Abstract
The diagnosis of amyotrophic lateral sclerosis (ALS) is mainly based on clinical and electrophysiological features. It is yet to be confirmed if cystatin C (Cys-C) can be a candidate diagnostic biomarker for ALS. This retrospective study aimed at investigating the changes in the level of Cys-C levels in the cerebrospinal fluid (CSF) of Chinese patients with ALS. CSF and serum samples obtained from patients with ALS, healthy controls (HC) and neurodegenerative disease controls from March 2012 to May 2014 were analyzed for levels of Cys-C using an immunoturbidimetric assay. The results were checked for the presence of meaningful correlations between Cys-C levels and variables such as the age of onset, site of symptoms onset, disease duration, and amyotrophic lateral sclerosis functional rating scale revised (ALSFRS-R) score, forced vital capacity (FVC) and rate of ALS disease progression. There was no difference in the Cys-C levels in CSF and serum between patients with ALS and controls. However, the serum Cys-C levels correlated with the ALSFRS-R score and the site of symptoms onset. The statistical analysis exhibited reduced levels of serum Cys-C in Upper limb-onset ALS (U-ALS) compared to Lower limb-onset ALS (L-ALS). The present data demonstrate that the level of Cys-C in CSF should not be considered as a biomarker of ALS. Cys-C in serum may be useful as an indicator of the severity of disease and site of symptoms onset although the specificity of serum Cys-C levels in ALS was not significant.
Keywords: Amyotrophic lateral sclerosis, cystatin C, cerebrospinal fluid, serum, interference factors
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurologic disease characterized by progressive motor neuron degeneration [1]. Currently, there is only one drug approved by the FDA to treat ALS and this therapy increases the life span by just two to three months, on an average [2]. Because CSF contains proteins and protein fragments released from ALS-affected neurons and neuroglia, it seems likely that profiles of CSF proteins may serve as biomarkers that are indicative of motor neuron degeneration in the spinal cord in patients with ALS [2]. Unfortunately, none of these reports has been fully validated or integrated into clinical practice so far [3]. Bunina bodies, the most specific histological hallmark of sporadic ALS (sALS) are immunonegative for ubiquitin, but have been found to be positive for cystatin C (Cys-C). Recently, it has been described that Cys-C is linked to the death of motor neurons in sALS [4]. However, there is no direct evidence that Cys-C is involved in ALS pathogenesis as no disease-causing mutations have been found within the CST3 gene of sporadic and familial ALS cases [5]. There have been reports of changes in Cys-C levels in neurodegenerative diseases such as Alzheimer’s disease and Creutzfeldt-Jakob disease and in models of pain [2]. There are three prior surface-enhanced laser desorption/ionization time of flight mass spectrometry studies reports of significant decreases in CSF Cys-C levels in ALS patients relative to healthy control (HC) and neurologic disease controls [1,2,6]. A recent study using small numbers of test subjects reported a significant reduction in CSF Cys-C and increased Cys-C levels in the plasma of ALS patients relative to individuals with polyneuropathy, as measured by ELISA [7]. Nevertheless, a study in Japan found that there was no difference in Cys-C levels between HCs and ALS or chronic inflammatory demyelinating polyneuropathy patients; It is interesting that Cys-C levels were significantly lower in multiple system atrophy (MSA) compared with HC. A new study indicates that in multivariable analyses only age, body mass index (BMI) and forced vital capacity (FVC) measured at the first clinic visit were independent prognostic indicators [8]. Recent studies suggest that BMI is an independent prognostic factor for survival after adjusting for markers of ALS disease severity [9,10]. A French multicenter cohort study revealed that a more significant worsening progression of weight (equivalent to BMI) and FVC loss in patients with bulbar onset [11]. Based on these prior studies, a larger study with a more comprehensive analysis of interference factors is required in order to compare prior results and assess if CSF Cys-C levels represent a candidate diagnostic biomarker for ALS. In this retrospective study, we have used an immuno-turbidimetric assay to check Cys-C levels and evaluate the diagnostic predictive value of Cys-C in both CSF and serum for ALS in the Chinese Han population.
