Abstract
Aims: Since the poor prognosis of glioma, our study was aimed to find out the role of HLA-DR in the prognosis of glioma patients that may contribute to the timely post-operative treatment on the glioma patients. Methods: 60 glioma patients were enrolled in the prospective cohort study. Western blotting was used to detect the content of HLA-DR. Kaplan-Meier curve was adopted to evaluate the effects of HLA-DR on the survival time of glioma patients. Cox regression analysis was used to evaluate the roles of clinical features and HLA-DR in the pathogenesis of glioma. Results: The expression level of HLA-DR was higher in tumor tissue, compared with normal tissues (P < 0.05). Moreover, expression levels of HLA-DR were correlated with the factors of pathological degree, Enneking staging and KPS score. The survival rate of patients with high content of HLA-DR was lower than those of patients with low content of HLA-DR. Cox regression analysis indicated that Enneking staging and HLA-DR were all associated with the prognosis of glioma (HR=14.43, 95% CI=1.05-199.16; HR=21.39, 95% CI=2.07-220.76). Conclusion: HLA-DR may serve as a biomarker for the prognosis of glioma patients.
Keywords: HLA-DR, glioma, prognosis
Introduction
Glioma, mainly deriving from neuroepithlial tissue, accounts for 40%-50% of intracranial tumors and 1.5% of malignant tumors of whole body and among 100,000 population, there are 3.5-4.5 individual suffering glioma [1,2]. It is well known that glioma is characterized with high malignant degree, poor prognosis and life-threat, which make it rank the 11th in various tumors with high mortality rate [3]. So it is urgent to find out a biomarker for glioma prognosis that contributes to comprehensive grasp on the state of patients and timely treatment. Some studies have investigated several potential indicators for the outcome of glioma [4-7]. However, the relationship of HLA-DR and glioma prognosis has not been identified.
HLA-DR belongs to type II cell-surface antigen encoded by major histocompatibility complex (MHC), which is exclusively distributed in immune cells with the function of stimulating allogenic immune reactions and regulating the intercellular immune response [8]. Recently, HLA class II antigen has been reported to serve as a favorable prognostic marker of colorectal carcinoma [9], which indicated that HLA-DR might be a predictive marker for prognosis of glioma.
So our study was conducted to detect the level of HLA-DR and then investigate whether there was significant association of HLA-DR and glioma prognosis.
Materials and methods
Subjects
From January 2006 until January 2008, relative data were obtained from 60 glioma patients with postoperative pathology confirmation in Neurosurgery Department of First Affiliated Hospital of Chongqing Medical University. Meanwhile, 30 samples of normal brain tissues resected in the surgery of internal decompression. The subjects in the survival analysis must meet the following demand: (1) operation on the first episode of supratentorial tumor without anti-tumor therapy; (2) treated with conventional radiotherapy after surgery; (3) fine follow-up compliance; (4) surgery and after-surgery review with magnetic resonance imaging (MRI) were operated by same group of senior physicians and tumors were confirmed as total or subtotal resection; (5) patients were all adult without obesity and diabetes. And the patients were excluded if they were incompliant in the observation, lost to follow-up, dying from other causes or receiving other anti-tumor therapies (e.g. operation) besides conventional radiotherapy.
Methods
Western blotting
Tumor tissues and normal tissues were completely homogenated, then were qualified by the method of Coomassie brilliant blue (CBB). Same amount of protein were tested by SDS-PAGE and transfered with semi-dry film method. After transfered on the nitrocellulose membrane, the samples were sealed by 5% skim milk, followed by warming process overnight with primary antibody, rinsing by PBS, reaction with second antibody labeled by HRP and color development. Based on the content of β-actin, the level of HLA-DR was measured.
Statistical methods
Mann-Whitney U test was applied to evaluate differences on HLA-DR expression between groups. χ2 test was used to analyze the correlation between HLA-DR expression levels and clinical pathologic features. Kaplan-Meier curve was used to evaluate survival rate between high content and low content of HLA-DR. Cox regression analysis was used to evaluate the roles of clinical features and HLA-DR in the pathogenesis of glioma. All the statistical analysis were performed with SPSS 18.0 software and P value < 0.05 indicates statistical significance.
