Abstract
Background: To investigated the diagnostic and prognostic value of engulfment and cell motility (ELMO3) in non small cell lung cancer (NSCLC). Methods: The expression of ELMO3 at mRNA levels were detected using reverse transcription quantitative real-time polymerase chain reaction (qRT-PCR) in 125 NSCLC patients’ tissues and adjacent tissues, as well as in the serum of 125 NSCLC patients and 89 healthy controls. Then, receiver operating characteristic curve (ROC), Kaplan-Meier and Cox regression analysis were adopted to estimate the potential diagnostic and prognostic value of ELMO3, respectively. Results: ELMO3 expression level was significantly up-regulated in NSCLC patients’ tissues and serum compared with controls (P<0.001). Moreover, the expression of ELMO3 was significantly associated with tumor size (P=0.020), TNM stage (P=0.017), lymph node metastasis (PP=0.045) and distance metastasis (P=0.033). ROC showed the AUC was 0.917, and the optimal cutoff value was 0.735, providing a sensitivity of 92.8% and a specificity of 84.3%. Furthermore, Kaplan-Meier analysis indicated the high expression of ELMO3 could lead to a shorter overall survival time. In multivariate analysis, ELMO3 expression (HR=3.378, 95% CI=1.326-8.587, P=0.011) was proved to be linked with the prognosis of NSCLC and might act as an independent prognostic marker. Conclusion: The over-expression of ELMO3 was a potential diagnostic and prognostic marker for NSCLC.
Keywords: Non small cell lung cancer, ELMO3, diagnosis, prognosis
Introduction
Lung cancer is a major cause of cancer-related death in worldwide, accounting for 18% (1.4 million) of cancer deaths in 2008 according to global cancer statistics [1]. It is traditionally classified into two major subtypes, small cell lung cancer and non-small cell lung cancer (NSCLC). Among them, NSCLC covers 80-85% [2]. Although the survival rate of patients with NSCLC has been improved due to the advances in surgical techniques and treatment strategies, the high rate of recurrence and metastasis were still lead to a short-term survival after surgical resection [3,4]. NSCLC is a slow-developing cancer with a complex pathogenesis and its progression get involve in several stages as well as activation of many oncogenes and inactivation of tumor suppressor genes [5]. Because of the late-stage diagnosis and other complex factors, NSCLC has a poor prognosis and low cure rate. Therefore, the research for effective molecular markers for diagnosis and prognosis of lung cancer is an important issue at present.
The engulfment and cell motility (ELMO) proteins family which is consists of ELMO1, ELMO2, and ELMO3, widely distribute in mammals and participate in a various of life action including cell migration, cell chemotaxis transfer, cell polarity, apoptosis, dendritic development and so on [6-10]. Ras GTPase-binding domain (RBD) that only present in Elmo proteins and ElmoD protein (Elmo domain) is the most important characteristic of ELMO [11,12]. In previous studies, most of the researches were about the function of ELMO1 and EMLO2, the investigation about ELMO3 was rarely. It was reported that ELMO3 plays important roles in the cell renewing and migration processes within the intestinal epithelia and in colorectal cancer [13]. Besides, ELMO3 had been considered to be a promoter of metastatic dissemination of NSCLC in the study of Sφes et al. [14]. However, the diagnostic and prognostic of ELMO3 in NSCLC remains unknown.
In this study, we investigated the expression level of ELMO3 in NSCLC tissues and serum through quantitative real-time polymerase chain reaction (qRT-PCR), and analyzed the association of ELMO3 with clinicopathological characteristics. Meanwhile, the diagnostic and prognostic values of ELMO3 were also estimated.
Materials and methods
Patients and samples
A total of 125 patients with NSCLC were recruited. The study was permitted by the Ethics Committee of the hospital. None of them had received any adjuvant chemotherapy or radiotherapy. Besides, 89 healthy volunteers matching age and gender were obtained as healthy controls. Meanwhile, written informed consents had been signed by each participators in advance.