Patients and methods
Demography and patient characteristics
Ninety-two consecutive patients with definite or probable ALS, admitted to the outpatient clinic of the Department of Neurology, Chinese PLA General Hospital from March 2012 to May 2014, were included in this study. Diagnosis of ALS was done by experienced neurologists specialized in motor neuron disease, according to the revised El Escorial criteria [12]. Seventy-eight patients had classic (spinal onset) disease and fourteen had bulbar onset. The median average time from clinical onset (when patient retrospectively reported initial symptoms) to diagnosis (when CSF was obtained) was 12.5 months for ALS subjects. This represents the typical time required for ALS patients to obtain a definitive diagnosis from the time of symptom onset. There were 59 (64.1%) men and 33 (35.8%) women with a mean (SD) age of 52.04 (10.07) years. For comparison, we also analyzed data from 43 multiple system atrophy (MSA) and 48 age-matched, unrelated HC who were enrolled in this study. The patients with MSA were diagnosed based on the Second Consensus Criteria [13]. Patients were divided into MSA-P or MSA-C based on the predominant symptoms and signs. HC subjects lacked any neurological symptoms and most of them were spouse or family friends of the hospitalized patients. Informed consent was obtained before collecting CSF and blood samples and the Institutional Review Board of the Chinese PLA General Hospital approved all procedures. All patients and controls were mainland Chinese Han population. At baseline, we collected demographic data (age, sex) and details of past medical conditions, ALSFRS-R, BMI on admission. Details of the patient demographic data are presented in Table 1. We excluded patients and controls with evidence of systemic complications (hypertension, coronary heart disease, urinary infection, rheumatoid arthritis) and those who received recent (≤ 3 months) glucocorticoid treatment, or those with any other major health event known to influence the levels of Cys-C.
Table 1.
Clinical characteristics of all study participants
| ALS (n=92) | MSA (n=43) | HC (n=48) | |
|---|---|---|---|
| Sex (Male/female) | 59/33 | 34/9 | 28/20 |
| Age (years) | |||
| 52.04±10.07 | 59.88±8.81 | 40.42±16.65 | |
| Relevant subgroups | 48 Upper limb-onset, 30 Lower limb-onset 14 Bulbar onset | 26 MSA-P, 17 MSA-C | NA |
| Age of onset (years) | 50.43±10.02 | 57.46±8.03 | NA |
| ALSFRS-R | 37.95±5.97 | NA | NA |
| Disease duration (months) | 19.30±15.48 | 29.08±25.39 | NA |
| FVC | 89.15±23.52 | NA | NA |
| BMI | 23.42±3.20 | 22.86±2.78 | 22.76±3.44 |
| Rate of disease progression (score/month) | 0.78±0.56 | NA | NA |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, the ALS functioning rating scale-revised; BMI, body mass index; FVC, forced vital capacity; HC, healthy controls; MSA, multiple system atrophy; MSA-C, multiple system atrophy-the cerebellar type; MSA-P, multiple system atrophy-Parkinsonian type; NA, not applicable.
Cys-C assay
We evaluated Cys-C levels in CSF and serum and correlated Cys-C levels with age at disease onset, site of onset, symptoms, disease duration and amyotrophic lateral sclerosis functional rating scale revised (ALSFRS-R), FVC and rate of ALS disease progression. ALS Patients were evaluated with the ALSFRS-R scale within 24 hours after admission by trained neurologists. All ratings performed by the same examiner were reproducible. The ALSFRS-R consists of 12 items to evaluate bulbar function, motor function and respiratory function and each item is scored on a scale of 0 (unable) to 4 (normal). The rate of ALS disease progression was calculated by the following formula:
ΔFS = (48-ALSFRS-R score at time of diagnosis)/Duration from the time of initial symptoms to diagnosis (months)
Our index analysis was based on results of previously published reports on Cys-C levels in ALS [1,2,5,6]. CSF and serum samples were obtained by lumbar puncture and venipuncture, respectively from 183 subjects at the Neurology Department of Chinese PLA General Hospital. All samples were spun at 4000 rpm at 4°C for 5 minutes to remove any cells and debris, aliquoted in small volumes, and stored in Eppendorf microcentrifuge tubes at -80°C. Only CSF samples without visible blood were processed by centrifugation. The concentrations of Cys-C in the CSF and serum samples were quantitatively measured employing an immuno-turbidimetric assay in an autoanalyzer.