Results
Expression level of HLA-DR
In contrast to normal tissues, content of HLA-DR in tumor tissue of giloma was much higher (P < 0.05) (Figure 1).
Figure 1.
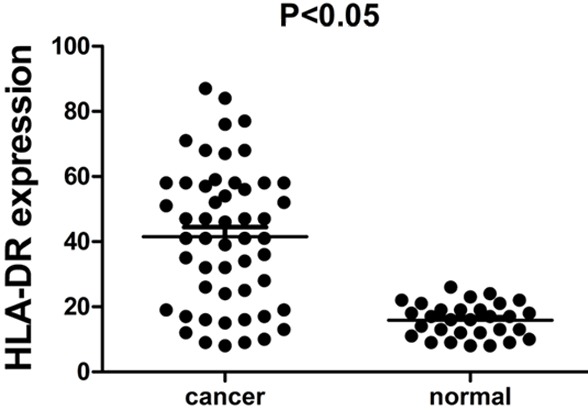
Comparison of HLA-DR expression level between cancer tissues and normal tissues.
Association of HLA-DR with clinical pathologic features
Clinical features of subjects were collected and listed in (Table 1).Then we analyzed the association of HLA-DR with clinical pathologic features, the results indicated that the expression level of HLA-DR was not correlated with factors of tumor site and tumor size (Figure 2A, 2B), however, there was a close relationship of expression of HLA-DR with factors of pathological degree, Enneking staging and KPS score (Figure 2C-E).
Table 1.
Clinical characteristics
| Variables | Glioma patients (n=60) |
|---|---|
| Tumor site | |
| Supratentorial | 27 |
| Subtentorial | 33 |
| Tumor size | |
| >5 cm | 38 |
| <5 cm | 22 |
| Pathological degree | |
| Low | 26 |
| High | 34 |
| Enneking staging | |
| IB | 18 |
| IIA | 26 |
| IIB | 16 |
| KPS score | |
| >70 | 18 |
| <70 | 42 |
Figure 2.
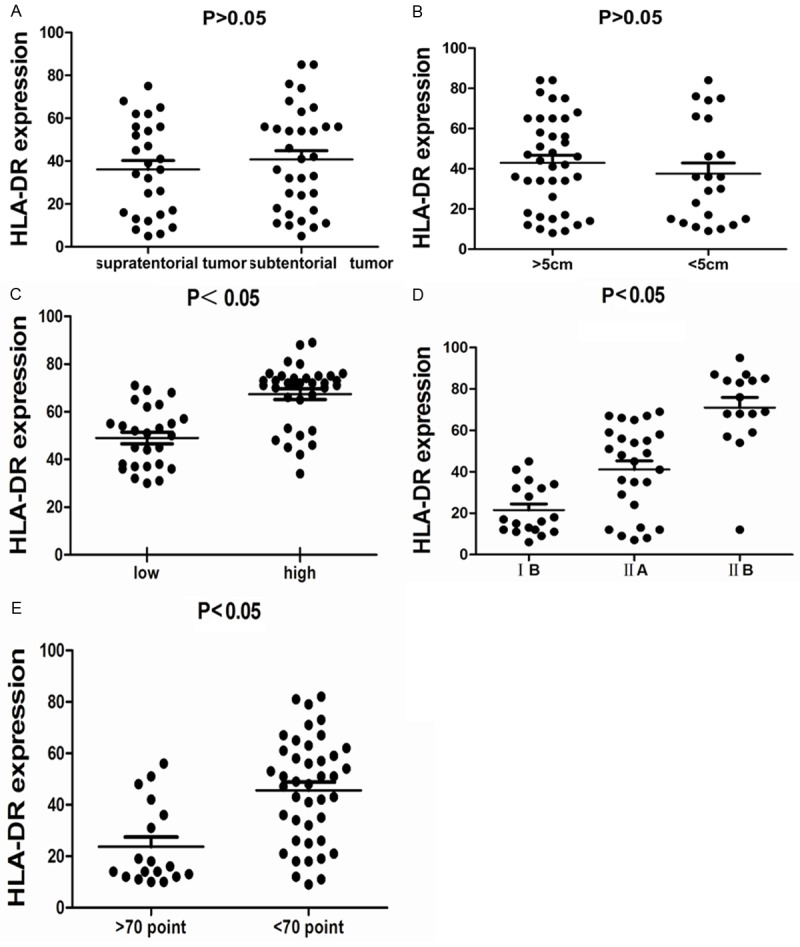
A. Association of HLA-DR expression level and tumor site factor. B. Association of HLA-DR expression level and tumor size factor. C. Association of HLA-DR expression level and pathological degree factor. D. Association of HLA-DR expression level and Enneking staging factor. E. Association of HLA-DR expression level and KPS score factor.
