Abstract
Lysosomal associated membrane protein 3 (LAMP3) is a newly identified tumor-specific and hypoxia-induced protein. It is a downstream target gene of tumor suppressor TP53 and its expression has been associated with hypoxia-induced metastasis and poor overall survival in cervical, breast and gastrointestinal cancers. However, little is known of LAMP3 protein expression in laryngeal squamous cell carcinoma (LSCC) and its prognostic value. We determined protein expression of LAMP3 and TP53 in LSCC tissues (n=117) by immunohistochemistry analysis on tissue microarray (TMA), their expression was correlated with patients’ clinical parameters and overall survival. LAMP3 and TP53 protein expression was significantly higher in cancerous tissues compared to adjacent normal surgical margin tissues. Both high LAMP3 and high TP53 protein expression was significantly associated with tumor stage and size. Significant correlation between LAMP3 and TP53 expression was observed. Patients with high LAMP3 or high TP53 expression had a poor overall survival. Our data suggest that both epithelial LAMP3 expression and TP53 expression are independent prognostic markers for LSCC.
Keywords: LAMP3, TP53, prognosis, laryngeal squamous cell carcinoma
Introduction
Laryngeal squamous cell carcinoma (LSCC) is the most common histological type of the head and neck cancer [1] and the second most common respiratory cancer after lung cancer [2]. Worldwide, there are an estimated 130,000 new laryngeal cancer cases and 82,000 deaths annually (GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012, International Agency for Research on Cancer). The estimated incidence and mortality rates of LSCC in China is 2.04/105 and 1.06/106 respectively during 2003-2007 [3]. LSCC is diagnosed predominantly in men and the rates are higher in urban areas than in rural areas [3]. Although LSCC is relatively rare in China compared to western developed countries, its incidence has been increasing over time due to increased consumption of tobacco and alcohol [2].
Patients with early stage LSCC are treated with either radiation therapy or laryngeal preservation surgery with the intent to preserve the function of larynx. Patients with advanced stage LSCC require multidisciplinary individualized modality therapy with the goal of balancing overall survival and the quality of life. Local recurrence and distant metastasis remain a major cause of mortality in LSCC, and current clinicopathological parameters have limited predictive and prognostic value for survival [4].
Genes involved in cell cycle regulation have been intensively investigated in LSCC, especially TP53. TP53 is one of the most important tumor suppressor genes that plays an essential role in the stability of human genome [5,6]. Mutations in TP53 are the most frequent genetic alterations identified in LSCC [7]. Wild type TP53 protein has a half-life of 6-20 minutes, which cannot be detected by immunohistochemistry, while mutant TP53 proteins are stabilized and show dominant-negative function, and can be detected by immunohistochemistry, which has been widely used as a surrogate marker for TP53 mutations [8,9]. TP53 overexpression has been detected in 40-60% of primary LSCC cases [7], patients with TP53 overexpression detected on the resection margins were more likely to experience local recurrence [1,10-12] and shorter disease free survival [11]. TP53 overexpression has also been shown to predict the malignant transformation from precursor lesions to LSCC [13]. However, the data are scant on the correlation between TP53 overexpression and overall survival in LSCC.
Tumor hypoxia refers to an inadequate supply of oxygen in the tumor microenvironment. It is frequently associated with poorly differentiated advanced stage tumors and presents a significant limitation for radiation therapy as radioresistance develops when the O2 partial pressure in a tumor is less than 25-30 mmHg [14,15]. Mechanistically, it has been hypothesized that reduced local oxygen concentrations can activate the hypoxic response pathway, which in turn remodels the extracellular matrix, induces epithelial-to-mesenchymal transition (EMT), promotes cancer stem cells and immune evasion [16]. It has been shown in vitro that hypoxia promotes radioresistance in LSCC [17]; however, the molecular markers involved in hypoxia-induced readioresistance have not been identified.
Novel molecular prognostic markers are needed to improve clinical outcome in LSCC patients as well as to enhance understanding of the mechanism of tumorigenesis and hypoxia-induced radioresistance. Lysosomalassociated membrane protein 3 (LAMP3) is a newly identified hypoxia regulated and TP53 downstream target gene involved in hypoxia-induced therapy resistance and metastasis. Its overexpression has been observed in several types of human cancers [18], including cervical, breast, and gastrointestinal cancer [19]. LAMP3 overexpression is associated with resistance to chemotherapy and radiotherapy [20-22], and LAMP3 expression has been associated with lymph node metastasis and poor overall survival [19,23-25].
