Abstract
CD133 and cancer-testis antigens (CTAs) may be potential predicted markers of adjuvant chemotherapy or immune therapy, and they may be the independent prognostic factor of NSCLC. Nowadays, there is still no predictive biomarker identified for the use of adjuvant chemotherapy in non-small cell lung cancer (NSCLC) patients. To clarify the role of CD133 and CTAs as a predictive marker for adjuvant chemotherapy or prognostic factors of overall survival, we performed a retrospective study in 159 stage Ib-IIIA NSCLC patients receiving adjuvant chemotherapy or observe from April 2003 to March 2004 in our institute. Clinical data and gene anaylisis results were collected, while CD133 and three CTAs (MAGE-A4, NY-ESO-1, MAGE-A10) were determined according to their monoclonal antibodies such as CD133, 57B, D8.38 and 3GA11 by immunohistochemistry. All CTAs were more frequently expressed in squamous cell carcinoma (SCC) (50.0%, 26.9%, 34.6%) than in adenocarcinoma (16.2%, 16.2%, 16.2%). CD133 was more frequently found in patients with adenocarcinoma (P=0.044). Negative expression of CD133 was associated with a significantly longer overall survival compared to positive expression of CD133 (62.5 vs. 48.5 months, P=0.035). When combined with MAGEA4, NY-ESO-1or MAGE-A10, patients’ OS showed significantly difference among different combination. (CD133-MAGEA4-/CD133-MAGEA4+/CD133+MAGEA4-/CD133+MAGEA4+: 65.6 months vs.51.5 months vs.32.2 months vs.19.8 months, P=0.000, CD133-NY-ESO-1-/ CD133+NY-ESO-1-/CD133-NY-ESO-1+/ CD133+NY-ESO-1+: 57.8 months vs. 55.7 months vs. 44.6 months vs. 28.5 months, P=0.000, CD133-MAGEA10-/CD133+ MAGEA10-/CD133-MAGEA10-/CD133+MAGEA10+: 66.2 months vs. 57.2 months vs. 48.8 months vs. 41.4 months, P=0.001). There is no difference between patients received adjuvant chemotherapy or not, but subgroup analysis showed that the patients with CD133+NY-ESO-1+ expression who received chemotherapy will survive longer than not receive adjuvant chemotherapy (received vs. not received, 52.1 vs. 27.1 months, P=0.020). In the subgroup with EGFR mutation/ALK translocation/Ros1 translocation/Ret fusion, the trend remained but without a statistically significant difference. Multivariate COX regression analysis showed that stage, CD133, CD133-MAGEA4- and CD133-NY-ESO-1- are independent prognostic factors. In conclusion, CTAs (MAGE-A4, NY-ESO-1, MAGE-A10) were more likely expressed in patients with squamous cell carcinoma and when CTAs combined with CD133, they can be better prognostic factors. Patients with CD133+NY-ESO-1+ expression may survive longer when treated with adjuvant chemotherapy, which indicates that the CD133 and CTAs might be a potential marker to guide adjuvant chemotherapy in this population.
Keywords: CD133, cancer-testis antigens, non-small cell lung cancer, adjuvant chemotherapy
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. A substantial proportion of NSCLC patients suffer a recurrence following curative tumor resection, even when they have early stage disease [1]. Although treatment of stage Ib-IIIA NSCLC is mainly due to surgery, a meta-analysis of 14 randomized trials demonstrated an absolute 5% of 5 years survival benefit plus cisplatin-based adjuvant chemotherapy [1,2], however, many unselected patients will suffer from side effect of chemotherapy instead of achieving survival benefit. Prediction of which population of patients will benefit from adjuvant chemotherapy is critical to decision making. Several prognostic gene signatures have been proposed to define which stage Ib patients are most likely to recur and which stage II and IIIA patients are least likely to recur [3]. But there are still no definitive biomarkers which have led to an increased demand for the discovery and identification of new definitive biomarkers.
In recent years, cancer stem cell (CSC) related biomarkers have been proposed [4,5]. CD133 is a trans-membrane glycoprotein, its expression in cell surface down-regulates quickly as cell differentiated [6]. CD133 has been widely used as a marker to identify CSC in colon, lung, brain, pancreatic cancer and so on [2-5]. Its prognostic value for cancer patients has also been found in lung cancers and being regard as one of represented lung cancer stem cell biomarkers [6-8], which may related to cancer metastasis, chemoresistance or radioresistance.
Several studies have indicated that CTAs may play a role in the “stemness” of various stem cells. [9,10]. However, little is known about their specific connection with each other. In this regard, it was recently reported that some CTAs such as NRAGE, NY-ESO-1, MAGE-1, and SSX are expressed in human mesenchymal stem cells of the bone marrow, suggesting that CTA expression may not only be a hallmark of gametogenesis but also a stem cell marker [11].
This study was to investigate the expressions of CD133, one of the representative biomarkers of lung cancer stem cells, and several cancer-testis antigens (CTAs) in early stage non-small cell lung cancer (NSCLC) and their relationship with clinical outcome. With this rational, the results may applies the possibility of utilizing CD133 and CTAs as predictive biomarkers of adjuvant chemotherapy or immune therapy.