Association of HLA-DR with outcome of glioma patients
In the follow-up observation, 13 patients were survived, while 41 were died and 6 were censored. Patients with high content of HLA-DR were found with survival rate of 16.7% (6/36) and patients with low content of HLA-DR was 38.9% (7/18) (Figure 3).
Figure 3.
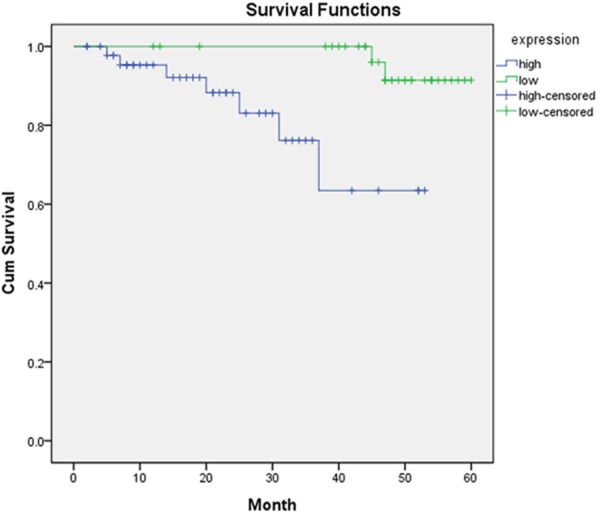
Kaplan-Meier survival curve.
Cox regression analysis
Cox regression suggested that Enneking staging appeared to be a predictive index for glioma prognosis (HR=14.43, 95% CI=1.05-199.16). Meanwhile, we also found that HLA-DR could be a biomarker for glioma prognosis (HR=21.39, 95% CI=2.07-220.76) (Table 2).
Table 2.
Cox regression analysis
| B | SE | Wald | df | Sig. | HR | 95.0% CI | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower | Upper | |||||||
| HLA-DR | 3.063 | 1.191 | 6.614 | 1 | 0.010 | 21.389 | 2.072 | 220.762 |
| Enneking staging | 2.670 | 1.339 | 3.974 | 1 | 0.046 | 14.433 | 1.046 | 199.159 |
Discussion
Glioma, a common type of intracranial tumors with high malignancy, is characterized by rapid growth, power invasiveness, easy to relapse and poor prognosis [10,11]. At present, there exist two huge problems in treatment for glioma. On the one hand, due to high malignancy, rapid development, short disease course and 3-5d tumor cell cycles, the postoperative recurrence of glioma cannot be avoided even after the total section. On the other hand, the tumor cells are adapt to strongly invade adjacent normal tissues, several lobes and even deep structures and may approach the contra hemisphere via corpus callosum [12,13]. So it is urgent to avoid metastasis and improve the outcome of glioma patients. Our study was aimed to explore the role of HLA-DR in glioma prognosis and provide a predictive index for glioma prognosis.