To our best knowledge, thus far, no studies have investigated the potential role of LAMP3 in LSCC. In the present study, we analyzed epithelial LAMP3 and TP53 expression by immunohistochemistry analysis in both malignant LSCC and adjacent normal tissues using tissue microarrays (TMAs). We correlated epithelial LAMP3 and TP53 expression with clinicopathological characteristics as well as overall survival in patients with LSCC.
Material and methods
Human tissue specimens and patient clinical information
A total of 159 formalin-fixed paraffin-embedded (FFPE) tissue samples were collected from 117 LSCC patients. These include 117 LSCC tissues and 42 matched normal tissues at surgical margins. All tissue blocks were obtained from the Department of Pathology, Affiliated Hospital of Nantong University from year 2003 to 2011. Clinical characteristics of cancer patients were extracted from their medical records, including: age, sex, histological type, differentiation grade, tumor stage, tobacco and alcohol consumption. None of the cancer patients received any types of treatments (radiation therapy, chemotherapy, or immunotherapy) before surgery. Overall survival (OS) was defined as the period from initial biopsy confirmed diagnosis to death. Patients who were alive at the last follow-up date were censored from the analysis. The study protocol was approved by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University, Jiangsu, China.
Tissue microarray (TMA) construction and immunohistochemical analysis (IHC)
TMA was generated using the manual Tissue Microarrayer System Quick Ray (UT06, UNITMA, Korea) in the Department of Clinical Pathology, Nantong University Hospital, Jiangsu, China. Specifically, core tissue biopsies (2 mm in diameter) were taken from ~70 individual FFPE blocks and arranged in a new recipient paraffin block. A total of three TMAs were made. Four-micron sections were cut and placed on super frost-charged glass microscope slides to generate TMA slides.
Tissue sections were deparaffinized and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by incubation in 3% H2O2. Antigen retrieval was carried out with 0.01 M citrate buffer pH 6.0 and microwave heat induction. LAMP3 was detected by rabbit polyclonal anti-human LAMP3 antibody (dilution 1:100) (Abcam, ab111090), and TP53 was detected by rabbit polyclonal anti-human TP53 antibody (dilution 1:100) (DAKO, M3629). Reactions were detected with Envision+TM peroxidase kit (Dako, Carpinteria, CA, USA). Color development was accomplished by incubating with 3,3’-diaminobenzidine plus (Dako, Carpinteria, CA, USA), counterstained with hematoxylin, dehydrated through graded alcohols, cleared in xylene, and coverslipped with permanent mounting media.
All cases were reviewed and scored without knowledge of clinical characteristics. The expression of LAMP3 and TP53 was scored using the semi-quantitative H-score method, taking into account both the staining intensity and the percentage of cells at that intensity [26]. The staining intensity was scored as 0 (no staining), 1+ (weak staining), 2+ (moderate staining), or 3+ (intense staining). For each of the four staining intensity scores, the percentage of cells stained at the respective intensity was determined and multiplied by the intensity score to yield an intensity percentage score. The final staining scores were then calculated from the sum of the four intensity percentage scores; thus the staining score had a minimum value of 0 (no staining) and a maximum of 300 (100% of cells with 3+ staining intensity).
Statistical analysis
For statistical analysis, the continuous LAMP3 and TP53 expression data from IHC were first converted into dichotic data (low vs. high) using specific cutoff values, which were selected to be significant in terms of overall survival (OS) using the X-tile software program (The Rimm Lab at Yale University; http://www.tissuearray.org/rimmlab) [25,27].
Student t test and Pearson χ2 test were used to determine the statistical significance of differences between comparison groups. The correlation between LAMP3 and TP53 protein expression was calculated using Spearman’s test. The cumulative patient survival was estimated using the Kaplan-Meier method, and a log-rank test was used to compare the survival curves. A Cox proportional hazards model was used to calculate univariate and multivariate hazard ratios for the variables. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were carried out using the SPSS 19.0 statistical software package (SPSS Inc., Chicago, IL).
