Abstract
It has recently been reported that transmembrane protease, serine 3 (TMPRSS3) is overexpressed in cancer. However, TMPRSS3 expression and its biological roles in breast cancer (BC) have not been reported. This study aims to investigate the TMPRSS3 expression in BC and its relation with the outcome of the BC. This study involves a total of 149 BC tissues and adjacent non-cancerous tissues that were diagnosed between 2004 and 2007. Immunohistochemistry is used to compare the pattern of TMPRSS3 expression in BC and in adjacent non-cancerous tissues. Kaplan-Meier and Cox regression analyses were used to assess the prognostic significance of TMPRSS3 expression among BC patients. The results are as follow: TMPRSS3 expression is significantly in BC compared to adjacent non-cancerous tissues. High TMPRSS3 expression was related to TNM stage, lymph node metastasis, and Ki-67 expression. Furthermore, the overall survival (OS) and disease-free survival (DFS) in BC patients with high TMPRSS3 expression were lower than those in patients with low TMPRSS3 expression. Based on multivariate analysis, lymph node metastasis, TNM stage and TMPRSS3 expression are independent prognostic factors for OS in BC, while lymph node metastasis and TMPRSS3 expression are independent prognostic factors for DFS in BC. This study proves that TMPRSS3 expression is an independent prognostic factor for BC patients. Bioinformatic analysis of potential TMPRSS3 binding proteins revealed that TMPRSS3 could be a key regulator of cancer pathways. This study helps us better understand the function of TMPRSS3 in cancer.
Keywords: TMPRSS3, immunohistochemistry, breast cancer, prognosis
Introduction
Breast cancer is one of the most frequently diagnosed cancers and one of the top leading causes of cancer related deaths among women. It makes up 23% of the total cancer cases and 14% of the cancer related deaths in developing as well as developed countries [1]. Despite the improvement of breast cancer prevention, diagnosis, and therapy, the prognosis and survival for most patients have not dramatically changed [2]. To date, prognostic and predictive factors, such as stage, grade, estrogen receptor (ER) and progesterone receptor (PR) status, and HER2 amplification are considered to guide adjuvant systemic therapy in women with early-stage BC. However, there is still heterogeneity in the specific subgroups of breast cancer. In these cases, using the expression profile of these traditional markers alone, it is impossible to successfully categorize all breast cancers into specific risk groups. Therefore, it is essential to consider new prognostic and predictive factors in order to optimize treatments among BC patients [3].
Type II transmembrane serine protease (TTSP) family is a class of membrane-bound proteolytic enzymes. They are important mediators of various biological processes [4], and they are related to cancer progression as well [5]. A range of proteolytic activities occur in malignant cells with TTSPs, which enable them to survive, grow, move, invade, and digest the extracellular matrix. TTSPs are therefore seen as perfect candidates for tumor markers [6]. Enteropeptidase, hepsin, spinesin (TMPRSS5), corin, matriptase, matriptase-2, TMPRSS2 and TMPRSS4 are all members of this class of enzymes [7].
The transmembrane protease, serine 3 (TMPRSS3) gene, was originally cloned and named tumor associated differentially-expressed gene-12 (TADG-12). It is one of the members of the TTSP family. It has been reported that in ovarian cancer, TMPRSS3 protease is up-regulated [8]. It was proposed as a target for immunotherapeutic vaccination strategies that would be used against chemotherapy resistant refractory ovarian carcinomas [9].
TMPRSS3 is a pro-metastatic mediator in other cancer types such as pancreatic, breast and colorectal cancer. TMPRSS3 is highly expressed in these cancers [10-12]. It is membrane bound with both an NH2-terminal signal-anchor sequence and a glycosylated extracellular region in addition to the serine protease domain. Because its locus is localized to chromosome 11 at q23.3.(10), TMPRSS3 is over expressed in cancer. This could contribute to the process of metastasis and tumor invasion. However, the pattern of TMPRSS3 expression in human breast cancer has not been explored yet.
In this study, we aimed to detect the expression levels and subcellular localization of TMPRSS3 protein by immunohistochemistry in a random sample of 149 patients with BC, exploring how it affects clinicopathological features and patient survival. Furthermore, we aim to determine which independent prognostic factors affect the disease-specific survival in patients with BC.
Materials and methods
Patients and ethics statement
The formalin-fixed, paraffin embedded specimens used for immunohistochemistry were collected from 149 breast cancer patients following surgical resections with no preoperative chemotherapy or radiotherapy in the First Affiliated Hospital of China Medical University from 2004 to 2007. Data were collected from the patients’ operative and pathological reports, and follow-up data were retrieved from the clinical database. Clinicopathological data and patient characteristics were obtained from medical archives by retrospective analysis.
