Abstract
In our previous work, apoptosis signal-regulating kinase 1 (ASK1), a member of the mitogen-activated protein kinase (MAPK) kinase family has been proved to be associated with the pro-apoptosis effect of tight junction protein claudin-6 in breast cancer. However, its expression in cervical carcinoma has not been reported. The ASK1 and claudin-6 expression in cervical carcinoma tissues and adjacent non-neoplastic tissues was examined by immunohistochemistry. The mRNA and protein expression of ASK1 and claudin-6 in cervical cancer carcinoma cells was detected by reverse transcription (RT)-PCR and western blot. The expression level of ASK1 was down-regulated in cervical carcinoma tissues compared with the adjacent non-neoplastic tissues and positively correlated with the level of claudin-6 in cervical carcinoma cells and tissues. Our present study reveals that ASK1 protein expression altered between human cervical carcinoma and adjacent non-neoplastic tissues. The expression of ASK1 is correlated with the level of claudin-6 in cervical carcinoma cells and tissues.
Keywords: Apoptosis, apoptosis signal-regulating kinase 1, cervical carcinoma, claudin-6
Introduction
Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen-activated protein (MAP) kinase which is required for apoptosis induced by TNF, oxidative stress and endoplasmic reticulum (ER) stress [1]. ASK1 induces cell apoptosis through activating the c-Jun N-terminal kinase (JNK) and p38 MAP kinase signaling cascades in human epithelial cells [2]. Additionally, ASK1 appears to be a pivotal component not only in stress-induced cell apoptosis but also in a broad range of biological activities in order for cells to adapt to or oppose various stresses [3,4]. In the early stage of our work, ASK1 has been proved to be associated with the pro-apoptosis effect of tight junction protein claudin-6 in breast cancer [5]. However, the expression of ASK1 in cervical carcinoma and its correlation with claudin-6 has not been discussed until now.
Tight junctions exist in the junctional complexes of epithelial and endothelial cells and play crucial roles in cell polarity, adhesion and permeability [6]. It is revealed that the expression of claudin proteins which are the main protein components of tight junction are often altered in human tumors [7]. In our earlier study, we have identified that claudin-6, a tight junctional protein, as a potential breast cancer suppressor gene, which contributed to the apoptosis of breast cancer cells [8]. In addition, we found that the expression level of claudin-6 is correlated with the level of ASK1 in breast cancer [5]. However, to date, it is unknown about the ASK1 and claudin-6 expression and the relationship between ASK1 and claudin-6 in cervical carcinoma. Therefore, in this research, the expression of ASK1 and claudin-6 in cervical carcinoma cells and tissues was investigated.
Patients and methods
Patients
Paraffin blocks from fifty specimens of cervical carcinoma and fifty specimens of histologically normal cervical tissue adjacent to the neoplasms were collected from patients being treated at the First Hospital of Jilin University during the period between July 2011 and June 2013. The patients’ medical records were reviewed to determine their age and gender. There are 36 cases of squamous cell carcinoma, 14 cases of endocervical adenocarcinoma. Sections of the tumor were analyzed to identify the histological grade, and the presence or absence of regional lymph node metastasis. Twenty-six tumors had well differentiated histological appearance, another twenty-four tumors were of moderately and poor differentiated. For the use of these clinical materials for research purposes, prior patient’s consent and approval from the Institute Research Ethics Committee was obtained. All the cancer cases were classified and staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical carcinoma.
Immunohistochemistry
Immunohistochemistry was performed as described elsewhere [9]. Tissue sections were immunostained with claudin-6 antibodies (Santa Cruz Biotechnology Inc) and ASK1 antibodies (Bioworld Technology) were used. The negative controls were handled in the same way except PBS instead of primary antibody. Diaminobenzidine (DAB) was used for color development. Claudin-6 expression is indicated in brown and is expressed in the membrane of cervical carcinoma cells and ASK1 is indicated in brown and is expressed in the cytoplasm of cervical carcinoma cells. Scoring was performed as follows: negative (-), < 10% positive tumor cells; positive (+), ≥ 10% positive tumor cells. Positive or negative reactions were determined in five random fields of each sample with image processing software Image-Pro Plus 6.0.