Results
LAMP3 or TP53 expression in laryngeal tissues
LAMP3 protein expression was mainly detected in tumor epithelial cells, only in rare occasions (<5 cases) was it also detected in tumor infiltrating lymphocyte. LAMP3 protein was localized in the cytoplasm while TP53 protein was localized in the nuclei (Figure 1). Using the X-tile software program for TMA data analysis (http://www.tissuearray.org/rimmlab), we first identified significant cutoff point in terms of overall survival in LSCC. The cutoff was 100 for both LAMP3 and TP53 proteins: score 0-100 was considered low expression while 101-300 was considered high expression. For all subsequent analyses, LAMP3 and TP53 protein expression levels were considered either as “Low” or “High” using these cutoff values.
Figure 1.
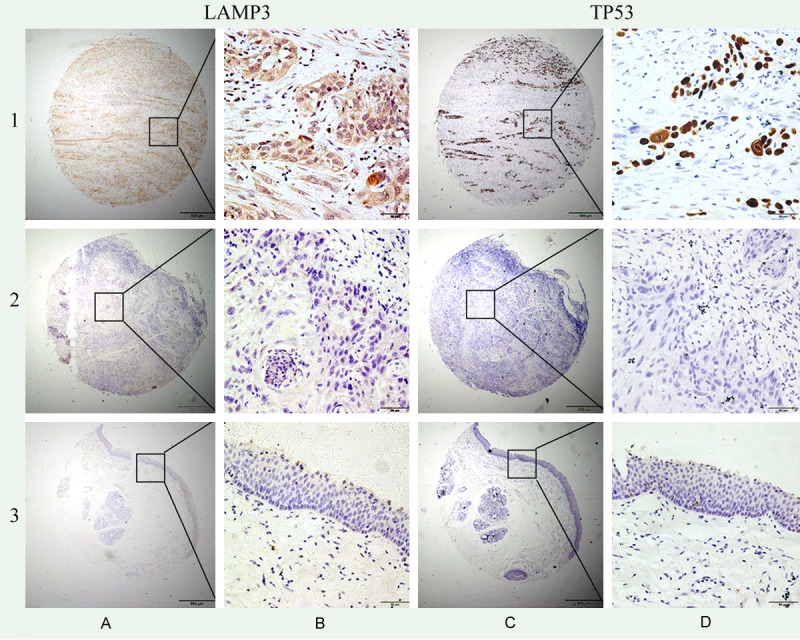
Representation of LAMP3 and TP53 protein expression in LSCC and normal margin tissues on TMA sections. Row 1: LSCC tissue with high LAMP3 expression and high TP53 expression; row 2: LSCC tissue with no LAMP3 expression and no TP53 expression; row 3: normal marginal tissue with no LAMP3 expression and no TP53 expression. Column A and B are LAMP3 staining with x40 (bar =500 μm) and x400 (bar =50 μm) magnification respectively, and column C and D are TP53 staining with x40 (bar =500 μm) and x400 (bar =50 μm) magnification respectively.
Of 117 LSCC tissues, 82 had high LAMP3 expression (LAMP3+, 70.9%), significantly higher than normal surgical margin tissues where only 12 out of 42 had high LAMP3 expression (28.6%) (P<0.001) (Table 1). Similarly, the frequency of high TP53 expression (TP53+) was significantly higher in LSCC tissues (67.5%) than normal surgical margin tissues (35.7%) (P<0.001). High LAMP3 and high TP53 expression (LAMP3+/TP53+) was more frequent in cancerous tissues (52.1%) than in adjacent normal surgical margins (20.5%) (P<0.001).
Table 1.
LAMP3 and TP53 protein expression in LSCC and noncancerous tissues
| Characteristic | n | LAMP3+ | Pearson χ2 | P | TP53+ | Pearson χ2 | P | LAMP3+/TP53+ | Pearson χ2 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Carcinoma | 117 | 82 (70.90) | 22.038 | <0.001* | 79 (67.52) | 12.937 | <0.001* | 61 (52.14) | 13.060 | <0.001* |
| Surgical margin | 42 | 12 (28.57) | 15 (35.71) | 9 (20.45) |
P<0.05.
Association of LAMP3 and TP53 expression with clinicopathologic characteristics in LSCC
Next, we examined the correlation between LAMP3 or TP53 protein expression and clinical parameters among LSCC patients. High LAMP3 expression was significantly associated with tumor stage (P=0.029), with both size (P=0.012) and regional lymph node metastasis (P=0.019); while high TP53 expression was significantly associated with tumor stage (P=0.009), tumor size (P=0.005), and marginally with regional lymph node metastasis (P=0.054) (Table 2). High LAMP3 and high TP53 expression (LA-MP3+/TP53+) was significantly associated with tumor stage (P<0.001), tumor size (P<0.001), and regional lymph node metastasis (P=0.008). Significant correlation between LAMP3 and TP53 expression was detected (P=0.019).