The tissues and adjacent tissues were collected from NSCLC patients and the serum was reserved. The serum from healthy controls were extracted, too. All the tissues were frozen by liquid nitrogen while the serum samples were put into blood collection tube of EDTA and stored at -80°C for RNA extraction. A 5-years’ follow-up was performed to estimate the prognosis of NSCLC patients who had undergone curative surgical resection. The clinicopathologic characteristics including age, gender, tumor size, TNM stage, lymph node metastasis, and distant metastasis were recorded in a database. Patients who died from unexpected events or other diseases were excluded from our study.
RNA extraction and quantitative real-time polymerase chain reaction (QRT-PCR)
Total RNA was isolated from the tissues and adjacent tissues of 125 patients with NSCLC as well as the serum samples using the Trizol reagent (Invitrogen). The RNA was purified to the OD A260/A280 ratio reach to 2.0, the analysis would be subsequently conducted. Reverse transcription was performed to synthesize the first chain of cDNA according to the TaqMan microRNA assay protocol (Applied Biosystems, Foster City, CA, USA). Then the RT-PCR reaction was carried out in the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, California, USA). ELMO3 primers were: forward; 5’-GGC CTT CTC AGA GCT CAT G-3’ and reverse; 5’-TGA GGT TCA TGT TCA CGT AGC-3’. β-actin was taken as an internal control. Each sample was examined in triplicate, and the relative quantification of ELMO3 expression was evaluated by the comparative cycle threshold (CT) method, normalized to β-actin.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software. The data was stated as mean ± SD. The differences of ELMO3 expression between NSCLC tissues and adjacent tissues as well between NSCLC serum and healthy serum were estimated via students’ T-test, respectively. The correlations between ELMO3 expression and clinicopathologic parameters were analyzed by chi-square test. The diagnostic value of serum ELMO3 expression was evaluated through the establishment of receiver operating characteristic curve (ROC). Kaplan-Meier analysis was used to determine the overall survival time of patients with different expression of ELMO3. The log-rank test was used to analyze the significance of the result. Multivariate analyses were performed using Cox regression analysis to determine the factors affecting the prognosis of NSCLC. The difference was considered to be statistically significant when the P value was less than 0.05.
Results
Expression of ELMO3 was increased in NSCLC tissues and serum
To detect the expression of ELMO3 in NSCLC patients, we analyzed the expression of ELMO3 at mRNA level by qRT-PCR in NSCLC tissues and adjacent tissues, as well as in NSCLC patients’ serum and healthy controls’ serum. The expression of ELMO3 at mRNA level in NSCLC tissues was significantly higher than that in adjacent tissues (P<0.05, Figure 1A). Similarly, the serum ELMO3 expression was also markedly up-regulated in patients with NSCLC compared with healthy controls (P<0.05, Figure 1B). These results indicated that ELMO3 could be an oncogene in NSCLC.
Figure 1.