Material and methods
Patient selection
All patients had histologically confirmed resected stage Ib-IIIA NSCLC, the histological diagnosis of tumors was based on criteria of the World Health Organization, and TNM stage was determined according to criteria revised in 1997. All the patients did not receive neo-adjuvant chemotherapy or radiation therapy before surgery. Resected lung cancer tissues were fixed immediately in 10% buffered formalin (Ph=7.0) and embedded in paraffin. The 4-μm-thick serial sections were prepared to do the following experiment.
A total of 159 patients who met the above criteria were identified in Shanghai Pulmonary Hospital, Tongji University from April 2003 to March 2004. The laboratory data were obtained and recorded independently and blinded from clinical data until analyses by a biostatistician. The study was approved by the Institutional Ethic Committee of Shanghai Pulmonary Hospital. All the patients signed an informed consent for the use of their tumor tissues.
IHC for CD133, MAGE-A, NY-ESO-1 and MAGE-A10
IHC was performed on formalin-fixed, paraffin-embedded samples. CD133, MAGE-A, NY-ESO-1 and MAGE-A10 immunostainings were assessed on whole tissue sections, respectively. Primary antibodies used were rabbit polyclonal to CD133 (1:100) (Kangwei Tech Co., China), the antibodies of CTAs were kindly provided by the University of Basal. Monoclonal antibodies (MAb) 57B, D8.38 and 3GA11 were generated by using as immunogens recombinant MAGE-A3, NY-ESO-1 and MAGE-A10 proteins, respectively (8-9). Briefly, after deparaffinization, sections were heated in a microwave oven (20 min at 90°C ) in citrate buffer for antigen retrieval. Then, they were washed in phosphate-buffered saline for 10 min and incubated overnight at 4°C in the presence of CD133, 57B, D8.38 and 3GA11. Bound antibodies were visualized by using the SP method according to the supplier’s recommendations (SP Kit; Kangwei Tech Co., China). Diaminobenzidine was used as chromogen. All specimens were evaluated independently by two observers to evaluate staining intensity of analyzed cells (on a scale of 0-3) and the fraction of cells staining at each intensity. Interobserver agreement was reached in all cases.
Interpretation of staining
We adopted a scoring system [12] to calculate the score of intensity times the percentage of the stained tumor cells. For sections with analyzable tumor cells, staining intensity was scored in four categories: no staining (0), weak staining (1+), intermediate staining (2+, between 1+ and 3+) and strong staining (3+). The percentages of tumor cells showing the different staining intensities were assessed visually and then recorded by trained pathologists. We used the prospectively collected IHC data to generate CD133, MAGE-A, NY-ESO-1 and MAGE-A10 IHC scores on a continuous scale of 0-300. By integration of the data relating to the intensity and frequency of staining, the IHC score was calculated with the formula: 1× (percentage of cells staining weakly [1+]) +2× (percentage of cells staining moderately [2+]) +3× (percentage of cells staining strongly [3+]). Our data for the cutoff point analysis showed that H score of 100 was the best value to predict both the DFS and OS for CD133 and all three CTAs. We choose H score of 100 as a cutoff point both for CD133 and for all three CTAs. The representative IHC figures are shown in Figure 1.
Figure 1.
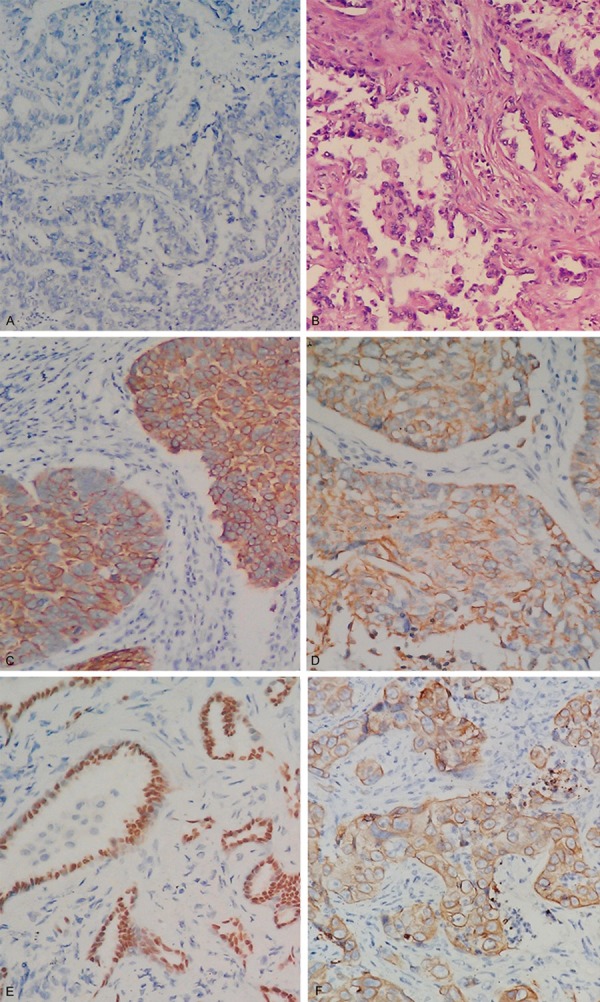
Examples of the four classifications of MAGE-A4, NY-ESO-1, MAGE-A10 and CD133 in NSCLC tumor cells based on the criteria described in the results. A. Negative control; B. HE staining; C. MAGE-A4 (cytoplasm); D. NY-ESO-1 (cytoplasm); E. MAGE-A10 (nuclear); F. CD133 (membrane).