Table 2.
Association of high expression of LAMP3 and TP53 with clinicopathological characteristics in LSCC patients
| Groups | No. | LAMP3+ (%) | Pearson χ2 | P value | TP53+ (%) | Pearson χ2 | P value | LAMP3+/TP53+ (%) | Pearson χ2 | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 117 | 82 (70.09) | 79 (67.52) | 61 (52.14) | ||||||
| Age | 0.005 | 0.946 | 1.219 | 0.311 | 0.549 | 0.567 | ||||
| ≤60 years | 44 | 31 (70.45) | 27 (61.36) | 21 (47.73) | ||||||
| >60 years | 73 | 51 (69.86) | 52 (71.23) | 40 (54.79) | ||||||
| Tobacco consumption | 0.241 | 0.624 | 0.001 | 1.000 | 0.004 | 1.000 | ||||
| No | 32 | 25 (78.13) | 23 (71.88) | 18 (56.25) | ||||||
| Yes | 72 | 53 (73.61) | 52 (72.22) | 41 (56.94) | ||||||
| Unknown | 13 | 4 (30.77) | 4 (30.77) | 2 (15.38) | ||||||
| Alcohol consumption | 2.517 | 0.113 | 0.001 | 1.000 | 3.371 | 0.077 | ||||
| No | 54 | 37 (68.52) | 39 (72.22) | 26 (48.15) | ||||||
| Yes | 50 | 41 (82.00) | 36 (72.00) | 33(66.00) | ||||||
| Unknown | 13 | 4 (30.77) | 4 (30.77) | 2(15.38) | ||||||
| TNM stage | 7.086 | 0.029* | 9.537 | 0.009* | 15.573 | <0.001* | ||||
| Stage I | 14 | 10 (71.43) | 8 (57.14) | 6 (42.86) | ||||||
| Stage II | 57 | 38 (66.67) | 37 (64.91) | 25 (43.86) | ||||||
| Stage III and IV | 33 | 30 (90.91) | 30 (90.91) | 28 (84.85) | ||||||
| Unknown | 13 | 4 (30.77) | 4 (30.77) | 2 (15.38) | ||||||
| T stage | 9.032 | 0.012* | 10.830 | 0.005* | 18.931 | <0.001* | ||||
| I | 13 | 9 (69.23) | 7 (53.84) | 5 (38.46) | ||||||
| II | 50 | 32 (64.00) | 31 (62.00) | 20 (40.00) | ||||||
| III and IV | 41 | 37 (90.24) | 34 (82.93) | 34 (82.93) | ||||||
| Unknown | 13 | 4 (30.77) | 4 (30.77) | 2 (15.38) | ||||||
| N-Regional lymph nodes | 5.479 | 0.019* | 3.892 | 0.054 | 7.277 | 0.008* | ||||
| N0 | 100 | 66 (66.00) | 64 (64.00) | 47 (47.00) | ||||||
| N1 | 17 | 16 (94.12) | 15 (88.24) | 14 (82.35) | ||||||
| Histopathological grade | 0.574 | 0.780 | 1.879 | 0.427 | 2.503 | 0.309 | ||||
| 1 | 51 | 34 (66.67) | 34 (66.67) | 25 (49.02) | ||||||
| 2 | 57 | 41 (71.93) | 37 (64.91) | 29 (50.88) | ||||||
| 3 | 9 | 7 (77.78) | 8 (88.89) | 7 (77.78) | ||||||
| LAMP3 | ||||||||||
| High | 82 | 61 (74.39) | 5.897 | 0.019* | ||||||
| Low | 35 | 18 (51.43) | ||||||||
| TP53 | 5.897 | 0.019* | ||||||||
| High | 79 | 61 (77.22) | ||||||||
| Low | 38 | 21 (55.26) |
P<0.05.