The Human Research Ethical Committee of the China Medical University Affiliated Stomatological Hospital had approved the use of these tissue samples for the study. All the patients included in the study agreed that their tumor samples could be used for the purpose of investigation during their initial diagnosis. Furthermore, written consents showing the patients’ willingness to take part in this study were obtained from all participants.
Immunohistochemical staining
First, paraffin sections were taken from the specimens and cut into 4-μm thick sections. They were then added onto poly-lysine coated slides and incubated at 65°C overnight. The incubated slides were then deparaffinized in xylene and rehydrated with graded alcohol. The next step was to retrieve the antigen using citrate buffer (pH 6.0) and store the slides in Tris buffered saline (TBS). In order to block endogenous peroxidase activity, 3% hydrogen peroxide was added to the slides. They were then incubated overnight at 4°C in monoclonal antibody (Novus, Littleton, CO, USA) solution at 1:200 dilution. Finally, the slides were incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin, and color was developed using the DAB Horseradish Peroxidase Color Development Kit (Maixin Co., Fuzhou, China).
Evaluation of immunostaining
Two independent pathologists separately dealt with the evaluation for positive DAB staining of all the immunoreactions. Every slide was examined five times, and 100 cells were observed during each examination using a medical microscope 400 times magnification. There was positive immunostaining of the tumor cell membranes. Tissue samples stained for TMPRSS3 expression were classified into five categories and given a score from 0-5 according to the percentage of positively stained cells in each sample: ‘0’ (0%), ‘1’ (1%-5%), ‘2’ (5%-25%), ‘3’ (25%-50%) and ‘4’ (50%-100%). Additionally, the staining intensity of tissue samples was used to divide them into four categories and assign them a score between 0-3: 0: negative, 1: weak, 2: moderate and 3: strong. Then, the sum of the first and second score was used to determine TMPRSS3 expression levels: 0-2, low expression and 3-7, high expression. In this way, breast cancer patients were categorized into two groups: TMPRSS3-high and TMPRSS3-low patients.
Bioinformatics analysis
Potential TMPRSS3 binding proteins were analyzed using the STRING program.
Statistical analysis
In this study, all statistical analyses were performed using the SPSS version 16.0 software (SPSS, Chicago, IL, USA). The possible connection between TMPRSS3 expression and clinicopathological features of BC patients was examined using the χ2 test. Ten-year Kaplan-Meier survival curves were generated, and the differences between the curves were estimated using the log-rank test. OS curves (overall survival), defined from breast cancer diagnosis to the date of death from any cause, and DFS curves (disease-free survival), recorded from breast cancer diagnosis to any event related to breast cancer, were generated to determine the survival differences between the TMPRSS3-high and TMPRSS3-low patients. Based on the results of these two curves, the effects of TMPRSS3 expression on patient survival was examined using the hazards regression (HR), with the calculation of both univariate and multivariate Cox proportional HR models. Differences were considered statistically significant when P values were less than 0.05.
Results
TMPRSS3 expression in breast cancer (BC) and non-cancerous tissues
BC and adjacent non-cancerous tissue samples were collected from 149 human subjects to examine the potential correlation between TMPRSS3 and the clinicopathological features of BC, which were summarized in Table 1. Using an immunohistochemical staining method, TMPRSS3 expression levels and subcellular localization in BC and adjacent non-cancerous tissue were determined. Specific TMPRSS3 protein staining took place mainly in the membrane of BC cells (Figure 1). TMPRSS3 expression in BC tissue was found to be increased compared to adjacent non-cancerous tissue. TMPRSS3 expression occurred at high frequency (70.5%, 105/149) in BC tissue. It is worth mentioning that the expression profiles of the estrogen receptor α (ERα), the progesterone receptor (PR), antigen Ki-67 and human epidermal growth factor receptor-2 (HER2) were also determined for BC tissues and included in the database.
Table 1.