Statistical analysis
All statistical analyses were carried out using SPSS 16.0 software. A general linear regression was used to estimate the CSF, serum Cys-C levels for each diagnostic group, with gender, age, and BMI included as control variables. We set three dummy variables to compare the differences among ALS and control subjects. The statistical design avoided interference factors, which included gender, age and BMI in between-group differences. Data were summarized and recorded as mean ± SD for the continuous variables. The relationship between Cys-C levels of CSF and serum versus the clinical indicators of the ALS patients were assessed and the partial correlation coefficient (rp) was used for variables to account for the influence of age, gender and BMI. For symptom onset subgroup comparison, one-way ANOVA was used to determine statistical significance, followed by the post-hoc pairwise comparison in Tukey’s method. Receiver operating characteristic (ROC) curves were used to examine the CSF and serum Cys-C levels in predicting ALS diagnosis. The area under the curve (AUC) was calculated from the ROC curve. For all data analysis, a P-value of < 0.05 was considered significant.
Results
CSF Cys-C levels and clinical indicators
We evaluated the CSF Cys-C levels and found that the estimated means for all the measures of CSF Cys-C were not significantly different from patients with ALS (4.17 mg/L; SD = 1.32 mg/L; range, 1.80-9.18 mg/L), HC (4.62 mg/L; SD = 1.94 mg/L; range, 2.10-7.82 mg/L) and MSA (4.10 mg/L; SD = 1.12 mg/L; range, 2.10-5.95 mg/L). Correlations between CSF Cys-C levels and clinical indicators were also compared. No meaningful correlations were observed between CSF Cys-C levels and each indicator (Table 2).
Table 2.
Correlations between CSF Cys-C levels and clinical indicators
| CSF Cys-C (mg/l) | FVC | ALSFRS-R | Disease duration | Age of onset | Site of onset symptoms | Rate of ALS disease progression |
|---|---|---|---|---|---|---|
| r-values | 0.010 | 0.126 | 0.081 | 0.096 | -0.103 | -0.002 |
| p-values | 0.940 | 0.355 | 0.553 | 0.192 | 0.452 | 0.991 |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, the ALS functioning rating scale-revised; FVC, forced vital capacity.
Serum Cys-C levels and clinical indicators
We repeated the group analysis for the Cys-C levels in serum and the association with clinical indicators. The levels of serum Cys-C were not significantly different from patients with ALS (1.03 mg/L; SD = 0.18 mg/L; range, 0.65-1.53 mg/L), HC (1.01 mg/L; SD = 0.38 mg/L; range, 0.46-2.39 mg/L) and MSA (1.07 mg/L; SD = 0.22 mg/L; range, 0.80-1.58 mg/L; Table 3). Measure of Cys-C in serum differed significantly by age and sex, while there were no significant differences based on BMI and diagnostic groups. We observed a significant negative correlation between serum Cys-C and ALSFRS-R (r = -0.355, P = 0.007; Figure 1), which suggested that Cys-C in serum may be a useful indicator of the severity of disease. We completed a linear regression and observed a positive correlation between serum Cys-C and site of symptoms onset (r = 0.274, P = 0.041). There was no significant correlation between serum Cys-C and any other indicator (Table 4).
Table 3.