Prognostic value of LAMP3 and TP53 protein expression in LSCC
We also determined prognostic factors in LSCC using both univariate and multivariate analysis. High LAMP3 expression (HR, 5.706, 95% CI, 2.011-16.191; P=0.001) was significantly associated with poor overall survival in univariate analysis, along with previously reported prognostic markers, including tumor stage (HR, 2.171, 95% CI, 1.221-3.861; P=0.008) and tumor size (HR, 2.073, 95% CI, 1.160-3.706; P=0.014) (Figure 2). High TP53 expression was significantly associated with poor overall survival (HR, 6.107, 95% CI, 1.874-19.900; P=0.003), and high LAMP3 and high TP53 (LAMP3+/TP53+) was significantly associated with poor overall survival (HR, 7.590, 95% CI, 3.134-18.382; P<0.001) in univariate analysis. In multivariate analysis, only high LAMP3 (HR, 9.481, 95% CI, 2.216-40.566; P=0.002) and high TP3 (HR, 6.436, 95% CI, 1.469-28.197; P=0.014) remained significantly associated with poor overall survival, but not tumor size, tumor stage, and combination of high LAMP3 and TP53 expression (Table 3).
Figure 2.
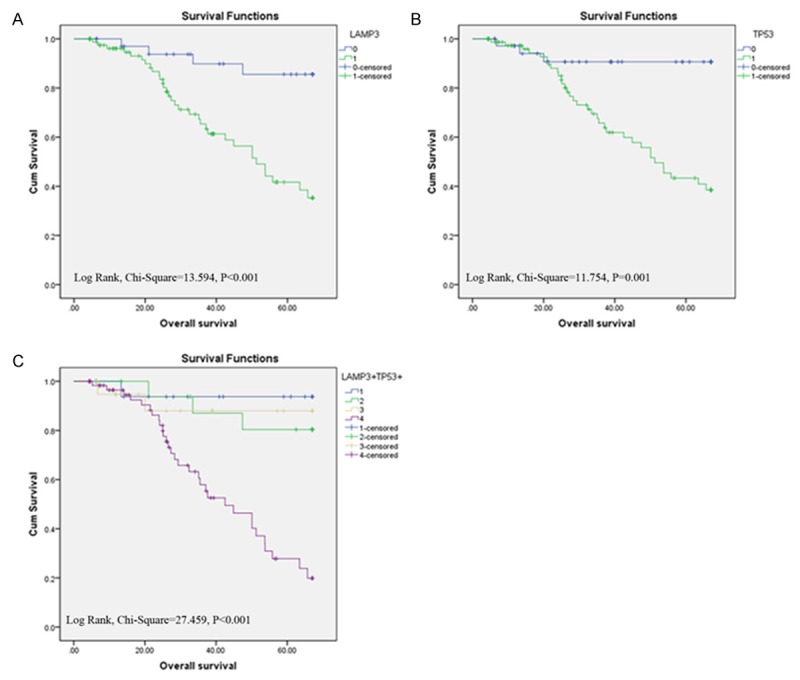
Survival curves of LSCC by the Kaplan-Meier method and the log-rank test. A: Overall survival curves of LAMP3+ (green line, 1) and LAMP3- (blue line, 0); B: Overall survival curves of TP53+ (green line, 1) and TP53- (blue line, 0); C: Overall survival curves of LAMP3+/TP53+ (purple line, 4), LAMP3+/TP53- (yellow line, 3), LAMP3-/TP53+ (green line, 2), and LAMP3-/TP53- (blue line, 1).
Table 3.
Univariate and multivariate analysis of prognostic markers for overall survival in LSCC patients
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | p value | 95% CI | HR | p value | 95% CI | |
| LAMP3 expression | 5.706 | 0.001* | 2.011-16.191 | 9.481 | 0.002* | 2.216-40.566 |
| high versus low | ||||||
| TP53 expression | 6.107 | 0.003* | 1.874-19.900 | 6.436 | 0.014* | 1.469-28.197 |
| high versus low | ||||||
| LAMP3/TP53 expression | 7.590 | <0.001* | 3.134-18.382 | |||
| LAMP3+/TP53+ versus Non- LAMP3+/TP53+ | ||||||
| Age (years) | 1.088 | 0.803 | 0.560-2.116 | |||
| ≤60 versus >60 | ||||||
| Tobacco consumption | 0.878 | 0.720 | 0.430-1.793 | |||
| No versus Yes | ||||||
| Alcohol consumption | 1.595 | 0.171 | 0.817-3.113 | |||
| No versus Yes | ||||||
| Differentiation | 1.528 | 0.213 | 0.784-2.977 | |||
| Well and middle versus poor | ||||||
| T stage | 2.073 | 0.014* | 1.160-3.706 | 0.747 | 0.727 | 0.146-3.833 |
| I versus II versus III and IV | ||||||
| N-Regional lymph nodes | 2.045 | 0.091 | 0.893-4.685 | |||
| N0 versus N1 | ||||||
| TNM stage | 2.171 | 0.008* | 1.221-3.861 | 1.590 | 0.571 | 0.320-7.903 |
| I versus II versus III and IV | ||||||
P<0.05.