Gene analyses
Genomic DNA was extracted from lung tumors using standard protocols (QiAamp DNA Mini Kit; Qiagen, Hilden, Germany). Cycle sequencing of the purified polymerase chain reaction (PCR) products was carried out with PCR primers using the commercially available ADx Mutation Detection Kits (Amoy Diagnostics Company, Xiamen, China), which is based on the ARMS technology. The assay can identify the 29 most common types of EGFR mutations currently described in lung cancers. All experiments were done by following the manufacturer’s protocols. The Ct values that we used to determine whether a sample was positive or negative were based on extensive validation. Briefly, 10 ng genomic DNA was added to 45 μL PCR master mix containing PCR buffer, DNA polymerase, PCR primers and fluorescent Taqman probe specific for each individual EGFR mutation. After 47 amplification cycles, the fluorescent signal was collected from FAM and HEX channels. We using multiplex real-time polymerase chain reaction assay to detect ALK/Ros1 gene translocation and RET fusion and validated all positive samples using direct sequencing. The details were described in our previous articles [13,14].
Statistical analysis
Pearson’s χ2 test was performed in the statistical analyses to evaluate whether CD133 and CTA expression correlated with clinical-pathological parameters. Spearman’s rank was used to see if there was any mutual relationship in the expression. For univariate survival analyses, the Kaplan-Meier method was applied, and the log-rank test correlation was used to determine statistical differences between life tables. All analyses were performed with the SPSS statistical package ver. 14.0 (SPSS Inc, Chicago,IL). P value less than 0.05 were regarded as statistically significant. All statistical tests were two-sided. Multivariate Cox analysis was used to analyze the effect of different factors selected from clinical characteristics on the survival time.
Results
Patient characteristics
The median age was 61 years (range, 39-82 years). The proportions of males, ever smokers and squamous carcinomas were 54.7%, 42.8% and 40.9%, respectively. Among which, 98 patients received adjuvant chemotherapy after surgery. All patients were followed till Nov, 2014. The mean follow-up time was 62 months.
Expression of CD133 and three CTAs and their relations with baseline characteristics
Immunohistochemical expression of CD133 was found not only in the membranous localization of the neoplastic cells, but also in the cytoplasmic localization, and was expressed at different levels and with various intracellular localizations. Immunoreactivity to MAGE-A4 and NY-ES0-1 were observed mostly in the cytoplasm and nuclear staining, Whereas, MAGE-A10 only in the nuclear site (Figure 1). MAGE and NY-ES0-1 was not detected in normal lung tissues, in accordance with previously published data [15,16]. The frequencies of CTAs expression was: MAGE-A4 29.6%, NY-ESO-1 25.2% and MAGE-A10 23.9%. This expression frequency is in line with previous studies reporting 8.3-25% NY-ESO- 1-positive NSCLC tumors [16-21]. Among the 159 surgically resected specimen, 77 (48.4%) were positively stained for CD133 and 46 (51.7%) of the CD133-positive tumors were adenocarcinoma, Details of CD133, MAGE-A4, NY-ESO-1 and MAGE-A10 expression in patients are shown in Table 1. The relations between IHC staining patterns and clinicopathological factors were examined (Table 1). The selected clinicopathological factors were as follows: age, sex, smoking history, pathology, postsurgery disease stage (pStage) and adjuvant chemotherapy (administered/not administered) (Table 1). NY-ESO-1 and MAGE-A10 expression did not correlate with age, sex or smoking history (data not shown), Neither MAGE-A4 nor NY-ESO-1 expression correlated with differentiation grade. CD133 and CTAs were more likely expressed in patients >61 years than patients ≤61 (P<0.05). All CTAs were more frequently expressed in squamous carcinoma (47.7%, 26.2%, 33.8%) than in adenocarcinoma (18.0%, 21.3%, 18.0%) (Table 1),while CD133 was more frequently expressed in adenocarcinoma 51.7% than in squamous carcinoma 40.0%. CT antigens have been proposed as markers of cancer stem cells [9], and further studies should be conducted to uncover the identity of this small subset of CTA-positive tumor cells. It is also notable that while the frequency of tumors positive for both negative MAGE-A4, NY-ESO-1 and MAGE-A10 proteins with negative CD133 suggested a prolonged survival compared with positive combined expression (P=0.000).
Table 1.