Main group results for CSF and serum Cys-C
| N | Serum Cys-C (mg/l) Mean ±SD | CSF Cys-C (mg/l) Mean ±SD | |
|---|---|---|---|
| ALS | 92 | 1.03±0.18 | 4.17±1.32 |
| HC | 48 | 1.01±0.38 | 4.62±1.94 |
| MSA | 43 | 1.07±0.22 | 4.10±1.12 |
Abbreviations: ALS, amyotrophic lateral sclerosis; HC, healthy control; MSA, multiple system atrophy.
Figure 1.
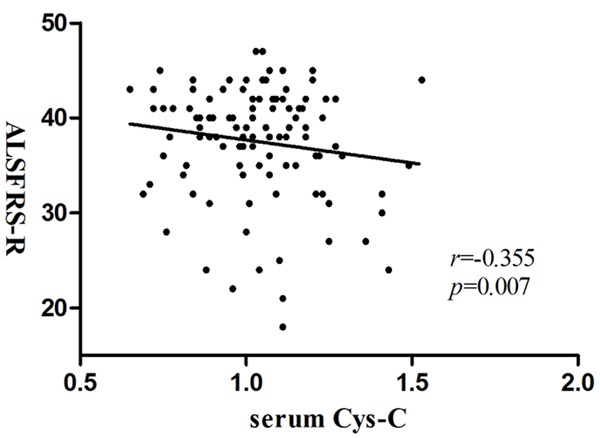
Correlation of serum Cys-C with ALSFRS-R: partial correlation coefficient is r = -0.355 (P = 0.007). (ALSFRS-R, Amyotrophic lateral sclerosis functional rating scale-Revised).
Table 4.
Correlations between serum Cys-C levels and clinical indicators
| Serum Cys-C (mg/L) | FVC | ALSFRS-R | Disease duration | Age at onset | Site of onset | Rate of ALS disease progression |
|---|---|---|---|---|---|---|
| r-values | -0.237 | -0.355 | 0.068 | -0.065 | 0.274 | 0.098 |
| p-values | 0.078 | 0.007 | 0.618 | 0.636 | 0.041 | 0.474 |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, the ALS functioning rating scale-revised; FVC, forced vital capacity.
Symptom onset subgroup results for Cys-C levels in CSF and serum
To further explore the relationship between Cys-C levels in CSF or serum and site of symptoms onset, we separated ALS patients into three ALS subgroups such as [Upper limb-onset ALS (U-ALS), Lower limb-onset ALS (L-ALS) and Bulbar-onset ALS (B-ALS)]. We evaluated the CSF Cys-C levels across the symptom onset subgroups and found that there was no significant difference among the three groups (P = 0.908). However, comparisons of the serum Cys-C levels and symptom onset were significant (P = 0.022) among the three groups. The post-hoc pairwise comparison exhibited reduced levels of serum Cys-C in the U-ALS group than that of the L-ALS group (Table 5). However, the comparisons between L-ALS and B-ALS or B-ALS and U-ALS were not statistically significant.
Table 5.
Symptom onset subgroup results for serum Cys-C
| N | Serum Cys-C (mg/l) Mean ±SD | Significance of Difference (p-values) | |
|---|---|---|---|
| Bulbar-onset ALS vs Upper limb-onset ALS | 14/48 | 1.02±0.22 vs 1.03±0.18 | 0.983 |
| Upper limb-onset ALS vs Lower limb-onset ALS | 48/30 | 1.03±0.18 vs 1.12±0.17 | 0.040 |
| Lower limb-onset ALS vs Bulbar-onset ALS | 30/14 | 1.12±0.17 vs 1.02±0.22 | 0.153 |
Abbreviation: ALS, amyotrophic lateral sclerosis.
Relationship between Cys-C levels in CSF and serum
We compared the mean Cys-C levels in CSF and serum of ALS patients and we found significant differences between both body fluids. To further characterize the relationship between CSF and serum Cys-C levels, we assessed the correlation between CSF and serum Cys-C levels for individual subjects. The results indicated that there was no correlation between CSF Cys-C and serum Cys-C levels (r = 0.07, P = 0.605).