Clinical characteristics according to TMPRSS3 expression in BC
| Clinicopathological variables | Number of patients | High TMPRSS3 expression n=105 | Low TMPRSS3 expression n=44 | P value |
|---|---|---|---|---|
| Age | ||||
| <50 years | 64 | 46 (43.8%) | 18 (40.9%) | 0.744 |
| ≥50 years | 85 | 59 (56.2%) | 26 (59.1%) | |
| Menopausal status | ||||
| Premenopausal | 80 | 55 (52.4%) | 25 (56.8%) | 0.620 |
| Postmenopausal | 69 | 50 (47.6%) | 19 (43.2%) | |
| TNM stage | ||||
| I-II | 108 | 71 (67.6%) | 37 (84.1%) | 0.040* |
| III-IV | 41 | 34 (32.4%) | 7 (15.9%) | |
| ER status | ||||
| Negative | 83 | 57 (54.3%) | 26 (59.1%) | 0.590 |
| Positive | 66 | 48 (45.7%) | 18 (40.9%) | |
| PR status | ||||
| Negative | 72 | 50 (47.6%) | 22 (50%) | 0.791 |
| Positive | 77 | 55 (52.4%) | 22 (50%) | |
| Her-2 status | ||||
| Negative | 40 | 25 (23.8%) | 15 (34.1%) | 0.196 |
| Positive | 109 | 80 (76.2%) | 29 (65.9%) | |
| LN metastasis | ||||
| Negative | 75 | 46 (43.8%) | 29 (65.9%) | 0.014* |
| Positive | 74 | 59 (56.2%) | 15 (34.1%) | |
| ki-67 status | ||||
| Negative | 36 | 31 (29.7%) | 23 (53.1%) | 0.008** |
| Positive | 60 | 74 (70.3%) | 21 (46.9%) |
P<0.05;
P<0.01.
Figure 1.
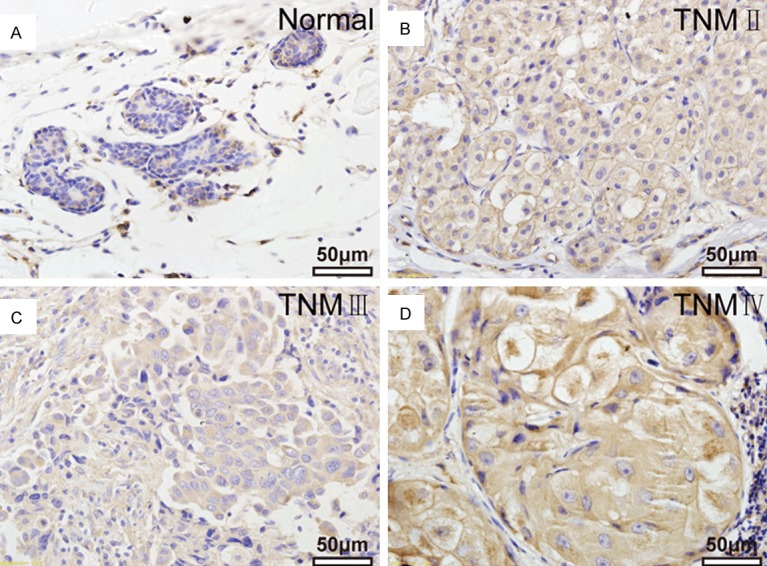
TMPRSS3 expression by immunohistochemistry in clinical specimens. A. TMPRSS3 staining of breast tissue. B-D. TMPRSS4 staining in different stages of breast cancer tissues.
Correlation between TMPRSS3 expression and clinicopathological features of BC patients
The correlation between TMPRSS3 expression and clinicopathological parameters of BC was studied to further determine how TMPRSS3 is involved in the development of BC. Statistical analysis (Table 1) illustrated the significant correlation between the level of TMPRSS3 expression and TNM stage (P = 0.040), lymph node metastasis (P = 0.014), and Ki-67 expression (P = 0.008), showing that high TMPRSS3 expression was associated with late clinical stage (32.4%), high possibility of lymph node metastasis (56.2%), and high Ki-67 expression levels (70.3%), whereas low TMPRSS3 expression group was not. However, no obvious association was observed between TMPRSS3 expression and age, menopausal status, ER, PR, Her-2 expression.
Analysis of the correlation between TMPRSS3 expression and patient survival in BC
Follow-up data was collected from 149 BC patients during a period of time ranging from 22 to 123 months, with an average of 93.4 months. TMPRSS3 expression levels and survival time of BC patients were recorded and their correlation was assessed by Kaplan-Meier survival analysis. While TMPRSS3 expression varied, survival curves stratified. Using log-rank tests (Figure 2), we have determined that OS and DFS of BC patients with high TMPRSS3 expression levels were lower than those with low TMPRSS3 expression levels (OS, P = 0.032; DFS, P = 0.042, respectively). Therefore, TMPRSS3 could be a significant biomarker for the evaluation of BC patient’s prognosis.