Cell lines and transfection protocol
HeLa and C33A, human cervical carcinoma cell lines were cultured at 37°C in a humidified 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO, Gibco BRL, Carlsbad, California, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Japan), 10 mmol/l HEPES. Full-length human claudin-6 cDNA fragment [coding region from nucleotide 1 to 685 (claudin-6 1-685), Genebank accession no. NM-021195] was amplified by KeyGEN BioTECH Company. The 0.685 kb fragments, digested by XhoI/EcoRI, were ligated into the response plasmid p-EGFP-C1 (+) to create the expression vector p-EGFP (+)-claudin-6. The final construct was confirmed by direct sequence analysis. We amplified the cloned claudin-6 expression vector and purified this plasmid DNA fragment according to Current Protocol in Molecular Biology. Five micrograms of each plasmid was transfected into cells using X-Fect Transfection Reagent (TaKaRa, Japan). G418 (Sigma, St. Louis, Missouri, USA)-resistant clones were expanded in culture as a monoclonal population. Cells transfected with an empty vector p-EGFP-C1 (+) were used as a vector control.
Reverse transcription polymerase chain reaction
Measurement of ASK1 and claudin-6 mRNA expression by semi quantitative RT-PCR. Total RNA was isolated from 5 × 106 cells using TRIzol reagent (Invitrogen, Carlsbad, California, USA). The reverse transcription reaction was performed with 5 mg of total RNA using M-MuLV reverse transcriptase (TaKaRa, Japan) at 42°C for 60 min, and 0.5 mg cDNA was used for RT-PCR. The PCR step was performed using Taq DNA polymerase (TaKaRa, Japan). As an internal control, human GAPDH was amplified to ensure cDNA quality and quantity for each RT-PCR reaction. The specific primer sequences of GAPDH were: 5’-TGTTGCCATCAATGACCCCTT-3’ (sense) and 5’-CTCCACGACGTACTCAGCG-3’ (antisense), ASK1 primer sequences were: 5’-TTCACACAAAACGGATGTAACATT-3’ (sense) and 5’-CCTAAACAGTTATGGTCACATTTTGG-3’ (antisense) and claudin-6 primer sequences were: 5’-TTCATCGGCAACAGCATCGT-3’ (sense) and 5’-GGTTATAGAAGTCCCGGATGA-3’ (antisense). Triplicate independent PCR reactions were carried out to ensure the reproducibility of expression. The result was analyzed by Quantity One 4.4.1 software (Bio-Rad Laboratories Inc, Hercules, California, USA).
Western blot analysis
Cells grown on dishes were rinsed with 0.01 mol/l phosphate-buffered saline (PBS) three times, scraped in 450 ml of lysis buffer (1 mmol/l NaHCO3 and 10 mmol/l phenylmethyl sulfonylfluoride) and collected in micro-centrifuge tubes. The protein concentration of each sample was determined using a BCA Protein Assay Kit (Pierce Chemical Co., Rockford, Illinois, USA). Twenty micrograms of denatured total protein was subsequently applied to one end of 10% SDS polyacrylamide gels submerged in a suitable buffer. Electrophoresis was terminated when the dyestuff had run to the edge of the other end of the gel. The proteins on the gel were then transferred onto a nitrocellulose membrane (Millipore, Temecular, California, USA). The membrane was blocked with 5% defatted milk (in 25 mmol/l Tris, pH 8.0, 125 mmol/l NaCl, 0.1% Tween 20) overnight at room temperature and incubated with 1:1000 diluted anti-β-actin (Santa Cruz Biotechnologies, California, USA), anti-claudin-6 (Santa Cruz Biotechnologies) and anti-ASK1 (Santa Cruz Biotechnologies) antibodies at room temperature for 1 h. After washing, the membrane was reacted with horse radish peroxidase-conjugated secondary antibodies at room temperature for 1 h and washed again. Finally the immunoreactive bands were visualized using an ECL western blotting system (GE, Fairfield, Connecticut, USA).
Statistical analyses
Origin 7.5 (Origin Lab Corp, Northampton, MA) laboratory data analysis software and image processing software (Image-Pro Plus6.0) were used to quantitate data. All data were expressed as mean ± SD. The Chi-square test/Chi-Square Goodness-of-Fit Test was used to determine the prognostic significance value for disease progression of each factor alone. One-way analysis of variance was employed with SPSS version 13.0 software to determine the significance of differences across treatment groups, and P < 0.05 was considered significant.
