentiated tumors [22]. Minervini et al. found that [18] pseudocapsule invasion rate increased significantly as tumor size increased.
Some studies reported that pseudocapsule invasion might correlate with focal residence and recurrence after TE [23,24]. However, Minervini et al. reported that [20] pseudocapsule invasion on the parenchymal side would not increase the risk of relapse compared with the intact one. Ficarra et al. [25] speculated that the chronic inflammation around the tumor in the normal renal tissue could tend to adhere to the pseudocapsule, causing its removal without tumor residue during enucleation. In our study, there was no significant difference in survival of patients with ccRCC treated by RN and TE.
A majority of Xp11.2 RCCs occurred in children and young adults [2,26]. In our study patients with Xp11.2 RCC were significantly younger than those with ccRCC. And 40.9% of patients with Xp11.2 RCC referred to gross hematuria at diagnosis, possibly due to tumor involvement of renal collecting system, while none of ccRCC had such manifestation. Xp11.2 RCC was significantly larger than ccRCC. It is insensitive to both radiotherapy and chemotherapy. And surgical resection was regarded as the most effective treatment [5]. Nineteen patients with Xp11.2 RCC underwent RN in our study and the 5-year overall survival rate was lower than that of ccRCC, due to the aggressive nature of Xp11.2 RCC in adult patients [3].
Considering that pseudocapsule incidence of Xp11.2 RCCs ≤7 cm was relatively high (76.5%) and pseudocapsule integrity rate of Xp11.2 RCC was comparable with that of ccRCC and all penetration of the pseudocapsule occurred on the parenchymal side in our study, TE might be a rational alternative treatment for small Xp11.2 RCC to preserve more renal function and to avoid higher risk of renal inadequacy. TE was a safe alternative for small renal masses locally confined on preoperative imaging and easily delineated intraoperatively without grossly invasion beyond the pseudocapsule [27]. Preoperative imaging such as CT can provide important information on tumor’s location, diameter, margin, pseudocapsule, local invasion and distant metastasis [19] when TE is regarded as a treatment choice. In our study, all subtypes of RCCs ≤7 cm locally confined with a clear margin and without lymph node or distant metastasis on preoperative CT imaging were regarded as candidates for TE therapy.
In our study, 3 patients with Xp11.2 RCC received TE treatment. In preoperative CT images, those three tumors measured 3.2~5.0 cm, with clear margins and without any sign of lymphatic or distant metastasis. TE was performed according to a standard protocol. In particular, one patient was treated with radiofrequency ablation assisted TE. TE is a relatively bloodless procedure without the need of hilar clamping [28]. Prior to TE, an electrode was inserted between the tumor and normal renal parenchyma so that radiofrequency can make surrounding parenchymal vessels occlusive. Radiofrequency ablation could also help to kill tumor cells in tumor bed. Postoperative pathologic studies confirmed the existence of intact pseudocapsule, negative surgical margins and a stage I disease in all three cases. During the follow up of 11~44 months, all of the patients were alive without any recurrence.
In this study, both TFE3 IHC and FISH assay were performed to eliminate false positive and false negative diagnosis in Xp11.2 RCC [29]. Xp11.2 RCCs were generally characterized by several translocations in chromosome Xp11.2 involving the TFE3 gene [30]. Since those translocations lead to overexpression of the TFE3 protein, IHC staining for TFE3 was widely used in the diagnosis of Xp11.2 RCC [31]. TFE3 IHC is undoubtedly helpful in diagnosis, but more strict evaluation criteria are required. Klatte et al. reported that the positive predictive value of TFE3 IHC staining is only 12% (2/17) for Xp11.2 RCC [32]. Chevallier et al. also found that TFE3 IHC technique was too sensitive and increased the false positive rate [33]. Definite diagnosis of Xp11.2 RCC might only be made by genetic analysis.
There were some limitations in this study. Firstly, the sample size was still small and the follow-up time was relatively short. Secondly, due to small sample size of patients with Xp11.2 RCC treated with TE, we couldn’t compare the survival difference between RN and TE in patients with Xp11.2 RCCs. Thirdly, the diagnostic performance of preoperative imaging in detecting the pseudocapsule was not evaluated. Further studies are required to solve these problems.
In conclusion, pseudocapsule incidence and integrity rate of small Xp11.2 RCC were relatively high. Although RN was the primary treatment for Xp11.2 RCC, TE is still a rational alternative treatment for small Xp11.2 RCC with intact pesudocapsule at an early stage in order to preserve renal function. Initial experience of TE in three patients with Xp11.2 RCC obtained favorable outcome.
Acknowledgements
This work was supported by a grant from National Natural Science Foundation of China (ID: 21377052), Natural Science Foundation of Jiangsu Province (ID: BK20131281) and Nanjing City Young Health Personnel Training Project (third level) (ID: QRX11178).
Disclosure of conflict of interest
None.