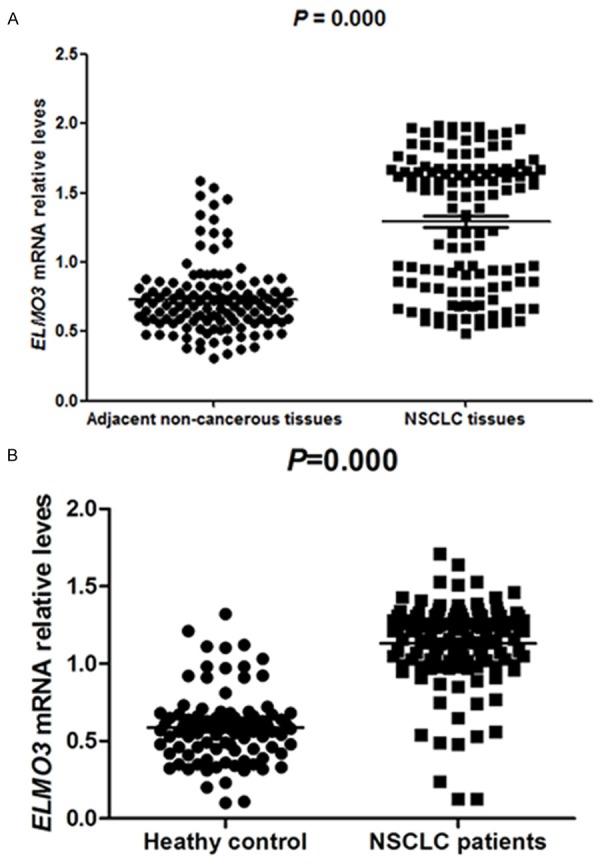
Expression level of ELMO3 in NSCLC tissues and serum detected. A. The expression of ELMO3 in NSCLC tissues was significantly higher than in adjacent tissues (P<0.05). B. The serum ELMO3 expression were also markedly up-regulated in patients with NSCLC compared with healthy controls (P<0.05).
Correlation between ELMO3 expression and clinicopathological characteristics
To explore whether EMLO3 expression was relevant to clinicopathological characteristics, we performed chi-square test. As indicated in Table 1, ELMO3 expression was significantly correlated with tumor size (P=0.020), TNM stage (P=0.017), lymph node metastasis (P=0.045) and distant metastasis (P=0.033). Nevertheless, no significant correlation was observed among ELMO3 expression with gender (P=0.322>0.05) and age (P=0.184>0.05).
Table 1.
Relationship between ELMO3 expression and clinicopathological characteristics in NSCLC
| Characteristic | Cases | ELMO3 expression | x2 | P-values | |
|---|---|---|---|---|---|
|
| |||||
| Low N=55 | High N=70 | ||||
| Gender | |||||
| Male | 72 | 30 | 42 | 0.982 | 0.322 |
| Female | 53 | 25 | 28 | ||
| Age (years) | |||||
| ≤60 | 83 | 40 | 43 | 1.762 | 0.184 |
| >60 | 42 | 15 | 27 | ||
| Tumor size | |||||
| ≤5 cm | 89 | 45 | 44 | 5.400 | 0.020 |
| >5 cm | 36 | 10 | 26 | ||
| TNM stage | |||||
| Stage I | 102 | 50 | 52 | 5.299 | 0.017 |
| Stage II/III | 23 | 5 | 18 | ||
| Lymph node metastasis | |||||
| Yes | 34 | 10 | 24 | 4.034 | 0.045 |
| No | 91 | 45 | 46 | ||
| Distant metastasis | |||||
| Absent | 98 | 48 | 50 | 4.566 | 0.033 |
| Present | 27 | 7 | 20 | ||
Diagnostic value of ELMO3 for NSCLC
ROC curve was established to estimate the diagnostic value of ELMO3 with the serum ELMO3 expression (Figure 2). The AUC value was 0.917 corresponding with a sensitivity of 92.8% and a specificity of 84.3% with a cutoff value was 0.735. This result might demonstrate that EMLO3 was a potential biomarker for differentiating NSCLC patients.
Figure 2.