Results
Expression of ASK1 and claudin-6 in adjacent non-neoplastic tissues and cervical carcinoma tissues
The expression of ASK1 and claudin-6 in adjacent non-neopalstic cervical tissues and cervical carcinoma was examined by immunohistochemistry. The expression of ASK1 and claudin-6 in cervical carcinoma tissues was significantly lower than adjacent tissues (Figure 1). Positive expression of ASK1 protein was found in 46.0% (23/50) of cervical carcinoma tissues and in 64.0% (32/50) of adjacent tissues (Table 1). The expression rate of ASK1 in cervical carcinoma tissues was lower than the rate in adjacent tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, P = 0.032 < 0.05) and the expression of ASK1 was not correlated with age (P = 1.000), histological grade (P = 1.000), or lymph node metastasis (P = 0.437).
Figure 1.
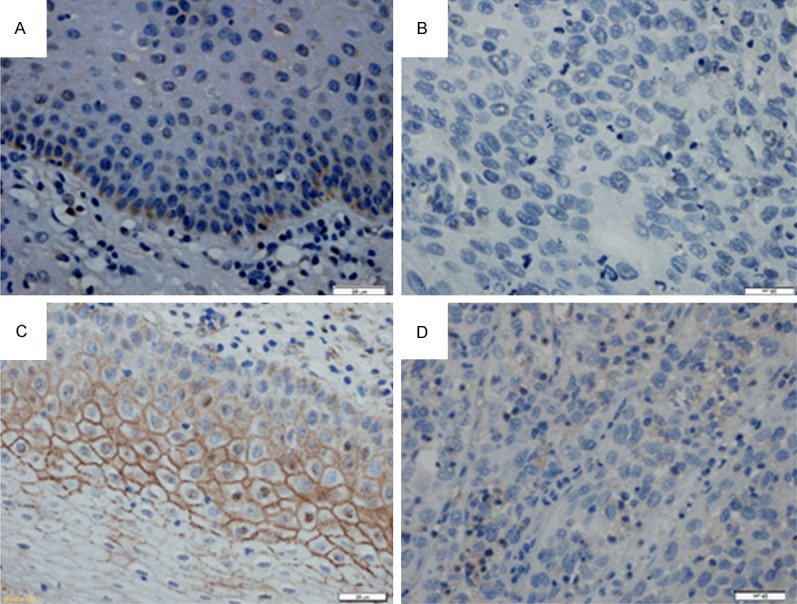
Immunohistochemical demonstration of ASK1 and claudin-6 protein expression in human cervical carcinoma and adjacent tissue (A). ASK1 was highly expressed in the cytoplasm of epithelial cells adjacent to cervical carcinoma but was expressed at low levels in cancer tissue itself (B, C). The strong expression of claudin-6 in the membrane of tissues adjacent to human cervical carcinoma compared to low expression of claudin-6 in human cervical carcinoma tissues (D). (Original magnification × 400).
Table 1.
Expression of ASK1 and claudin-6 in cervical carcinoma patients
| Item | n | ASK1 (+) | ASK1 (-) | P | n | Claudin-6 (+) | Claudin-6 (-) | P |
|---|---|---|---|---|---|---|---|---|
| cervical carcinoma tissue | 50 | 23 | 27 | < 0.05 | 50 | 13 | 37 | < 0.001 |
| Adjacent tissue | 50 | 32 | 18 | 50 | 37 | 13 | ||
| Age (year) | ||||||||
| ≤ 60 | 23 | 11 | 12 | 1.000* | 23 | 6 | 17 | 1.000* |
| > 60 | 27 | 12 | 15 | 27 | 7 | 20 | ||
| Histological grade | ||||||||
| Well -differentiated | 26 | 13 | 13 | 1.000* | 26 | 8 | 18 | 0.275* |
| Moderately and poor Differentiated | 24 | 10 | 14 | 24 | 5 | 19 | ||
| Lymph node metastasis | ||||||||
| + | 10 | 4 | 6 | 0.437* | 10 | 3 | 7 | < 0.05 |
| - | 40 | 19 | 21 | 40 | 10 | 30 |
No statistical significance.