References
- 1.Su H, Sung M, Chiang P, Cheng Y, Chen Y. The preliminary experiences of translocation renal cell carcinoma and literature review. Kaohsiung J Med Sci. 2014;30:402–8. doi: 10.1016/j.kjms.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kmetec A, Jeruc J. Xp 11.2 translocation renal carcinoma in young adults; recently classified distinct subtype. Radiol Oncol. 2014;48:197–202. doi: 10.2478/raon-2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asaki HE, Moshero G, Stanton ML, Humphreys MR. Xp11.2 translocation tumor. JAAPA. 2014;27:24–7. doi: 10.1097/01.JAA.0000442700.87975.0b. [DOI] [PubMed] [Google Scholar]
- 4.Rao Q, Guan B, Zhou XJ. Xp11.2 translocation carcinomas have a poorer prognosis than non-xp11.2 translocation carcinomas in children and young adults: a meta-analysis. Int J Surg Pathol. 2010;18:458–64. doi: 10.1177/1066896910375565. [DOI] [PubMed] [Google Scholar]
- 5.Song HC, Sun N, Zhang WP, He L, Fu L, Huang C. Biological characteristics of pediatric renal cell carcinoma associated with Xp11.2 translocations/TFE3 gene fusions. J Pediatr Surg. 2014;49:539–42. doi: 10.1016/j.jpedsurg.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Bjurlin MA, Walter D, Taksler GB, Huang WC, Wysock JS, Sivarajan G, Loeb S, Taneja SS, Makarov DV. National trends in the utilization of partial nephrectomy before and after the establishment of AUA guidelines for the management of renal masses. Urology. 2013;82:1283–9. doi: 10.1016/j.urology.2013.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carini M, Minervini A, Lapini A, Masieri L, Serni S. Simple enucleation for the treatment of renal cell carcinoma between 4 and 7 cm in greatest dimension: progression and long-term survival. J Urol. 2006;175:2022–6, 2026. doi: 10.1016/S0022-5347(06)00275-8. [DOI] [PubMed] [Google Scholar]
- 8.Minervini A, Tuccio A, Lapini A, Lanzi F, Vittori G, Siena G, Tosi N, Serni S, Carini M. Review of the current status of tumor enucleation for renal cell carcinoma. Arch Ital Urol Androl. 2009;81:65–71. [PubMed] [Google Scholar]
- 9.He J, Huan Y, Qiao Q, Zhang J, Zhang JS. Renal carcinomas associated with Xp11.2 translocations: are CT findings suggestive of the diagnosis? Clin Radiol. 2014;69:45–51. doi: 10.1016/j.crad.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Altinok G, Kattar MM, Mohamed A, Poulik J, Grignon D, Rabah R. Pediatric renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions and clinicopathologic associations. Pediatr Dev Pathol. 2005;8:168–80. doi: 10.1007/s10024-004-9106-3. [DOI] [PubMed] [Google Scholar]
- 11.Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, Dalrymple NC, Chintapalli KN. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006;26:1795–806. 1806–10. doi: 10.1148/rg.266065010. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Gospodariwicz M, Wittekind C, editors. UICC International Union Against Cancer. 7th edition. Wiley-Blackwell; 2009. TNM Classification of Malignant Tumors. [Google Scholar]
- 13.Antonelli A, Cozzoli A, Nicolai M, Zani D, Zanotelli T, Perucchini L, Cunico SC, Simeone C. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7 cm. Eur Urol. 2008;53:803–9. doi: 10.1016/j.eururo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, Sinescu IC European Association of Urology Guideline Group for renal cell carcinoma. Renal cell carcinoma guideline. Eur Urol. 2007;51:1502–10. doi: 10.1016/j.eururo.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Laryngakis NA, Guzzo TJ. Tumor enucleation for small renal masses. Current Opinion in Urology. 2012;22:365–71. doi: 10.1097/MOU.0b013e3283551f84. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka T, Obara W. Evidence-based clinical practice guideline for renal cell carcinoma: the Japanese Urological Association 2011 update. Int J Urol. 2012;19:496–503. doi: 10.1111/j.1442-2042.2012.03031.x. [DOI] [PubMed] [Google Scholar]
- 17.Li QL, Guan HW, Zhang QP, Zhang LZ, Wang FP, Liu YJ. Optimal margin in nephron-sparing surgery for renal cell carcinoma 4 cm or less. Eur Urol. 2003;44:448–51. doi: 10.1016/s0302-2838(03)00310-5. [DOI] [PubMed] [Google Scholar]