HLA gene, located on the short arm of chromosome 6, is a 4000 kb gene complex composed of hundreds of tightly connected gene groups, which is known for maximum allele polymorphisms so far and is closely related to the functions of immune system in humans [8,14-17]. At present, approximately 224 genes within HLA have been identified, whereas specific functions of majority of genes remains unknown [18]. Based on sequence, classic HLA gene is generally divided into three regions: HLA-I, HLA-II and HLA-III [19]. Antigen encoded by HLA-II gene mainly appears on the cytomembrane of macrophage and B lymphocyte. The whole cytomembrane is crossed over by α and β chains of HLA-II antigen. HLA-II was divided into HLA-DP, HLA-DQ, HLA-DR, HLA-DN and HLA-DO [20].
Nowadays, the research on HLA-DR primarily concentrates on diseases of immune system [21-24]. However, there was no report on the function of HLA-DR in the glioma prognosis. Our result suggested that the expression level of HLA-DR was closely associated with the malignant degree of glioma, which indicates that HLA-DR could serve as a biomarker for the malignant degree of glioma.
The prognosis of glioma patients is influenced by many factors, so our study took the factors of tumor site, tumor size, pathological degree, Enneking staging, KPS score and HLA-DR expression into consideration. Cox regression result indicated that Enneking staging and HLA-DR expression exhibited prognostic values for glioma. For Enneking staging, we labeled the staging of benign tumors with Arabic numerals and malignant tumors with roman numerals. IA and IB stood for low malignant, and IIA and IIB for high malignant. The higher the degree was, the poor prognosis the glioma patients would get.
Our study found that expression level of HLA-DR was high in tumor tissue of glioma compared with that of normal tissues and HLA-DR expression was significantly associated with outcome of glioma patients, high content of HLA-DR corresponding to increased mortality. In summary, up-regulated expression of HLA-DR involves in the pathogenesis of glioma, which can serve as a biomarker for glioma prognosis.
Disclosure of conflict of interest
None.
References
- 1.Birner P, Gatterbauer B, Oberhuber G, Schindl M, Rossler K, Prodinger A, Budka H, Hainfellner JA. Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer. 2001;92:165–171. doi: 10.1002/1097-0142(20010701)92:1<165::aid-cncr1305>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Shim JW, Koh YC, Ahn HK, Park YE, Hwang DY, Chi JG. Expression of bFGF and VEGF in brain astrocytoma. J Korean Med Sci. 1996;11:149–157. doi: 10.3346/jkms.1996.11.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nutt CL, Mani DR, Betensky RA, Tamayo P, Cairncross JG, Ladd C, Pohl U, Hartmann C, McLaughlin ME, Batchelor TT, Black PM, von Deimling A, Pomeroy SL, Golub TR, Louis DN. Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer Res. 2003;63:1602–1607. [PubMed] [Google Scholar]
- 4.Liu X, Wang X, Du W, Chen L, Wang G, Cui Y, Liu Y, Dou Z, Wang H, Zhang P, Chang L, Yi L, Cai J, Jiang C. Suppressor of fused (Sufu) represses Gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget. 2014;5:11681–94. doi: 10.18632/oncotarget.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding D, Song T, Jun W, Tan Z, Fang J. Decreased expression of the SPOP gene is associated with poor prognosis in glioma. Int J Oncol. 2015;46:333–341. doi: 10.3892/ijo.2014.2729. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M, Xu H, Liang F, He J, Zhang J. Association of osteopontin expression with the prognosis of glioma patient: a meta-analysis. Tumour Biol. 2015;36:429–36. doi: 10.1007/s13277-014-2645-y. [DOI] [PubMed] [Google Scholar]
- 7.Strojnik T, Smigoc T, Lah TT. Prognostic value of erythrocyte sedimentation rate and C-reactive protein in the blood of patients with glioma. Anticancer Res. 2014;34:339–347. [PubMed] [Google Scholar]