Figure 2.
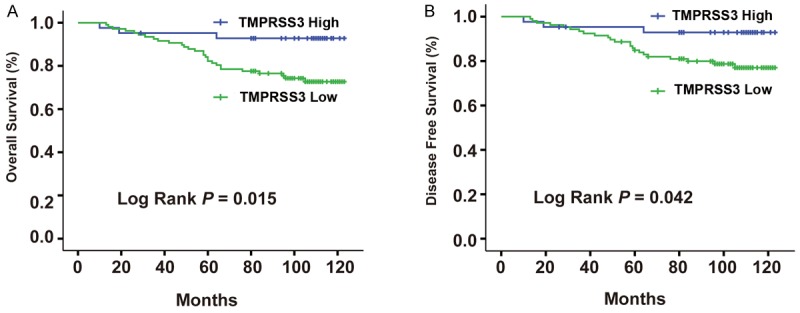
Correlation between survival analyses of TMPRSS3 expression in BC with clinicopathological characteristics. A. Overall survival (OS). B. Disease-free survival (DFS).
The impact of TMPRSS3 expression and clinicopathological features on BC patient’s prognosis was assessed using Cox proportional hazard model univariate and multivariate analyses. In Table 2, univariate Cox regression analysis demonstrated poor OS and DFS were significantly associated with the following elements: TNM stage (P = 0.031 for OS), lymph node metastasis (P = 0.015 for OS; P = 0.017 for DFS), Ki-67 expression (P = 0.039 for OS), as well as TMPRSS3 expression (P = 0.032 for OS; P = 0.042 for DFS). Similarly, multivariate analyses allowed us to conclude that TNM stage (P = 0.044 for OS), lymph node metastasis (P = 0.006 for OS; P = 0.002 for DFS), and TMPRSS3 expression (P = 0.043 for OS; P = 0.049 for DFS) were related to poor OS and DFS.
Table 2.
Univariable and multivariable analysis of BC survival using Cox’s proportional hazards model
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| OS | ||||||
| Age (<50 vs. ≥ 50 years) | 0.289 | 0.049-1.701 | 0.170 | |||
| Menopausal status (pre vs. post) | 3.237 | 0.0558-18.774 | 0.190 | |||
| TNM stage (I, II vs. III, IV) | 3.222 | 0.391-5.817 | 0.031* | 3.946 | 0.335-5.297 | 0.044* |
| ER status (negative versus positive) | 1.886 | 0.623-5.712 | 0.261 | |||
| PR status (negative versus positive) | 0.395 | 0.134-1.166 | 0.093 | |||
| Her-2 status (negative versus positive) | 0.662 | 0.245-1.785 | 0.415 | |||
| Lymph nodes metastasis (Yes vs. No) | 4.963 | 1.364-18.058 | 0.015* | 4.672 | 1.542-14.161 | 0.006** |
| Ki-67 status (negative versus positive) | 7.725 | 0.952-62.705 | 0.039* | 6.940 | 0.910-52.927 | 0.062 |
| TMPRSS3 expression (High vs. Low) | 10.163 | 1.221-84.586 | 0.032* | 7.540 | 1.004-56.597 | 0.043* |
| DFS | ||||||
| Age (<50 vs. ≥ 50 years) | 0.164 | 0.020-1.320 | 0.089 | |||
| Menopausal status (pre vs. post) | 3.510 | 0.466-26.461 | 0.223 | |||
| TNM stage (I, II vs. III, IV) | 1.227 | 0.364-4.138 | 0.742 | |||
| ER status (negative vs. positive) | 3.058 | 0.868-10.779 | 0.082 | |||
| PR status (negative vs. positive) | 0.295 | 0.086-1.009 | 0.052 | |||
| Her-2 status (negative vs. positive) | 0.682 | 0.227-2.049 | 0.495 | |||
| Lymph nodes metastasis (Yes vs. No) | 6.150 | 1.389-27.225 | 0.017* | 7.500 | 2.146-26.218 | 0.002** |
| Ki-67 status (negative vs. positive) | 7.678 | 0.919-64.165 | 0.060 | |||
| TMPRSS3 expression (High vs. Low) | 9.959 | 0.965-83.138 | 0.042* | 7.370 | 0.974-55.762 | 0.049* |
OS, overall survival; DFS, disease-free survival; HR, hazard ratio.
P<0.05;
P<0.01.