- 18.Minervini A, di Cristofano C, Lapini A, Marchi M, Lanzi F, Giubilei G, Tosi N, Tuccio A, Mancini M, della Rocca C, Serni S, Bevilacqua G, Carini M. Histopathologic analysis of peritumoral pseudocapsule and surgical margin status after tumor enucleation for renal cell carcinoma. Eur Urol. 2009;55:1410–8. doi: 10.1016/j.eururo.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 19.Tsili AC, Argyropoulou MI, Gousia A, Kalef-Ezra J, Sofikitis N, Malamou-Mitsi V, Tsampoulas K. Renal cell carcinoma: value of multiphase mdct with multiplanar reformations in the detection of pseudocapsule. Am J Roentgenol. 2012;199:379–86. doi: 10.2214/AJR.11.7747. [DOI] [PubMed] [Google Scholar]
- 20.Minervini A, Raspollini MR, Tuccio A, Di Cristofano C, Siena G, Salvi M, Vittori G, Sebastianelli A, Lapini A, Serni S, Carini M. Pathological characteristics and prognostic effect of peritumoral capsule penetration in renal cell carcinoma after tumor enucleation. Urol Oncol. 2014;32:15–50. doi: 10.1016/j.urolonc.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Dehner LP, Leestma JE, Price EJ. Renal cell carcinoma in children: a clinicopathologic study of 15 cases and review of the literature. J Pediatr. 1970;76:358–68. doi: 10.1016/s0022-3476(70)80474-7. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal CL, Kraft R, Zingg EJ. Organ-preserving surgery in renal cell carcinoma: tumor enucleation versus partial kidney resection. Eur Urol. 1984;10:222–8. doi: 10.1159/000463796. [DOI] [PubMed] [Google Scholar]
- 23.Franks ME, Hrebinko RL, Konety BR. Surgical enucleation for the treatment of renal tumors. Urol Int. 2003;71:184–9. doi: 10.1159/000071844. [DOI] [PubMed] [Google Scholar]
- 24.Berdjis N, Hakenberg OW, Zastrow S, Oehlschlager S, Novotny V, Wirth MP. Impact of resection margin status after nephron-sparing surgery for renal cell carcinoma. Bju Int. 2006;97:1208–10. doi: 10.1111/j.1464-410X.2006.06157.x. [DOI] [PubMed] [Google Scholar]
- 25.Ficarra V, Galfano A, Cavalleri S. Is simple enucleation a minimal partial nephrectomy responding to the EAU guidelines’ recommendations? Eur Urol. 2009;55:1315–8. doi: 10.1016/j.eururo.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 26.Malouf GG, Camparo P, Molinie V, Dedet G, Oudard S, Schleiermacher G, Theodore C, Dutcher J, Billemont B, Bompas E, Guillot A, Boccon-Gibod L, Couturier J, Escudier B. Transcription factor E3 and transcription factor EB renal cell carcinomas: clinical features, biological behavior and prognostic factors. J Urol. 2011;185:24–9. doi: 10.1016/j.juro.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 27.Laryngakis NA, Van Arsdalen KN, Guzzo TJ, Malkowicz SB. Tumor enucleation: a safe treatment alternative for renal cell carcinoma. Exp Rev Anticancer Therapy. 2011;11:893–9. doi: 10.1586/era.11.68. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Zhang S, Liu G, Ji C, Wang W, Chang X, Chen J, Li X, Gan W, Zhang G, Minervini A, Guo H. Zero ischemia laparoscopic radio frequency ablation assisted enucleation of renal cell carcinoma: experience with 42 patients. J Urol. 2012;188:1095–101. doi: 10.1016/j.juro.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury T, Prichard-Jones K, Sebire NJ, Bier N, Cherian A, Sullivan MO, O’Meara A, Anderson J. Persistent complete response after single-agent sunitinib treatment in a case of TFE translocation positive relapsed metastatic pediatric renal cell carcinoma. J Pediatr Hematol Oncol. 2013;35:e1–3. doi: 10.1097/MPH.0b013e318266bf34. [DOI] [PubMed] [Google Scholar]
- 30.Kothari KS, Sathe PA, Naik LP, Kandalkar BM. Xp11 translocation renal cell carcinoma. Indian J Pathol Microbiol. 2013;56:471–2. doi: 10.4103/0377-4929.125383. [DOI] [PubMed] [Google Scholar]
- 31.Komai Y, Fujiwara M, Fujii Y, Mukai H, Yonese J, Kawakami S, Yamamoto S, Migita T, Ishikawa Y, Kurata M, Nakamura T. Adult Xp11 translocation renal cell carcinoma diagnosed by cytogenetics and immunohistochemistry. Clin Cancer Res. 2009;15:1170–6. doi: 10.1158/1078-0432.CCR-08-1183. [DOI] [PubMed] [Google Scholar]
- 32.Klatte T, Streubel B, Wrba F, Remzi M, Krammer B, de Martino M, Waldert M, Marberger M, Susani M, Haitel A. Renal cell carcinoma associated with transcription factor E3 expression and Xp11.2 translocation: incidence, characteristics, and prognosis. Am J Clin Pathol. 2012;137:761–8. doi: 10.1309/AJCPQ6LLFMC4OXGC. [DOI] [PubMed] [Google Scholar]
- 33.Gaillot-Durand L, Chevallier M, Colombel M, Couturier J, Pierron G, Scoazec JY, Mege-Lechevallier F. Diagnosis of Xp11 translocation renal cell carcinomas in adult patients under 50 years: interest and pitfalls of automated immunohistochemical detection of TFE3 protein. Pathol Res Pract. 2013;209:83–9. doi: 10.1016/j.prp.2012.10.013. [DOI] [PubMed] [Google Scholar]