Correlations between baseline characteristics and expression of MAGE-A4, NY-ESO-1, MAGE-A10 and CD133 in the 159 resected NSCLC patients
| Number (%), Characteristics | N=159 | MAGE-A4 (%), n=70 | NY-ESO-1 (%), n=70 | MAGE-A1 (%), n=700 | CD133 (%), n=70 | p value | |
|---|---|---|---|---|---|---|---|
| Age (years) | > 61 | 80 (50.3) | 22 (27.5) | 29 (36.3) | 26 (32.5) | 41 (51.6) | < 0.05 |
| ≤ 61 | 79 (49.7) | 25 (31.6) | 11 (13.9) | 12 (15.2) | 36 (45.6) | ||
| Gender | Male | 87 (54.7 | 27 (31.0) | 26 (29.9) | 21 (24.1) | 40 (46.0) | > 0.05 |
| Female | 72 (45.3) | 20 (27.8) | 14 (19.4) | 17 (23.6) | 37 (51.4) | ||
| Smoking history | Never smoker | 91 (57.2) | 23 (25.3) | 13 (14.3) | 18 (19.8) | 44 (48.4) | > 0.05 |
| Smoker | 68 (42.8) | 24 (35.3) | 27 (39.7) | 20 (29.4) | 33 (48.5) | ||
| Pathology | Squamous | 65 (40.9) | 31 (47.7) | 18 (26.2) | 22 (33.8) | 26 (40.0) | < 0.01 |
| Non- Squamous | 94 (59.0) | 16 (22.8) | 22 (31.4) | 16 (22.8) | 51 (72.8) | ||
| Adjuvant | not received | 61 (38.4) | 19 (31.1) | 11 (18.0) | 16 (18.0) | 34 (55.7) | > 0.05 |
| Chemotherapy received | 98 (61.6) | 28 (28.6) | 29 (29.6) | 22 (22.4) | 43 (43.9) | ||
| EGFR mutation | Activated | 68 (42.8) | 22 (32.4) | 19 (28.0) | 22 (32.4) | 35 (51.5) | > 0.05 |
| Wild type | 87 (54.7) | 24 (27.6) | 17 (19.5) | 15 (17.2) | 41 (47.1) | ||
| Unknown | 4 (2.5) | 1 (25.0) | 4 (100) | 1 (25) | 1 (25.0) | ||
EGFR unknown patients were not included in the statistical analysis for the limited number. Abbreviations: Adeno-squa2: adeno-squamous carcinoma.
Relationship between gene analysis and CTAs
The EGFR mutation rate of the 159 patients were 45.3%. For patients with CD133 positive expression, EGFR mutation rate was 46.8%. For patients with MAGE-A4, NY-ESO-1 and MAGE-A10 positive expression, EGFR mutation rate was 48.9%, 52.5% and 42.1%, respectively. The ALK translocation/Ros1 translocation/Ret fusion rate of the 159 patients were 1.9%, 1.3%, 1.3% respectively. There is no relationship between EGFR mutation/ALK translocation/Ros1 translocation/Ret fusion and the expression of CD133, MAGE-A4, NY-ESO-1 or MAGE-A10 (data not shown).
Comparison of survival according to the expression of CD133 and CTAs
The relationships between other clinicopathological factors and survival were also examined. CD133-negative expressers showed a significantly longer OS than CD133-positive expressers (62.5 vs. 48.5 months, P=0.035). (Figure 2A) CD133 and MAGE-A4 negativity (P=0.000, CD133-MAGEA4-/CD133+MAGEA4+: 65.6 months vs. 19.8 months) were significant independent prognostic factors of a prolonged survival (Figure 2B). CD133 and NY-ESO-1 negativity (P=0.000, CD133- NY-ESO-1-/CD133+NY-ESO-1+: 57.8 months vs. 28.5 months) were significant independent prognostic factors of a prolonged survival (Figure 2C). CD133 and MAGE-A10 negativity were significant independent prognostic factors of a prolonged survival (Figure 2D) (CD133-MAGEA10-/CD133+MAGEA10-/CD133-MAGEA10-/CD133+MAGEA10+: 66.2 months vs. 57.2 months vs. 48.8 months vs. 41.4 months, P=0.001).
Figure 2.