- 8.Complete sequence and gene map of a human major histocompatibility complex. The MHC sequencing consortium. Nature. 1999;401:921–923. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- 9.Sconocchia G, Eppenberger-Castori S, Zlobec I, Karamitopoulou E, Arriga R, Coppola A, Caratelli S, Spagnoli GC, Lauro D, Lugli A, Han J, Iezzi G, Ferrone C, Ferlosio A, Tornillo L, Droeser R, Rossi P, Attanasio A, Ferrone S, Terracciano L. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 2014;16:31–42. doi: 10.1593/neo.131568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froberg MK, Gerhart DZ, Enerson BE, Manivel C, Guzman-Paz M, Seacotte N, Drewes LR. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. Neuroreport. 2001;12:761–765. doi: 10.1097/00001756-200103260-00030. [DOI] [PubMed] [Google Scholar]
- 11.Adesina AM, Nalbantoglu J, Cavenee WK. p53 gene mutation and mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res. 1994;54:5649–5651. [PubMed] [Google Scholar]
- 12.Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Nakamura I, Han T, Aderca I, Isomoto H, Garrity-Park MM, Shire AM, Li J, Sanderson SO, Adjei AA, Fernandez-Zapico ME, Roberts LR. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology. 2010;52:1680–1689. doi: 10.1002/hep.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vortmeyer AO, Stavrou T, Selby D, Li G, Weil RJ, Park WS, Moon YW, Chandra R, Goldstein AM, Zhuang Z. Deletion analysis of the adenomatous polyposis coli and PTCH gene loci in patients with sporadic and nevoid basal cell carcinoma syndrome-associated medulloblastoma. Cancer. 1999;85:2662–2667. [PubMed] [Google Scholar]
- 14.Zeggini E, Donn RP, Ollier WE, Thomson W. Evidence for linkage of HLA loci in juvenile idiopathic oligoarthritis: independent effects of HLA-A and HLA-DRB1. Arthritis Rheum. 2002;46:2716–2720. doi: 10.1002/art.10551. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48:17–26. doi: 10.1016/s0165-0378(00)00070-x. [DOI] [PubMed] [Google Scholar]
- 16.Le Bouteiller P, Blaschitz A. The functionality of HLA-G is emerging. Immunol Rev. 1999;167:233–244. doi: 10.1111/j.1600-065x.1999.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 17.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 18.Nepom GT, Byers P, Seyfried C, Healey LA, Wilske KR, Stage D, Nepom BS. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989;32:15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- 19.von Landenberg P, Scholmerich J. Tissue-associated autoantigens in rheumatoid arthritis. Tissue-antigens detected by autoantibodies in synovial fluid and sera of RA patients. Clin Rev Allergy Immunol. 2000;18:59–71. doi: 10.1385/CRIAI:18:1:59. [DOI] [PubMed] [Google Scholar]
- 20.Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:S4–11. [PubMed] [Google Scholar]
- 21.Gao XJ, Brautbar C, Gazit E, Segal R, Naparstek Y, Livneh A, Stastny P. A variant of HLA-DR4 determines susceptibility to rheumatoid arthritis in a subset of Israeli Jews. Arthritis Rheum. 1991;34:547–551. doi: 10.1002/art.1780340506. [DOI] [PubMed] [Google Scholar]
- 22.Jamshidi J, Movafagh A, Emamalizadeh B, Zare Bidoki A, Manafi A, Ghasemi Firouzabadi S, Shahidi GA, Kazeminasab S, Petramfar P, Fazeli A, Motallebi M, Mortazavi-Tabatabaei SA, Kowsari A, Jafarian Z, Darvish H. HLA-DRA is associated with Parkinson’s disease in Iranian population. Int J Immunogenet. 2014;41:508–511. doi: 10.1111/iji.12151. [DOI] [PubMed] [Google Scholar]
- 23.Hasan ZN, Zalzala HH, Mohammedsalih HR, Mahdi BM, Abid LA, Shakir ZN, Fadhel MJ. Association between human leukocyte antigen- DR and demylinating Guillain-Barre syndrome. Neurosciences (Riyadh) 2014;19:301–305. [PMC free article] [PubMed] [Google Scholar]
- 24.Nada AM, Hammouda M. Immunoregulatory T cells, LFA-3 and HLA-DR in autoimmune thyroid diseases. Indian J Endocrinol Metab. 2014;18:574–581. doi: 10.4103/2230-8210.137524. [DOI] [PMC free article] [PubMed] [Google Scholar]