BMI levels and clinical indicators
Recent studies of ALS suggest that BMI predicts survival in a curvilinear manner [14]. As mentioned above, in our analysis neither measure of Cys-C in CSF nor serum differed significantly by BMI. To determine the potential contribution of BMI to the levels of Cys-C in CSF and serum, we paid close attention to the relationship between BMI of ALS and clinical indicators and found that the BMI values correlated with FVC (Pearson r = 0.321, P = 0.003) (Figure 2), indicating that the decline in the FVC was associated with lower BMI. However, BMI did not correlate with the other clinical indicators (data not shown).
Figure 2.
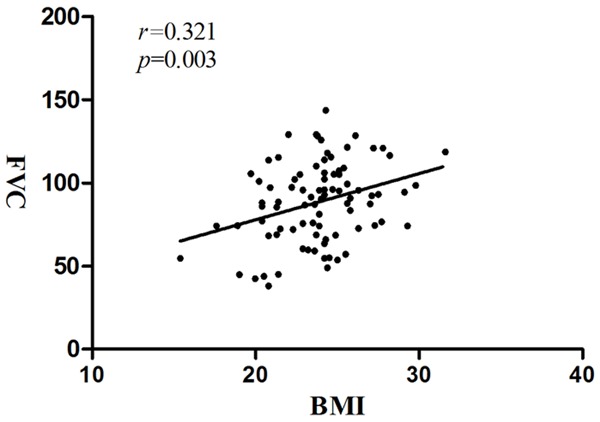
Correlation of BMI with FVC in ALS patients: Correlation of BMI with FVC in ALS patients, Pearson correlation coefficient is r = 0.321 (P = 0.003). (ALS, amyotrophic lateral sclerosis; BMI, body mass index; FVC, forced vital capacity).
Usefulness of CSF and Cys-C levels in predicting ALS diagnosis
ROC curve analysis demonstrated that CSF Cys-C levels (P = 0.893) and serum Cys-C levels (P = 0.380) fails to differentiate ALS patients from MSA and HC subjects. ROC curves were used to calculate the AUC to evaluate the usefulness of CSF and serum Cys-C levels in predicting ALS diagnosis. The AUC for CSF and serum Cys-C levels were 0.506 ± 0.043 and 0.538 ± 0.043, respectively (Figure 3).
Figure 3.
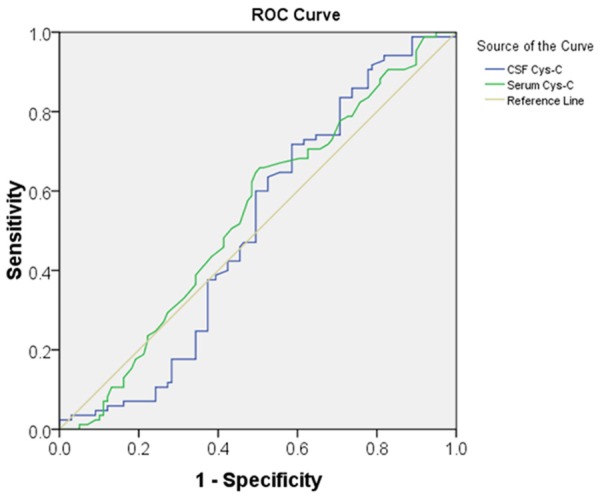
Receiver operating characteristic (ROC) curves for cerebrospinal fluid (CSF and serum Cys-C in the ALS cohort: The Area under Curve for CSF and serum Cys-C levels were 0.506 ± 0.043 (P = 0.893) and 0.538 ± 0.043 (P = 0.380), respectively.