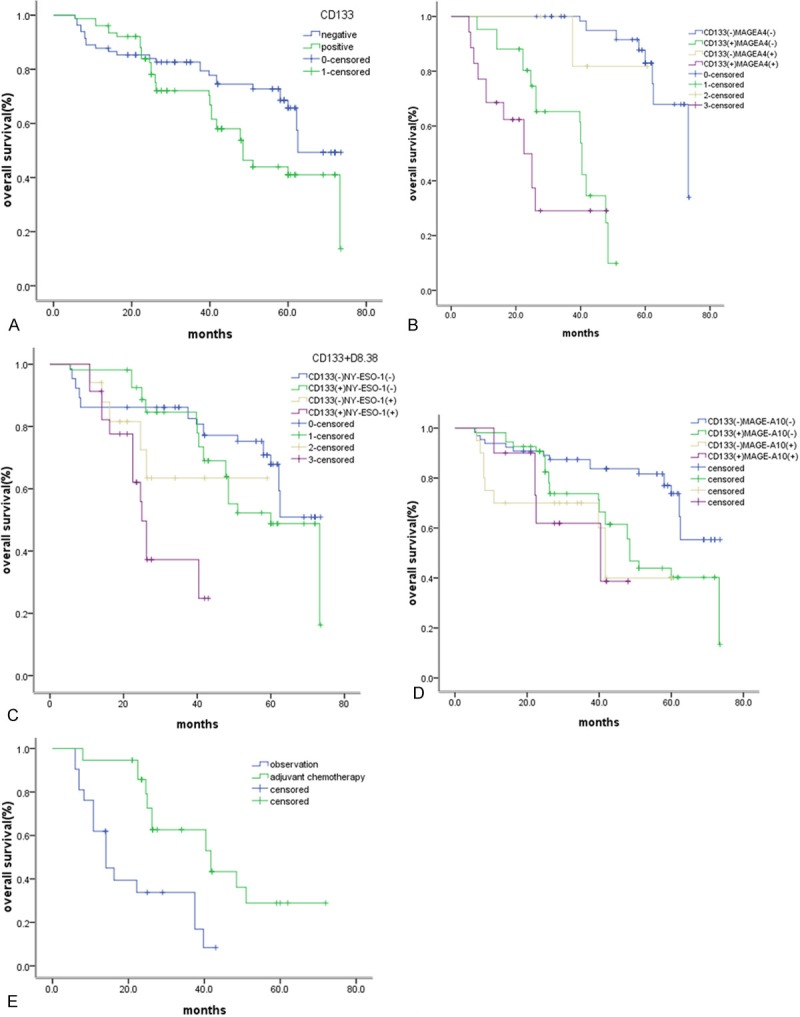
Kaplan-Meier survival curve according to the expression of CTAs and CD133 in patients. A. CD133-/CD133+: 62.5 months vs. 48.5 months, P=0.035) did not receive chemotherapy. B. CD133-MAGEA4-/CD133-MAGEA4+/CD133+MAGEA4-/CD133+MAGEA4+: 65.6 months vs. 51.5 months vs. 32.2 months vs. 19.8 months, P=0.000. C. CD133-NY-ESO-1-/CD133+NY-ESO-1-/CD- 133-NY-ESO-1+/CD133+NY-ESO-1+: 57.8 months vs. 55.7 months vs. 44.6 months vs. 28.5 months, P=0.000. D. CD133-MAGEA10/CD133+ MAGEA10/CD133- MAGEA10/CD133+ MAGEA10+: 66.2 months vs. 57.2 months vs. 48.8 months vs. 41.4months, P=0.001. E. CD133+NY-ESO-1+ patients received chemotherapy or not (received vs. not received, 52.1 vs. 27.1 months, P=0.020.
Survival outcomes
Postoperative adjuvant chemotherapy was performed in 98 of the 159 patients. Univariate analysis found that there is no difference between patients received adjuvant chemotherapy or not (P=0.265), but subgroup analysis showed that the patients with CD133+NY-ESO-1+ expression who received chemotherapy will survive longer than not receive adjuvant chemotherapy (received vs. not received, 52.1 vs. 27.1 months, P=0.020) (Figure 2E). In the subgroup with EGFR mutation/ALK translocation/Ros1 translocation/Ret fusion , the trend remained but without a statistically significant difference. Multivariate COX regression analysis showed that stage, CD133, CD133-MAGEA4- and CD133-NY-ESO-1- are independent prognostic factors. In conclusion, CTAs (MAGE-A4, NY-ESO-1, MAGE-A10) were more likely expressed in patients with squamous cell carcinoma and when CTAs combined with CD133, they can be better prognostic factors. Patients with CD133+NY-ESO-1+ expression may survive longer when treated with adjuvant chemotherapy, which indicates that the CD133 and CTAs might be a potential marker to guide adjuvant chemotherapy in this population (Table 2).
Table 2.
Survival analysis in the whole population
| OS(overall survival) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variables | Univariate HR (95% CI) | P* | Multivariate HR (95% CI) | P* | ||
| Age (years) | > 61 | 1.0 | 0.831 | 1.0 | 0.081 | |
| ≤ 61 | 0.965 (0.733-1.923) | 0.548 (0.304-1.098) | ||||
| Gender | Male | 1.0 | 0.443 | 1.0 | 0.351 | |
| Female | 0.677 (0.423-1.883) | 1.357 (0.714-2.577) | ||||
| Smoking history | Never smoker | 1.0 | 0.993 | 0.484 (0.245-1.050) | 0.053 | |
| Smoker | 0.666 (0.563-1.965) | 1.0 | ||||
| Pathology | Squamous | 1.0 | 0.412 | 1.0 | 0.082 | |
| Adenocarcinoma | 0.552 (0.364-1.324) | 2.643 (1.455-2.800) | ||||
| Stage | I | 1.0 | 0.000 | 1.0 | 0.000 | |
| II | ||||||
| III | 1.965 (1.634-2.438) | 1.838 (1.346-2.510) | ||||
| Adjuvant | not received | 1.0 | 0.265 | 1.0 | 0.095 | |
| received | 1.242 (0.973-1.839) | 1.503 (0.915-1.936) | ||||
| CD133 | negative | 1.0 | 0.035 | 1.0 | 0.010 | |
| Positive | 1.965 (1.723-2.475) | 0.370 (0.174-0.788) | ||||
| CD133/MAGE-A4 | negative | 1.0 | 0.000 | 1.0 | 0.000 | |
| Positive | 1.834 (1.572-2.436) | 1.944 (1.396-2.707) | ||||
| CD133/NY-ESO-1 | negative | 1.0 | 0.000 | 1.0 | 0.002 | |
| Positive | 0.973 (0.678-1.823) | 1.660 (0.949-1.965) | ||||
| CD133/MAGE-A10 | negative | 1.0 | 0.001 | 1.0 | 0.094 | |
| Positive | 1.892 (0.932-2.421) | 1.365 (0.932-2.421) | ||||
| Gene analysis | Activated | 1.0 | 1.0 | |||
| Wild type | 0.624 (0.238-1.214) | 0.421 | 0.573 (0.325-1.011) | 0.054 | ||
| Unknown | ||||||
Represent P < 0.05.