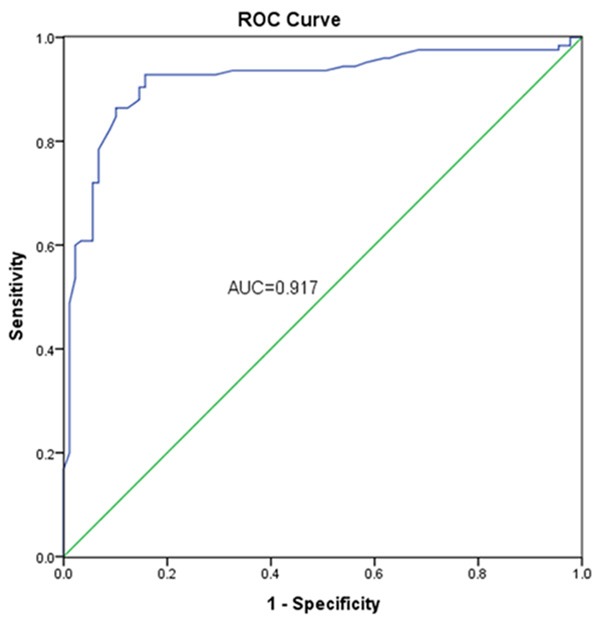
Diagnostic value of ELMO3 via ROC which had a AUC of 0.917, combing with a sensitivity of 92.8% and a specificity of 84.3%.
Association between EMLO3 expression and overall survival
The relationship between ELMO3 expression and overall survival time of the patients with NSCLC was evaluated by Kaplan-Meier analysis. As determined by the log-rank test in Figure 3, the overall survival time of patients with high ELMO3 expression was significantly lower than those with low ELMO3 expression (P=0.001). Besides, whether ELMO3 as well as clinicopathological characteristics could be used as prognostic markers for NSCLC patients were estimated via Cox regression analysis. The outcome indicated that no clinicopathological characteristics but ELMO3 expression (P=0.011, HR=3.378, CI=1.326-8.587) alone was related to the prognosis of NSCLC. Moreover, it could be an independent prognostic markers in NSCLC (Table 2).
Figure 3.
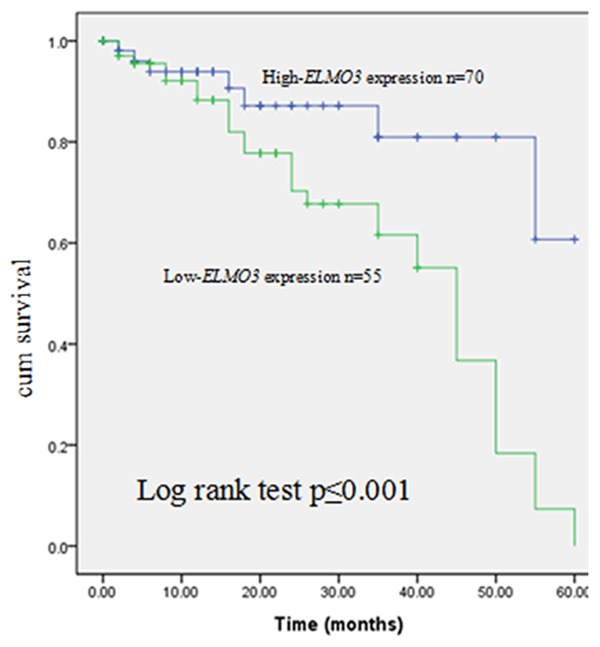
Association between ELMO3 and overall survival time of NSCLC patients according to Kaplan-Meier analysis.
Table 2.
Cox regression analysis of ELMO3 and clinicopathological characteristics
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| ELMO3 gene | 3.378 | 1.326-8.587 | 0.011 |
Discussion
NSCLC is the leading cause of lung cancer related deaths in world with a characteristic of a long asymptomatic latency and poor prognosis [15 16]. The 5-year survival rate was different along with the change of its stage and the rate could reach to 50% if it was early detected and received treatment [17]. Novel bio-markers with high sensitivity and specificity are therefore urgently needed to the early diagnosis of NSCLC and to promote the development of new treatments [6]. Wang et al. had reported that circulating MACC1 was expressed higher in NSCLC patients than in benign disease patients or healthy volunteers, represents a potential noninvasive, diagnostic and prognostic marker for NSCLC [18]. Ulivi et al. found peripheral blood miR-328 expression could be as a potential biomarker for the early diagnosis of NSCLC according to relative analysis [19]. Although there are a lot of bio-markers such as mutations in the KRAS, epidermal growth factor receptor, TP53 genes, and changes in the expression levels of carcinoembryonic antigen (CEA), cytokeratin-19 fragment (CK19), cancer antigen-125 (CA125), and neuron-specific enolase (NSE) have been confirmed in NSCLC now [20-22], the clinical value of them were still limited [23,24].