Bioinformatics analysis results
Figure 3 showed that TMPRSS3 may participate in several cancer related pathways including the UBASH3A, CDH23, MYO15A, and GRXCR1 pathways.
Figure 3.
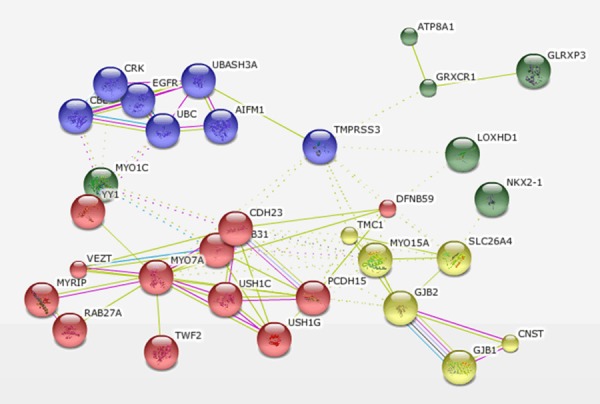
Bioinformatics analysis of TMPRSS3 binding protein.
Discussion
TTSPs activities on the surface of tumor cells can break down the surrounding extracellular matrix components, thereby promoting the process of metastasis. In this way they contribute to tumor growth, invasion and metastasis [13]. The human TTSP family consists of 17 members. Some of them have significant functions in development [14], and are involved in tumorigenesis and metastasis [5,15]. TMPRSS3 is a member of the TTSP family and was found to be highly expressed in several cancer tissues, enhancing cancer cell metastasis and tumor invasion [10]. TMPRSS3 expression is significantly associated with breast cancer [13].
In this study, TMPRSS3 expression was detected in BC tissues for the first time using immunohistochemistry. Our results showed that it was mainly localized in the membrane of cancer cells, and that TMPRSS3 expression was significantly correlated with TNM stage, lymph node metastasis and Ki-67 expression in BC patients. In addition, the results showed that the TMPRSS3 expression levels were inversely correlated with the OS and DFS of BC patients. Therefore, TMPRSS3 was an independent prognostic factor for OS and DFS according to multivariate analysis. Finally, the results showed that overexpression of TMPRSS3, a contributor to BC invasion, metastasis, and cell proliferation, was a poor prognostic factor for BC patients and that suppression of TMPRSS3 expression would be a potential target for therapeutic intervention in BC. Overall, high TMPRSS3 expression levels were significantly associated with tumor progression, metastasis, and poor prognosis. Therefore, TMPRSS3 may be used as a potential biomarker to identify a subgroup of BC patients with more aggressive tumors. It could also become a prognosis tool, and serve as a potential therapeutic target for BC patients in the future.
Bioinformatics analysis suggests TMPRSS3 could interact with UBASH3A (ubiquitin associated and SH3 domain containing, A), CDH23, MYO15A, GRXCR1 and epidermal growth factor receptor (EGFR) signaling pathway. Interfering with CBL-mediated down-regulation and degradation of receptor-type tyrosine kinases, UBASH3A promotes the accumulation of activated target receptors, such as T-cell receptors, EGFR and PDGFRB [16]. UBASH3A is overexpressed in TNBC, supporting malignant growth, invasion, and metastasis mainly by modulating EGFR [17]. Cadherins are calcium dependent cell adhesion proteins, which preferentially interact with each other in a homophilic manner in connecting cells. Cadherin 23 (CDH23) functions in establishing and/or maintaining the proper organization of the stereocilia bundle of hair cells in the cochlea and the vestibule during late embryonic and early postnatal development. According to a recent study, CDH23 may play an important role in the early stages of metastasis [18]. MYO15A is an actin-based motor molecule with ATPase activity [19]. With a highly divergent tail, it is presumed to bind to membranous compartments, allowing it to be moved relative to actin filaments. Glutaredoxin, cysteine rich 1 (GRXCR1) contains GRX-like domains that play a part in the S-glutathionylation of proteins and are possibly involved in actin organization. Taken together these studies suggest that TMPRSS3 may play the role of a crucial node bridging multiple invasion-promoting pathways and it could be a novel gene associated with resistance of trastuzumab. This study provides us with a potential therapeutic target for TNBC.