Discussion
In this study, we have determined LAMP3 and TP53 protein expression in laryngeal tissues by immunohistochemistry analysis on tissue microarray (TMA). We found that both LAMP3 and TP53 protein expression were significantly higher in LSCC tissues than in normal adjacent tissues. We found high LAMP3 protein expression was associated with tumor stage, tumor size and regional lymph node metastasis, and we detected correlation between LAMP3 and TP53 expression. In both univariate and multivariate analysis, we found high LAMP3 and high TP53 was significantly associated with patients’ poor overall survival.
Our study confirms and extends previous findings on the important role of TP53 in LSCC. TP53 overexpression was more frequent in LSCC cancerous tissues (67.5%) than in the adjacent normal surgical margins (35.7%), TP53 overexpression was also more frequent in advanced stage patients (90.9% in stage III and IV vs. 64.9% in stage II and 57.1% in stage I), and more frequent in patients with regional lymph node metastasis (88.2% in N1 vs. 64% in N0). Our study is the first to show that TP53 overexpression is a prognostic marker for overall survival: TP53 overexpression was associated with worse overall survival in both univariate (HR, 6.107, CI%, 1.874-19.900; P=0.003) and multivariate analyses (HR, 6.436, CI%, 1.469-28.197; P=0.014).
To our best knowledge, this is the first study investigating epithelial LAMP3 protein expression as well as potential LAMP3 and TP53 protein interaction in LSCC. LAMP3 was originally characterized as a molecular marker for mature interdigitating dendritic cells (DC) (CD208, DC-LAMP) [28], as well as a lung specific gene (TSC403) [18]. It is overexpressed in several types of human cancers, including cancer of cervix, breast, stomach, ovary, colon, and liver [18,19]. It has been hypothesized that LAMP3 may be important for tumor metastasis and resistance to therapy: LAMP3 induces migration and invasion of tumor cells in vitro [29-30]; LAMP3 expression has been associated with resistance to chemotherapy and radiotherapy [20-22]; and LAMP3 expression has been associated with lymph node metastasis and poor overall survival [23-25]. Expression of LAMP3 protein in tumor epithelial cell had prognostic value in breast cancer [23,31], and gastrointestinal cancer from our previous study [19].
We also identified significant correlation between LAMP3 and TP53 overexpression. Of 117 LSCC cases, 61 (52.1%) had high expression of both LAMP3 and TP53. This is consistent with previous report on the identification of LAMP3 as one of the downstream target genes of TP53 involved in 5-fluorouracil (5-FU) resistance in colon cancer [32], but in contrast to our previous study in gastrointestianal cancer [19], where no correlation had been identified between LAMP3 and TP53 expression. Future in vitro mechanistic studies are needed to confirm the interaction between LAMP3 and TP53 in LSCC.
Radiation therapy is a valuable treatment option for LSCC patients. However, radioresistance represents a serious problem in LSCC patients, even among early stage patients [17]. Elucidating the underlying mechanism of tumor hypoxia-induced radioresistance will ultimately improve patients’ survival and quality of life. LAMP3 is a novel hypoxia-regulated gene and a mediator of hypoxia induced metastasis [33]. Both LAMP3 mRNA and protein are induced by hypoxia. In cervical cancer, expression of LAMP3 is associated with hypoxia and mediates hypoxia-driven nodal metastasis through regulating cell migration [24,30]. In breast cancer xenografts, LAMP3 protein expression colocalizes with hypoxic areas and is associated with locoregional recurrence [23]. Mechanistically, it has been shown that tumor hypoxia induces unfolded protein response (UPR) pathway, which in turn induces LAMP3 via the PKR-like ER kinase (PERK)/activating transcription factor 4 (ATF4)-arm of the UPR [29,33].