ELMO3 has the most different protein sequences in ELMO family, but modular protein domains are similar with other members [25]. Previous reports have demonstrated that ELMO is up-regulated in several cancer tissues, such as human glioma and lung cancer [14,26,27]. Molecular alterations between normal lung tissues and tumor tissues, are true drivers of the metastatic process, which are specific to the cohort of patients. ELMO3 has been a negative impact on the survival of lung cancer patients with distant metastases causing additional morbidities. However, its diagnostic and prognostic role in NSCLC is still unclear.
In the study, we performed qRT-PCR to detect the ELMO3 expression in NSCLC tissues and adjacent tissues as well as the serum ELMO3 expression was tested at mRNA level. The result showed that ELMO3 in NSCLC was significantly higher than that in adjacent tissues and the trend was similar with in serum, implying that ELMO3 might serve as an oncogene in NSCLC. Besides, ELMO3 was possible to be related to the occurrence and development of NSCLC through the analysis of the relationship between it and patients’ clinicopathological characteristics including tumor size, TNM staging, lymph node metastasis and distance metastasis. The results also verified that ELMO3 is essential for the progress and metastasis of NSCLC which was consistent with the outcome of previous study [28].
As its abnormal expression, we inferred ELMO3 could be involved in the diagnosis or prognosis or both of them. Hence, we estimated the diagnostic and prognostic value of ELMO3 furthermore. For a high AUC value, sensitivity and specificity on the basis of ROC, the diagnostic value of ELMO3 was considered to be valuable. Meanwhile, the prognostic value was confirmed by Kaplan-Meier and Cox regression analysis. ELMO3 might be a new potential bio-marker for the early detection and prognosis of NSCLC.
In conclusion, ELMO3 is over-expressed in NSCLC patients and its expression is influenced by many clinicopathological characteristics including tumor size, TNM stage, lymph node metastasis and distant metastasis. Besides, ELMO3 is identified to be an independent diagnostic and prognostic indicator in our NSCLC. To our knowledge, this is the first study to investigate the diagnostic and prognostic value of ELMO3 in NSCLC. There are still several limitations in the study. On the one hand, the sample size of the study is small, and further studies with more patients are required to confirm the results. On the other hand, all of the patients in this study come from one hospital and the results may differ according to the techniques used. Hence, further studies are needed.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 2008;13(Suppl 1):5–13. doi: 10.1634/theoncologist.13-S1-5. [DOI] [PubMed] [Google Scholar]
- 3.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Zhang Y, Bai X, Peng Y, He P. TRIM31 is downregulated in non-small cell lung cancer and serves as a potential tumor suppressor. Tumour Biol. 2014;35:5747–52. doi: 10.1007/s13277-014-1763-x. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 6.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Jin T. A shortcut from GPCR signaling to Rac-mediated actin cytoskeleton through an ELMO/DOCK complex. Small GTPases. 2012;3:183–5. doi: 10.4161/sgtp.20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handa Y, Suzuki M, Ohya K, Iwai H, Ishijima N, Koleske AJ, Fukui Y, Sasakawa C. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat Cell Biol. 2007;9:121–8. doi: 10.1038/ncb1526. [DOI] [PubMed] [Google Scholar]