Discussion
In this study we have used an immuno-turbidmetric assay to evaluate the diagnostic predictive value of Cys-C for ALS among the Chinese population. Mass spectrometry is a semi-quantitative method; in contrast, the enzyme-linked immunoabsorbent assay (ELISA) and the immuno-turbidimetric assay is quantitative methods and widely recommended. The immuno-turbidimetric assay is a fully automated, one-step, reproducible and easy to perform; thus, it can be a useful tool in the measurement of Cys-C. We also analyzed if any direct relationship between levels of Cys-C in CSF and serum and clinical indicators were present. Prior studies have reported that Cys-C concentrations are decreased in the CSF of ALS patients relative to HC and neurologic disease controls [2,6,7,15]. Meghan et al reported that increased Cys-C levels were observed in the plasma of ALS patients [7]. Recent in vitro and in vivo data have demonstrated that Cys-C plays protective roles and may have a therapeutic role that could potentially prevent brain damage and neurodegeneration [16]. In the present study, data were collected and analyzed in an attempt to estimate the difference between ALS patients and controls and clarify the potential effect of altered levels of Cys-C in CSF and serum. However, we observed no significant difference in levels of Cys-C in CSF and serum between ALS and control groups. We analyzed the relationship between Cys-C levels of CSF and serum versus the clinical indicators of ALS patients. As reported earlier, no meaningful correlations were observed between CSF Cys-C levels and each indicator [7]. However, in this study, we observed a direct correlation between serum Cys-C and ALSFRS-R scores, this finding suggested that Cys-C in serum may be useful as an indicator of the severity of disease. However, the exact mechanisms behind the observed correlations have not been conclusive; further studies will be pursued to explore potential functional links between Cys-C and ALSFRS-R. We observed that a positive correlation between serum Cys-C and site of symptoms onset, which can be clinically useful as a diagnostic biomarker to differentiate between the ALS onset types that are classified as U-ALS, L-ALS and B-ALS. This analysis revealed serum Cys-C levels in U-ALS were lower as compared to L-ALS. However, the comparisons between L-ALS and B-ALS or B-ALS and U-ALS were not statistically significant. This may have resulted because of patients with different sites of disease onset, as B-ALS patients typically have shorter survival times than limb-onset patients. Interestingly, Cys-C levels correlated with symptom onset in limb-onset ALS, this suggests that Cys-C levels in the serum may be useful as a marker of symptom onset in specific subtypes of ALS. However, B-ALS patients in our study exhibited a significantly rapid disease progression when compared with limb-onset patients (data not shown). Unfortunately, there were inadequate numbers of U-ALS, L-ALS and B-ALS patients to analyze these individual subgroups in this study. Further studies are required to explore this possibility. Hence, Cys-C in serum may be useful as an indicator of the severity of disease and site of symptoms onset, although the specificity of serum Cys-C levels in ALS was not significant. We also assessed the relationship between Cys-C levels in CSF and serum in ALS patients. Cys-C levels were previously reported to be 5-fold higher in the CSF than in the bloodstream [17]. However, the results of this study suggest that Cys-C is 4-fold higher in the CSF than in the serum, which was marginally lower than that reported in published literature. Because Cys-C levels are higher in the CSF than in serum, it has been speculated that Cys-C is produced in the central nervous system (CNS) or, alternatively, it is transported actively to the CNS through the blood brain barrier by an unknown mechanism [18]. As hypothesized, our data indicated that there is no correlation between CSF Cys-C and serum Cys-C levels, as observed by the absence of a relationship between Cys-C levels in concurrently drawn CSF and serum samples. We also analyzed the relationship between BMI and clinical indicators in ALS patients and found that the BMI values correlated with FVC, it has been observed that the negative prognostic impact of weight loss might also