Our study is a retrospective observational study; the conclusions might not be applicable to the general population. Larger prospective studies are needed to confirm our findings. Our results might be biased because we have used TMA to analyze LAMP3 and TP53 protein level, the expression pattern might not represent the expression pattern of the whole tissue, and IHC data are semiquantitative, additional methods are needed to evaluate and confirm LAMP3 and TP53 expression in tumor cells. Finally, we do not know whether LAMP3 protein is associated with radioresistance in LSCC. Future prospective studies are needed to investigate the association between LAMP3 overexpression and radioresistance in LSCC.
In conclusion, we have shown that high LAMP3 protein expression is an independent prognostic marker in LSCC. Because of the role of LAMP3 in tumor-associated hypoxia, future research is warranted to investigate whether LAMP3 plays a role in hypoxia-associated radioresistance and whether LAMP3 is both a prognostic marker and a valid novel therapy target in LSCC.
Acknowledgements
This study was supported by the Six talent peaks project (WSW-030) in Jiangsu, China, the postdoctoral fellowship (140220C) from the Human Resources and Social Security Department of Jiangsu, China, the postdoctoral fellowship (2014-23-02) and from the Affiliated Hospital of Nantong University, Jiangsu, China.
Disclosure of conflict of interest
None.
References
- 1.Yang JQ, Liu HX, Liang Z, Sun YM, Wu M. Over-expression of p53, p21 and Cdc2 in histologically negative surgical margins is correlated with local recurrence of laryngeal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:4295–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Cattaruzza MS, Maisonneuve P, Boyle P. Epidemiology of laryngeal cancer. Eur J Cancer B Oral Oncol. 1996;32B:293–305. doi: 10.1016/0964-1955(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Du LB, Mao WM, Chen WQ, Zhang SW, Yu CD, Zheng RS, Xia QM, Wang XH. [Incidence and mortality of larynx cancer in China during 2003-2007] . Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:395–8. [PubMed] [Google Scholar]
- 4.Rodrigo JP, Martínez P, Allonca E, Alonso-Durán L, Suárez C, Astudillo A, García-Pedrero JM. Immunohistochemical markers of distant metastasis in laryngeal and hypopharyngeal squamous cell carcinomas. Clin Exp Metastasis. 2014;31:317–25. doi: 10.1007/s10585-013-9630-5. [DOI] [PubMed] [Google Scholar]
- 5.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 6.Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–6. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 7.Nathan CO, Sanders K, Abreo FW, Nassar R, Glass J. Correlation of p53 and the proto-oncogene eIF4E in larynx cancers: prognostic implications. Cancer Res. 2000;60:3599–604. [PubMed] [Google Scholar]
- 8.Casey G, Lopez ME, Ramos JC, Plummer SJ, Arboleda MJ, Shaughnessy M, Karlan B, Slamon DJ. DNA sequence analysis of exons 2 through 11 and immunohistochemical staining are required to detect all known p53 alterations in human malignancies. Oncogene. 1996;13:1971–81. [PubMed] [Google Scholar]
- 9.Havrilesky L, Darcy kM, Hamdan H, Priore RL, Leon J, Bell J, Berchuck A Gynecologic Oncology Group Study. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2003;21:3814–25. doi: 10.1200/JCO.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 10.Allegra E, Puzzo L, Cutrona D, Trichini A, Garozzo A, Serra A. p53 overexpression on the resection margins as a marker of local recurrence in glottic T1a carcinoma. Acta Otorhinolaryngol Ital. 2003;23:454–8. [PubMed] [Google Scholar]
- 11.Jalali MM, Heidarzadeh A, Zavarei MJ, Sarmast H. p53 overexpression impacts on the prognosis of laryngeal squamous cell carcinomas. Asian Pac J Cancer Prev. 2011;12:1731–4. [PubMed] [Google Scholar]
- 12.Gorban’ NA, Kudaĭbergenova AG, Pankratov VA. [Prognostic value of markers for proliferative activity and apoptotic regulation in laryngeal squamous cell carcinoma] . Arkh Patol. 2013;75:3–9. [PubMed] [Google Scholar]