Positive expression of claudin-6 protein was found in 26.0% (13/50) of cervical carcinoma tissues and in 64% (37/50) of adjacent non-neoplastic tissues, the expression of claudin-6 was not correlated with age (P = 1.000), histological grade (P = 0.275), but correlated with lymph node metastasis (P < 0.05) (Table 1).
ASK1 and claudin-6 may be simultaneously expressed in adjacent non-neoplastic tissues and cervical carcinoma
We investigated the correlation between ASK1 and claudin-6 expression in cervical adjacent non-neoplastic tissues and carcinoma tissues using The Chi-square test/Chi-Square Goodness-of-Fit Test. We found that the expression of ASK1 was positively correlated with the expression of claudin-6 in adjacent non-neoplastic tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, φ = 0.781, P < 0.01) and cervical carcinoma tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, φ = 0.693, P < 0.01). The detailed results of the analysis are described in Tables 2 and 3.
Table 2.
Correlation between the expression of ASK1 and claudin-6 in non-neoplastic cervical tissues
| Item | ASK1 (+) | ASK1 (-) | φ* | P |
|---|---|---|---|---|
| claudin-6 (+) | 25 | 12 | 0.781 | < 0.01 |
| claudin-6 (-) | 7 | 6 |
φ Phi coefficient.
Table 3.
Correlation between the expression of ASK1 and claudin-6 in cervical carcinoma patients
| Item | ASK1 (+) | ASK1 (-) | φ* | P |
|---|---|---|---|---|
| claudin-6 (+) | 8 | 5 | 0.672 | < 0.01 |
| claudin-6 (-) | 15 | 22 |
φ Phi coefficient.
Claudin-6 enhances the expression of ASK1 in cervical carcinoma cells
After transfection with p-EGFP (+)/claudin-6, G418-resistant HeLa and C33A clones were expanded in culture as monoclonal populations. Two of HeLa and C33A clones were selected to examine claudin-6 expression by quantitative RT-PCR, western blot analysis. These clones were named C1 and C2. The ASK1 and claudin-6 expression in cervical carcinoma cell line was examined. HeLa and C33A cells which expressed claudin-6 stably have a higher level of ASK1 expression than vector group. Quantitative RT-PCR and western blot analysis showed that the level of ASK1 mRNA and protein positively correlated with the level of claudin-6 mRNA and protein (Figure 2).
Figure 2.

Claudin-6 up-regulates the expression of ASK1 in cervical carcinoma cells. A. RT-PCR assay examined the mRNA levels of ASK1 and claudin-6 in HeLa cells. B. RT-PCR assay examined the mRNA levels of ASK1 and claudin-6 in C33A cells. C. Western blot assay detected ASK1 and claudin-6 protein expression in HeLa cells. D. Western blot assay detected ASK1 and claudin-6 protein expression in C33A cells.
Discussion
To date, c-Jun N-terminal kinases (JNK) and p38 MAP kinases have been characterized as regulators of several cellular functions including cell apoptosis [10]. Apoptosis signal-regulating kinase 1 (ASK1), a ubiquitously expressed MAPK kinase (MAP3K), can activate the JNK and p38 signaling pathways and is required for both oxidative stress and cytokine-induced apoptosis [11,12].
Several studies have discussed the role of ASK1 in tumorigenesis [13,14]. For instance, ASK1 has been shown to participate in both colon and skin tumorigenesis through the regulation of apoptosis [15,16]. Phosphorylation of ASK1 on Ser-83 can induce the apoptosis of pancreatic cancer cell and the activation of ASK1-JNK signaling leads to the apoptosis in human bladder cancer T24 cells [17]. In addition, ASK1 affects multiple cellular functions, such as survival, differentiation, and the innate immune response [18,19]. It is revealed that over-expression of ASK1 could inhibit the adipocytic differentiation process of the Malignant fibrous histiocytomas (MFHs) cells [20]. In summary, these observations indicate that ASK1 is involved in tumorigenesis and may function as a tumor suppressor by exerting proapoptotic activity in these aggressive tumors [21].