influence this result. However, BMI did not correlate with the other indicators. Hence, it is imperative that the BMI values of ALS patients are considered when levels of Cys-C in the CSF or serum are measured, especially when respiratory muscles were involved. Nadia et al. described elevation of serum Cys-C in obese individuals, and strongly opined that such an increased production of Cys-C could be because of enlarged adipose tissues [19]. It has been reported that in men, hypertension, coronary heart disease, urinary infection, rheumatoid arthritis, glucocorticoid treatment, advancing age and lower functional status were found to be significant predictors of higher serum Cys-C values, whereas among women, the corresponding factors were hypertension, glucocorticoid treatment, age, functional status and BMI for elevated serum Cys-C [20]. Reports from previous studies have demonstrated that BMI was one of the significant predictors of high serum Cys C values for women and/or men [21]. Although no significant correlations between BMI and Cys-C levels in CSF and serum were found in our study. However, it is likely that this disparity is the result of varied techniques and sample maintenance conditions between our study and the reported studies. However, a subgroup analysis based on sex, age, BMI, and other interference factors for all the subjects augur well for the authenticity of our findings. With reference to the ability of CSF and serum Cys-C levels to confirm ALS diagnosis, we failed to demonstrate a statistically significant improvement in the diagnostic accuracy, as well as in distinguishing between ALS patients with MSA and HC, with a relatively low AUC value of 0.506 and 0.538, respectively. These findings confirm that Cys-C from CSF and serum cannot be potentially used as a biomarker for ALS. As in most Cys-C-related ALS studies, our cohort predominantly consisted of a large sample size, which could accurately observe the fluctuations in CSF and serum Cys-C levels, along with the corresponding clinical symptoms indicating ALS. Our analysis included BMI, a calculation using height and weight, site of onset symptoms and FVC. It is important to note that although additional studies using a similar quantitative assay such as ELISA or more quantitative mass spectrometry techniques are recommended, this will require a greater degree of sensitivity and reproducibility across laboratories to generate a clinically useful diagnostic assay. However, a past study suggests that the immunoturbidimetric assay is also a reproducible and easy assay [5]. In our study, the difference in Cys-C levels in CSF between the diagnostic groups was not statistically significant; however, this finding does not eliminate the possibility that changes in CSF Cys-C levels correlate with more subtle biochemical changes associated with disease progression, as these may not be accurately reflected by overt functional measures of clinical disease status. Finally, large prospective studies would be required to evaluate the utility of any ALS-specific biomarker for global application. There are a number of potential limitations in this study. First, all the serum samples were collected in the morning before breakfast. In contrast, the CSF samples were collected at the time of randomization; there exists the possibility of varying metabolite concentration in CSF at differing times of the day. For example, the effects of diurnal rhythms, diet, alcohol, and drugs of various types may also be important [22]. Another potential limitation is not including a sufficient number of control groups. The present study would have been more robust if the number of controls had matched that of ALS patients. In summary, our results indicate that Cys-C levels in CSF and serum when measured by an immunoturbidimetric assay, suggest that Cys-C levels in CSF are not a useful biomarker of ALS.
Acknowledgements
The authors would like to thank all the neurologists of the Chinese PLA General Hospital for assistance with the collection and storage of CSF and serum samples, and the guidance from the Neurology Department Laboratory. We also thank Dr. Jinyang Cai and Dr. Chao Zhang from School of Management and Economics, Beijng Institute of Technology for their assistance in statistical data analysis.
Disclosure of conflict of interest
None.