Acknowledgements
Thanks for grants from the Science and Technology project of Liaoning Province to support the study (Nos. 2012225002).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wei G, Wang Y, Zhang P, Lu J, Mao JH. Evaluating the prognostic significance of FBXW7 expression level in human breast cancer by a meta-analysis of transcriptional profiles. J Cancer Sci Ther. 2012;4:299–305. doi: 10.4172/1948-5956.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dechaphunkul A, Phukaoloun M, Kanjanapradit K, Graham K, Ghosh S, Santos C, Mackey JR. Prognostic significance of tissue inhibitor of metalloproteinase-1 in breast cancer. Int J Breast Cancer. 2012;2012:290854. doi: 10.1155/2012/290854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo R, Wu Q, Dickson RB, Netzel-Arnett S, Antalis TM, Bugge TH. Type II transmembrane serine proteases. Thromb Haemost. 2003;90:185–193. doi: 10.1160/TH03-02-0071. [DOI] [PubMed] [Google Scholar]
- 5.Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- 6.Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–860. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- 7.Choi SY, Bertram S, Glowacka I, Park YW, Pohlmann S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol Med. 2009;15:303–312. doi: 10.1016/j.molmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Underwood LJ, Shigemasa K, Tanimoto H, Beard JB, Schneider EN, Wang Y, Parmley TH, O’Brien TJ. Ovarian tumor cells express a novel multi-domain cell surface serine protease. Biochim Biophys Acta. 2000;1502:337–350. doi: 10.1016/s0925-4439(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 9.Bellone S, Anfossi S, O’Brien TJ, Cannon MJ, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. Induction of hu- man tumor-associated differentially expressed gene-12 (TADG-12/TMPRSS3)-specific cytotoxic T lymphocytes in human lymphocyte antigen-A2.1-positive healthy donors and patients with advanced ovarian cancer. Cancer. 2009;115:800–811. doi: 10.1002/cncr.24048. [DOI] [PubMed] [Google Scholar]
- 10.Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, Lerch MM, Adler G, Gress TM. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60:2602–2606. [PubMed] [Google Scholar]
- 11.Carlsson H, Petersson S, Enerback C. Cluster analysis of S100 gene expression and genes correlating to psoriasin (S100A7) expression at different stages of breast cancer development. Int J Oncol. 2005;27:1473–1481. [PubMed] [Google Scholar]
- 12.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 13.Luostari K, Hartikainen JM, Tengstrom M, Palvimo JJ, Kataja V, Mannermaa A, Kosma VM. Type II transmembrane serine protease gene variants associate with breast cancer. PLoS One. 2014;9:e102519. doi: 10.1371/journal.pone.0102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto T, Kato M, Shimomura T, Kitamura N. TMPRSS13, a type II transmembrane serine protease, is inhibited by hepatocyte growth factor activator inhibitor type 1 and activates pro-hepatocyte growth factor. FEBS J. 2010;277:4888–4900. doi: 10.1111/j.1742-4658.2010.07894.x. [DOI] [PubMed] [Google Scholar]
- 15.Webb SL, Sanders AJ, Mason MD, Jiang WG. Matriptase-2 inhibits HECV motility and tubule formation in vitro and tumour angiogenesis in vivo. Mol Cell Biochem. 2013;375:207–217. doi: 10.1007/s11010-012-1544-z. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Gallo LM, Sanchez E, Ortego-Centeno N, Sabio JM, Garcia-Hernandez FJ, de Ramon E, Gonzalez-Gay MA, Torsten W, Anders HJ, Gonzalez-Escribano MF, Martin J. Evidence of new risk genetic factor to systemic lupus erythematosus: the UBASH3A gene. PLoS One. 2013;8:e60646. doi: 10.1371/journal.pone.0060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee ST, Feng M, Wei Y, Li Z, Qiao Y, Guan P, Jiang X, Wong CH, Huynh K, Wang J, Li J, Karuturi KM, Tan EY, Hoon DS, Kang Y, Yu Q. Protein tyrosine phosphatase UBASH3B is overexpressed in triple-negative breast cancer and promotes invasion and metastasis. Proc Natl Acad Sci U S A. 2013;110:11121–11126. doi: 10.1073/pnas.1300873110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo HM, Park HJ, Park MH, Kim BY, Shin JW, Yoo WG, Koo SK. Identification of CDH23 mutations in Korean families with hearing loss by whole-exome sequencing. BMC Med Genet. 2014;15:46. doi: 10.1186/1471-2350-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird JE, Takagi Y, Billington N, Strub MP, Sellers JR, Friedman TB. Chaperone-enhanced purification of unconventional myosin 15, a molecular motor specialized for stereocilia protein trafficking. Proc Natl Acad Sci U S A. 2014;111:12390–12395. doi: 10.1073/pnas.1409459111. [DOI] [PMC free article] [PubMed] [Google Scholar]