Discussion
CTAs are extensively expressed in a variety of malignant neoplasms, including melanomas, and esophageal, breast, prostate, urinary tract, ovarian, and non-small cell lung cancer [8-11]. Patients with melanoma frequently express CT antigen genes while colon, renal and leukuemia /lymphoma rarely express these genes. CTAs often show heterogeneous expression within individual tumors, as determined by immunohistochemistry, the majority of the colorectal cancer and renal cell carcinoma come from the early stage, while the melanoma from the late stage.
In this study, All CTAs were more frequently expressed in squamous cell carcinoma (SCC) (50.0%, 26.9%, 34.6%) than in adenocarcinoma (16.2%, 16.2%, 16.2%). Which consistent with Ayyoub M et al’s study. CD133 was more frequently found in patients with adenocarcinoma (P=0.044).
Chen et al. reported that MAGE-A4 and NY-ESO-1 are both expressed in cytoplastic and nuclear site, which consistent with our result. The discrepancy in reported frequencies of NY-ESO-1 expression may be due to multiple parameters such as variation in clinical material (sampling, stage, subtype, treatment), staining protocol, scoring system/personnel etc. [22]. MAGE-A10 expressed in the nuclear site, which implies it may be a nuclear protein.
The expressions of CTAs in NSCLC are various according to different study. The mRNA expression rate of MAGE A4 ranging between 28.4% and 40%, NY-ESO-1 between 8.3% and 26.7%, MAGE-A10 between 27.2% and 66.7% in patient with NSCLC, respectively [23,24]. MAGE TAAs are expressed with frequencies ranging between 22.7% (larynx) and 50% of cases (lung) in squamous cell carcinomas from different anoatomic areas and in large cell carcinomas of the lung [25]. Expression of NY-ESO-1 was detectable in large cell carcinomas and adenocarcinomas of the lung. (17.8% and 10.5%, respectively) [23] Study showed MAGE-A3/4 and NY-ESO-1 positive staining in 65.1% and 23.3% of squamous cell carcinomas and 18.9% and 10.8% of adenocarcinomas, respectively [24]. The frequency of these two antigens expression in NSCLC in this study was higher than that in previous studies. The reasons for this difference in CT antigen expression are unclear, but the genetic difference, different research methods, different immunohistochemical sensitivity which depending on laboratory conditions and tumor cell heterogeneity are speculated as possible reasons. MAGE A10 expression is high in NSCLC, which may imply the possibility of its value in immunotherapy.
It is possible that CTAs are true hallmarks of cancer stem cells and provide unique targets to treat recurrences and metastatic cancer. In spite of being rare and exposed to immune attack, stem-cell like tumor cells are capable to escape the immune system of the host. Of particular interest is the frequent expression of MAGE A4 in squamous cell carcinomas than in adenocarcinoma. These data suggested the possibility of immunotherapeutic approaches, especially for squamous cell carcinomas of NSCLC. Although CTAs are expressed in different tumors, the positive rate have been both lower in mRNA or protein level. Using single antigen for immunotherapy may be insubstantial [25,26]. At least one CTA was expressed by 47.0%, two or three CTAs co-expression was 25.0%. This may applies the possibility for multiple vaccine immunotherapy.
Neither MAGE-A4, MAGE-A10nor NY-ESO-1 expression correlated with differentiation grade. But the relationship between CTAs expression and disease development and tumor malignancy is still unclear. There is a need to study the relationship between mRNA and protein expression of CTAs. The results suggested a possibility to use multiple vaccines, involved MAGE A10, which may be more suitable to moderately or poorly differentiated disease of NSCLC.
There is no difference between patients received adjuvant chemotherapy or not, but subgroup analysis showed that patients with CD133+NY-ESO-1+ expression who received chemotherapy will survive longer than not receive adjuvant chemotherapy, which implies that if immunotherapy are given to patients with NY-ESO-1 positive expression who unwilling to receive chemotherapy or unsuitable to chemotherapy, they may survive longer. This information is of critical relevance in the selection of patients potentially eligible for receiving adjuvant chemotherapy and for the monitoring of their effectiveness [27,28].