References
- 1.Pasinetti GM, Ungar LH, Lange DJ, Yemul S, Deng H, Yuan X, Brown RH, Cudkowicz ME, Newhall K, Peskind E, Marcus S, Ho L. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66:1218–1222. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- 2.Ranganathan S, Williams E, Ganchev P, Gopalakrishnan V, Lacomis D, Urbinelli L, Newhall K, Cudkowicz ME, Brown RH Jr, Bowser R. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95:1461–1471. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell RM, Freeman WM, Randazzo WT, Stephens HE, Beard JL, Simmons Z, Connor JR. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology. 2009;72:14–19. doi: 10.1212/01.wnl.0000333251.36681.a5. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, Devidze N, Wang X, Grubb A, Gan L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto-Watanabe Y, Watanabe M, Jackson M, Akimoto H, Sugimoto K, Yasujima M, Wakasaya Y, Matsubara E, Kawarabayashi T, Harigaya Y, Lyndon AR, Shoji M. Quantification of cystatin C in cerebrospinal fluid from various neurological disorders and correlation with G73A polymorphism in CST3. Brain Res. 2010;1361:140–145. doi: 10.1016/j.brainres.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Ryberg H, An J, Darko S, Lustgarten JL, Jaffa M, Gopalakrishnan V, Lacomis D, Cudkowicz M, Bowser R. Discovery and verification of amyotrophic lateral sclerosis biomarkers by proteomics. Muscle Nerve. 2010;42:104–111. doi: 10.1002/mus.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson ME, Boumaza I, Lacomis D, Bowser R. Cystatin C: a candidate biomarker for amyotrophic lateral sclerosis. PLoS One. 2010;5:e15133. doi: 10.1371/journal.pone.0015133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: Analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3:313–320. doi: 10.1212/CPJ.0b013e3182a1b8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, Walker J, Katsovskiy I, Schoenfeld D, Cudkowicz M, Leitner M. The PRO-ACT database: design, initial analyses and predictive features. Neurology. 2014;83:1719–1725. doi: 10.1212/WNL.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavelou P, Blanquet M, Peyrol F, Ouchchane L, Gerbaud L. Rates of progression of weight and forced vital capacity as relevant measurement to adapt amyotrophic lateral sclerosis management for patient Result of a French multicentre cohort survey. J Neurol Sci. 2013;331:126–131. doi: 10.1016/j.jns.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 13.Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 14.Reich-Slotky R, Andrews J, Cheng B, Buchsbaum R, Levy D, Kaufmann P, Thompson JL. Body mass index (BMI) as predictor of ALSFRS-R score decline in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:212–216. doi: 10.3109/21678421.2013.770028. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji-Akimoto S, Yabe I, Niino M, Kikuchi S, Sasaki H. Cystatin C in cerebrospinal fluid as a biomarker of ALS. Neurosci Lett. 2009;452:52–55. doi: 10.1016/j.neulet.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier S, Kaur G, Mi W, Tizon B, Levy E. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci (Schol Ed) 2011;3:541–554. doi: 10.2741/s170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson ME, Boumaza I, Bowser R. Measurement of cystatin C functional activity in the cerebrospinal fluid of amyotrophic lateral sclerosis and control subjects. Fluids Barriers CNS. 2013;10:15. doi: 10.1186/2045-8118-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono S, Shimizu N, Imai T, Mihori A, Nagao K. Increased cystatin C immunoreactivity in the skin in amyotrophic lateral sclerosis. Acta Neurol Scand. 2000;102:47–52. doi: 10.1034/j.1600-0404.2000.102001047.x. [DOI] [PubMed] [Google Scholar]
- 19.Naour N, Fellahi S, Renucci JF, Poitou C, Rouault C, Basdevant A, Dutour A, Alessi MC, Bastard JP, Clement K, Guerre-Millo M. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring) 2009;17:2121–6. doi: 10.1038/oby.2009.96. [DOI] [PubMed] [Google Scholar]
- 20.Wasen E, Isoaho R, Mattila K, Vahlberg T, Kivela SL, Irjala K. Serum cystatin C in the aged: relationships with health status. Am J Kidney Dis. 2003;42:36–43. doi: 10.1016/s0272-6386(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 21.Galteau MM, Guyon M, Gueguen R, Siest G. Determination of serum cystatin C: biological variation and reference values. Clin Chem Lab Med. 2001;39:850–857. doi: 10.1515/CCLM.2001.141. [DOI] [PubMed] [Google Scholar]
- 22.Cudkowicz ME, Swash M. CSF markers in amyotrophic lateral sclerosis: has the time come? Neurology. 2010;74:949–950. doi: 10.1212/WNL.0b013e3181d72c31. [DOI] [PubMed] [Google Scholar]