- 9.van Ham TJ, Kokel D, Peterson RT. Apoptotic cells are cleared by directional migration and elmo1- dependent macrophage engulfment. Curr Biol. 2012;22:830–6. doi: 10.1016/j.cub.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2004;2:E6. doi: 10.1371/journal.pbio.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–74. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 12.Laurin M, Cote JF. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes Dev. 2014;28:533–47. doi: 10.1101/gad.236349.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coskun M, Boyd M, Olsen J, Troelsen JT. Control of intestinal promoter activity of the cellular migratory regulator gene ELMO3 by CDX2 and SP1. J Cell Biochem. 2010;109:1118–28. doi: 10.1002/jcb.22490. [DOI] [PubMed] [Google Scholar]
- 14.Soes S, Daugaard IL, Sorensen BS, Carus A, Mattheisen M, Alsner J, Overgaard J, Hager H, Hansen LL, Kristensen LS. Hypomethylation and increased expression of the putative oncogene ELMO3 are associated with lung cancer development and metastases formation. Oncoscience. 2014;1:367–74. doi: 10.18632/oncoscience.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 16.Guo S, Yan F, Xu J, Bao Y, Zhu J, Wang X, Wu J, Li Y, Pu W, Liu Y, Jiang Z, Ma Y, Chen X, Xiong M, Jin L, Wang J. Identification and validation of the methylation biomarkers of non-small cell lung cancer (NSCLC) Clin Epigenetics. 2015;7:3. doi: 10.1186/s13148-014-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–21. [PubMed] [Google Scholar]
- 18.Wang Z, Cai M, Weng Y, Zhang F, Meng D, Song J, Zhou H, Xie Z. Circulating MACC1 as a novel diagnostic and prognostic biomarker for nonsmall cell lung cancer. J Cancer Res Clin Oncol. 2014 doi: 10.1007/s00432-014-1903-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulivi P, Foschi G, Mengozzi M, Scarpi E, Silvestrini R, Amadori D, Zoli W. Peripheral Blood miR-328 Expression as a Potential Biomarker for the Early Diagnosis of NSCLC. Int J Mol Sci. 2013;14:10332–42. doi: 10.3390/ijms140510332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arya SK, Bhansali S. Lung cancer and its early detection using biomarker-based biosensors. Chem Rev. 2011;111:6783–809. doi: 10.1021/cr100420s. [DOI] [PubMed] [Google Scholar]
- 21.Cabrera-Alarcon JL, Carrillo-Vico A, Santotoribio JD, Leon-Justel A, Sanchez-Gil R, Gonzalez-Castro A, Guerrero JM. CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin Lab. 2011;57:1011–4. [PubMed] [Google Scholar]
- 22.Gridelli C, Rossi A, Maione P. 2010 Consensus on Lung Cancer, new clinical recommendations and current status of biomarker assessment--first-line therapy. Eur J Cancer. 2011;47(Suppl 3):S248–57. doi: 10.1016/S0959-8049(11)70171-X. [DOI] [PubMed] [Google Scholar]
- 23.Cho JY, Sung HJ. Proteomic approaches in lung cancer biomarker development. Expert Rev Proteomics. 2009;6:27–42. doi: 10.1586/14789450.6.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Wang K, Xu J, Huang J, Zhang T. Prognostic significance of circulating tumor cells in non-small-cell lung cancer patients: a meta-analysis. PLoS One. 2013;8:e78070. doi: 10.1371/journal.pone.0078070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyette MA, Cote JF. NSCLC metastasis: going with ELMO3. Oncotarget. 2014;5:5850–1. doi: 10.18632/oncotarget.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarzynka MJ, Hu B, Hui KM, Bar-Joseph I, Gu W, Hirose T, Haney LB, Ravichandran KS, Nishikawa R, Cheng SY. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007;67:7203–11. doi: 10.1158/0008-5472.CAN-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapa E, Hill SK, Morten KJ, Potter M, Mitchell C. The over-expression of cell migratory genes in alveolar rhabdomyosarcoma could contribute to metastatic spread. Clin Exp Metastasis. 2012;29:419–29. doi: 10.1007/s10585-012-9460-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell. 2001;1:477–89. doi: 10.1016/s1534-5807(01)00058-2. [DOI] [PubMed] [Google Scholar]