Multivariate COX regression analysis showed that stage, CD133, CD133-MAGEA4- and CD133-NY-ESO-1- are independent prognostic factors. There are different opinions about the CTAs and the prognosis, majority studies showed that MAGE A4 is the prognostic factors of NSCLC [29-36]. This may related with the regulation mechanisms of the CTA. This finding, together with the inclination of global hypomethylation in cancer, suggests CPG island hypomethylation at the promoter regions as the likely mechanism for transcriptional activation of CT genes in cancer.
To evaluate CT antigens as therapeutic cancer vaccine targets, multiple clinical trials have been carried out, in melanoma, ovarian, prostate, esophagus and bladder cancers, but not in lung cancer. MAGE-A3 recombination protein phase II/III trial was administered to NSCLC patients, for which an improvement of disease-free survival was observed at the interim analysis. Our results also showed the three CTAs are expressed in NSCLC, and the expression different in different histology type. The multiple vaccines will play an important role in the treatment of NSCLC. Currently, studies of CSC and CTAs expressions in NSCLC are at both gene and protein level. Investment focus on the expression mechanism and biologic behavior will be conducted in the coming future. It is tempting to speculate that a multiple CTA recombination vaccine might prove useful in the implementation of clinical effective antigen specific immunotherapy in NSCLC. Together, the reported results provide guidance for the design of combination therapies in patients with NSCLC.
Acknowledgements
The authors would like to thank to the Tissue Typing Laboratory of Basel University Hospital for their kindly provide the antibodies and unwavering support over the years. This study was supported by grants from National Natural Science Fund (81402486, 81401890), Science and Technology Commission of Shanghai Municipality (Grant numbers: 132R1434600) and Shanghai City Health Bureau (Grant numbers: 20124259).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Suehisa H, Toyooka S. Adjuvant chemotherapy for completely resected non-small-cell lung cancer. Acta Med Okayama. 2009;63:223–30. doi: 10.18926/AMO/31842. [DOI] [PubMed] [Google Scholar]
- 3.Ioannidis G, Georgoulias V, Souglakos J. How close are we to customizing chemotherapy in early non-small cell lung cancer? Ther Adv Med Oncol. 2011;3:185–205. doi: 10.1177/1758834011409973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 5.Abols A, Ducena K, Zayakin P, Silina K, Kalnina Z, Sadovska L, Tars J, Vilmanis J, Narbuts Z, Eglitis J, Pirags V, Line A. Survey of autoantibody responses against tumor-associated antigens in thyroid cancer. Cancer Biomark. 2014;14:361–9. doi: 10.3233/CBM-140413. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 7.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–9. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–9. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 9.Costa FF, Le Blanc K, Brodin B. Concise review: cancer/testis antigens, stem cells, and cancer. Stem Cells. 2007;25:707–711. doi: 10.1634/stemcells.2006-0469. [DOI] [PubMed] [Google Scholar]
- 10.Saldanha-Araujo F, Haddad R, Zanette DL, De Araujo AG, Orellana MD, Covas DT, Zago MA, Panepucci RA. Cancer/testis antigen expression on mesenchymal stem cells isolated from different tissues. Anticancer Res. 2010;30:5023–5027. [PubMed] [Google Scholar]
- 11.Cronwright G, Le Blanc K, Gotherstrom C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: Down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65:2207–2215. doi: 10.1158/0008-5472.CAN-04-1882. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Su C, Li X, Fan L, Zheng L, Fei K, Zhou C. KIF5B-RET fusions in Chinese patients with non-small cell lung cancer. Cancer. 2013;119:1486–94. doi: 10.1002/cncr.27940. [DOI] [PubMed] [Google Scholar]
- 13.Ren S, Chen X, Kuang P, Zheng L, Su C, Li J, Li B, Wang Y, Liu L, Hu Q, Zhang J, Tang L, Li X, Zhou C, Schmid-Bindert G. Association of EGFR mutation or ALK rearrangement with expression of DNA repair and synthesis genes in never-smoker women with pulmonary adenocarcinoma. Cancer. 2012;118:5588–94. doi: 10.1002/cncr.27603. [DOI] [PubMed] [Google Scholar]