Similarly, it is reported that the activation of the p38 MAPK and JNK pathway is required for the induction of apoptosis in cervical carcinoma cells [22]. However, no reported study has examined the expression of ASK1 in cervical carcinoma. In our study, the ASK1 expression is significantly down-regulated in cervical carcinoma compared with the adjacent non-neoplastic cervical tissues, and the correlation between the expression of ASK1 and claudin-6 in cervical carcinoma was observed, suggesting that the pro-apoptosis effect of claudin-6 on cervical carcinoma cells may related with the ASK signal pathway.
In summary, our data revealed that ASK1 is down-regulated in cervical carcinoma tissues compared with the adjacent non-neoplastic tissues and the expression of ASK1 in cervical carcinoma is correlated with the claudin-6 in cervical carcinoma cells and tissues. Our observation suggested that ASK1 is a likely candidate for a tumor-suppressor gene in cervical carcinoma. However, our present study could not explain the role of ASK1 in the progression of cervical carcinoma and the mechanisms by which claudin-6 regulates the expression of ASK1 in cervical carcinoma cells. Further studies will address the exact molecular mechanisms by which ASK1 induces the apoptosis of cervical carcinoma cells.
In conclusion, this study reveals that ASK1 expression was obviously down-regulated in cervical carcinoma. Additionally, the expression of ASK1 is positively correlated with claudin-6 expression in cervical carcinoma tissues and cells.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Code: 81172499) and Jilin Province Science and Technology Development Projects (20140414036GH).
Disclosure of conflict of interest
None.
References
- 1.Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct. 2003;28:23–29. doi: 10.1247/csf.28.23. [DOI] [PubMed] [Google Scholar]
- 2.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 3.Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1–mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67:2766–2772. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa Y, Hirata Y, Nakagawa H, Sakamoto K, Hikiba Y, Kinoshita H, Nakata W, Takahashi R, Tateishi K, Tada M. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci U S A. 2011;108:780–785. doi: 10.1073/pnas.1011418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y, Xu X, Liu Z, Zhang T, Zhang X, Wang L, Wang M, Liu Y, Lu Y, Liu Y. Apoptosis signal-regulating kinase 1 is associated with the effect of claudin-6 in breast cancer. Diagn Pathol. 2012;7:1–6. doi: 10.1186/1746-1596-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Mariscal L, Bautista P. Tight junctions. Springer; 2006. [Google Scholar]
- 7.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y, Quan C. Tight junction protein, claudin-6, downregulates the malignant phenotype of breast carcinoma. Eur J Cancer Prev. 2010;19:186–194. doi: 10.1097/CEJ.0b013e328337210e. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z, Zhang X, Liu Z, Liu Q, Wang L, Lu Y, Liu Y, Wang M, Yang M, Jin X. The distinct expression patterns of claudin-2, -6, and-11 between human gastric neoplasms and adjacent non-neoplastic tissues. Diagn Pathol. 2013;8:133–139. doi: 10.1186/1746-1596-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner EF, Nebreda ÁR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 11.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita Ki, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan J, Chang Q, Wang X, Son Y, Zhang Z, Chen G, Luo J, Bi Y, Chen F, Shi X. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem Res Toxicol. 2010;23:568–577. doi: 10.1021/tx9003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 14.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 15.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 16.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 17.Zheng QY, Li PP, Jin FS, Yao C, Zhang GH, Zang T, Ai X. Ursolic acid induces ER stress response to activate ASK1–JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell Signal. 2013;25:206–213. doi: 10.1016/j.cellsig.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K, Hatai T, Hamazaki TS, Nishitoh H, Saitoh M, Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J Bio Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- 20.Chibon F, Mariani O, Derré J, Mairal A, Coindre JM, Guillou L, Sastre X, Pédeutour F, Aurias A. ASK1 (MAP3K5) as a potential therapeutic target in malignant fibrous histiocytomas with 12q14-q15 and 6q23 amplifications. Genes Chromosomes Cancer. 2004;40:32–37. doi: 10.1002/gcc.20012. [DOI] [PubMed] [Google Scholar]
- 21.Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 22.Kang YH, Lee SJ. The role of p38 MAPK and JNK in Arsenic trioxide-induced mitochondrial cell death in human cervical cancer cells. J Cell Physiol. 2008;217:23–33. doi: 10.1002/jcp.21470. [DOI] [PubMed] [Google Scholar]