- 13.López F, Alvarez-Marcos C, Alonso-Guervós M, Domínguez F, Suárez C, Hermsen MA, Llorente JL. From laryngeal epithelial precursor lesions to squamous carcinoma of the larynx: the role of cell cycle proteins and beta-catenin. Eur Arch Otorhinolaryngol. 2013;270:3153–62. doi: 10.1007/s00405-013-2476-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Lin Q, Glazer PM, Yun Z. Hypoxic tumor microenvironment and cancer cell differentiation. Curr Mol Med. 2009;9:425–34. doi: 10.2174/156652409788167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 16.Pistollato F, Giampieri F, Battino M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food Chem Toxicol. 2015;75C:58–70. doi: 10.1016/j.fct.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Li X, Qu Y, Xu O, Sun Q. Hypoxia promotes radioresistance of CD133-positive Hep-2 human laryngeal squamous carcinoma cells in vitro. Int J Oncol. 2013;43:131–40. doi: 10.3892/ijo.2013.1929. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki K, Nagata M, Suzuki M, Fujiwara T, Ueda K, Miyoshi Y, Takahashi E, Nakamura Y. Isolation and characterization of a novel human lung-specific gene homologous to lysosomal membrane glycoproteins 1 and 2: significantly increased expression in cancers of various tissues. Cancer Res. 1998;58:3499–503. [PubMed] [Google Scholar]
- 19.Sun R, Wang X, Zhu H, Mei H, Wang W, Zhang S, Huang J. Prognostic value of LAMP3 and TP53 overexpression in benign and malignant gastrointestinal tissues. Oncotarget. 2014;5:12398–409. doi: 10.18632/oncotarget.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennati M, Lopergolo A, Profumo V, De Cesare M, Sbarra S, Valdagni R, Zaffaroni N, Gandellini P, Folini M. miR-205 impairs the autophagic flux and enhances cisplatin cytotoxicity in castration-resistant prostate cancer cells. Biochem Pharmacol. 2014;87:579–97. doi: 10.1016/j.bcp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Nagelkerke A, Bussink J, van der Kogel AJ, Sweep FC, Span PN. The PERK/ATF4/LAMP3-arm of the unfolded protein response affects radioresistance by interfering with the DNA damage response. Radiother Oncol. 2013;108:415–21. doi: 10.1016/j.radonc.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Nagelkerke A, Sieuwerts AM, Bussink J, Sweep FC, Look MP, Foekens JA, Martens JW, Span PN. LAMP3 is involved in tamoxifen resistance in breast cancer cells through the modulation of autophagy. Endocr Relat Cancer. 2014;21:101–12. doi: 10.1530/ERC-13-0183. [DOI] [PubMed] [Google Scholar]
- 23.Nagelkerke A, Mujcic H, Bussink J, Wouters BG, van Laarhoven HW, Sweep FC, Span PN. Hypoxic regulation and prognostic value of LAMP3 expression in breast cancer. Cancer. 2011;117:3670–81. doi: 10.1002/cncr.25938. [DOI] [PubMed] [Google Scholar]
- 24.Kanao H, Enomoto T, Kimura T, Fujita M, Nakashima R, Ueda Y, Ueno Y, Miyatake T, Yoshizaki T, Buzard GS, Tanigami A, Yoshino K, Murata Y. Overexpression of LAMP3/TSC403/DC-LAMP promotes metastasis in uterine cervical cancer. Cancer Res. 2005;65:8640–5. doi: 10.1158/0008-5472.CAN-04-4112. [DOI] [PubMed] [Google Scholar]
- 25.Zhai X, Zhu H, Wang W, Zhang S, Zhang Y, Mao G. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol. 2014;31:970. doi: 10.1007/s12032-014-0970-z. [DOI] [PubMed] [Google Scholar]
- 26.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni S, Xu L, Huang J, Feng J, Zhu H, Wang G, Wang X. Increased ZO-1 expression predicts valuable prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2887–95. [PMC free article] [PubMed] [Google Scholar]
- 28.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Aït-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–36. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 29.Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, Span PN. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP, Koritzinsky M, Wouters BG. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–37. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 31.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–74. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 32.Adamsen BL, Kravik KL, Clausen OP, De Angelis PM. Apoptosis, cell cycle progression and gene expression in TP53-depleted HCT116 colon cancer cells in response to short-term 5-fluorouracil treatment. Int J Oncol. 2007;31:1491–500. [PubMed] [Google Scholar]
- 33.Mujcic H, Rzymski T, Rouschop KM, Koritzinsky M, Milani M, Harris AL, Wouters BG. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol. 2009;92:450–9. doi: 10.1016/j.radonc.2009.08.017. [DOI] [PubMed] [Google Scholar]