- 14.Jian G, Songwen Z, Ling Z, Qinfang D, Jie Z, Liang T, Caicun Z. Prediction of epidermal growth factor receptor mutations in the plasma/pleural effusion to efficacy of gefitinib treatment in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:1341–7. doi: 10.1007/s00432-010-0785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulie PG, Karanikas V, Lurquin C, Colau D, Connerotte T, Hanagiri T, Van Pel A, Lucas S, Godelaine D, Lonchay C, Marchand M, Van Baren N, Boon T. Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol Rev. 2002;188:33–42. doi: 10.1034/j.1600-065x.2002.18804.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4 (+) and CD8 (+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101:10697–702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tajima K, Obata Y, Tamaki H, Yoshida M, Chen YT, Scanlan MJ, Old LJ, Kuwano H, Takahashi T, Takahashi T, Mitsudomi T. Expression of cancer/testis (CT) antigens in lung cancer. Lung Cancer. 2003;42:23–33. doi: 10.1016/s0169-5002(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 18.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 19.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 20.Cuffel C, Rivals JP, Zaugg Y, Salvi S, Seelentag W, Speiser DE, Liénard D, Monnier P, Romero P, Bron L, Rimoldi D. Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer. 2011;128:2625–2634. doi: 10.1002/ijc.25607. [DOI] [PubMed] [Google Scholar]
- 21.Schultz-Thater E, Noppen C, Gudat F, Dürmüller U, Zajac P, Kocher T, Heberer M, Spagnoli GC. NY-ESO-1 tumour associated antigen is a cytoplasmic protein detectable by specific monoclonal antibodies in cell lines and clinical specimens. Br J Cancer. 2000;83:204–8. doi: 10.1054/bjoc.2000.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Lee S, Lee CH, Lee MK, Kim YD, Shin DH, Choi KU, Kim JY, Park do Y, Sol MY. Expression of Cancer-Testis Antigens MAGE-A3/6 and NY-ESO-1 in Non-Small-Cell Lung Carcinomas and Their Relationship with Immune Cell Infiltration. Lung. 2009;187:401–11. doi: 10.1007/s00408-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liang Z, Yuan YH, Han Y, Liu YX, Liu N, Chen YT, Ritter G, Old LJ, Zhang LJ. Expression of multiple cancer-testis antigen genes in non-small cell lung cancer treated by chemotherapy prior surgery. Zhonghua Yi Xue Za Zhi. 2004;84:464–8. [PubMed] [Google Scholar]
- 24.Grah J, Samija M, Juretić A, Sarcević B, Sobat H. Immunohystochemical expression of cancer/testis antigens (MAGE-A3/4, NY-ESO-1) in non-small cell lung cancer: the relationship with clinical-pathological features. Coll Antropol. 2008;32:731–736. [PubMed] [Google Scholar]
- 25.Ayyoub M, Memeo L, Alvarez-Fernández E, Colarossi C, Costanzo R, Aiello E, Martinetti D, Valmori D. Assessment of MAGE-A expression in resected non-small cell lung cancer in relation to clinicopathologic features and mutational status of EGFR and KRAS. Cancer Immunol Res. 2014;2:943–8. doi: 10.1158/2326-6066.CIR-13-0211. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer. 2009;10:371–4. doi: 10.3816/CLC.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 27.Kocher T, Schultz-Thater E, Gudat F, Schaefer C, Casorati G, Juretic A, Willimann T, Harder F, Heberer M, Spagnoli GC. Identification and intracellular location of MAGE-3 gene product. Cancer Res. 1995;55:2236–9. [PubMed] [Google Scholar]
- 28.Bolli M, Schultz-Thater E, Zajac P, Guller U, Feder C, Sanguedolce F, Carafa V, Terracciano L, Hudolin T, Spagnoli GC, Tornillo L. NY-ESO-1/LAGE-1 coexpression with MAGE-A cancer/testis antigens: a tissue microarray study. Int J Cancer. 2005;115:960–966. doi: 10.1002/ijc.20953. [DOI] [PubMed] [Google Scholar]
- 29.Yao J, Caballero OL, Yung WK, Weinstein JN, Riggins GJ, Strausberg RL, Zhao Q. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res. 2014;2:371–9. doi: 10.1158/2326-6066.CIR-13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YT, Hsu M, Lee P, Shin SJ, Mhawech-Fauceglia P, Odunsi K, Altorki NK, Song CJ, Jin BQ, Simpson AJ, Old LJ. Cancer/testis antigen CT45: analysis of mRNA and protein expression in human cancer. Int J Cancer. 2009;124:2893–2898. doi: 10.1002/ijc.24296. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Richter UD, Dowejko A, Reuther T, Kleinheinz J, Reichert TE, Driemel O. Analysis of expression profiles of MAGE-A antigens in oral squamous cell carcinoma cell lines. Head Face Med. 2009;9:10. doi: 10.1186/1746-160X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastorcic-Grgic M, Sarcevic B, Dosen D, Juretic A, Spagnoli GC, Grgic M. Prognostic value of MAGE-A and NY-ESO-1 expression in pharyngeal cancer. Head Neck. 2010;32:1178–84. doi: 10.1002/hed.21314. [DOI] [PubMed] [Google Scholar]
- 33.Grah JJ, Katalinic D, Juretic A, Santek F, Samarzija M. Clinical significance of immunohistochemical expression of cancer/testis tumor-associated antigens (MAGE-A1, MAGE-A3/4, NY-ESO-1) in patients with non-small cell lung cancer. Tumori. 2014;100:60–8. doi: 10.1700/1430.15817. [DOI] [PubMed] [Google Scholar]
- 34.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groeper C, Gambazzi F, Zajac P, Bubendorf L, Adamina M, Rosenthal R. Cancer/testis antigen expression and specific cytotoxic T lymphocyte responses in non small cell lung cancer. Int J Cancer. 2006;120:337–343. doi: 10.1002/ijc.22309. [DOI] [PubMed] [Google Scholar]
- 36.Shigematsu Y, Hanagiri T, Shiota H, Kuroda K, Baba T, Mizukami M. Clinical significance of cancer/testis antigens expression in patients with non-small cell lung cancer. Lung Cancer. 2010;68:105–110135. doi: 10.1016/j.lungcan.2009.05.010. [DOI] [PubMed] [Google Scholar]


