Abstract
We performed a study to investigate the role of ERCC1, ERCC2, ERCC5, XPA and XPC polymorphisms from perspective of the whole NER pathway in the prognosis of gastric cancer. A total of 410 gastric cancer patients were recruited between January 2010 and December 2011. Restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR) was used to analyze genotypes of ERCC1 rs11615 and rs3212986, ERCC2 rs13181 and s1799793, ERCC5 rs17655, XPA rs1800975 and XPC rs2228001. Our study found that carriers of ERCC1 rs3212986 TT genotype showed significantly favorable survival than wide-type GG genotype in multivariate analysis (OR=6.38, 95% CI=2.54-19.03), and patients with variant CC genotype of ERCC2 rs13181 exhibited better response to chemotherapy than those with AA genotype (OR=2.21, 95% CI=1.17-4.25). By Cox proportional hazards model, patients with variant TT genotype of ERCC1 rs3212986 exhibited longer PFS and OS than those who had GG genotype (for PFS, HR=0.37, 95% CI=0.17-0.75; for OS, HR=0.36, 95% CI=0.13-0.87). For ERCC2 rs13181 polymorphism, carriers with CC genotype demonstrated significantly increased hazards of progression of disease and death in multivariate model (for PFS, HR=0.48, 95% CI=0.26-0.88; for OS, HR=0.44, 95% CI=0.20-0.91). In conclusion, our finding suggests that ERCC1 rs3212986 and ERCC2 rs13181 gene polymorphism could influence the response to chemotherapy and clinical outcome of gastric cancer.
Keywords: DNA repair gene, polymorphism, gastric cancer, clinical outcome
Introduction
Gastric cancer is the fourth most common cancer and the second most common cause of cancer-related death all over the world, especially in East Asia [1]. Although remarkable improvement has been made in surgical treatment, the survival of GC still remains poor, with the overall 5-year survival rate for gastric cancer approximately 27.4% in China [2]. Moreover, patients with the same tumor stage and treatment could show different clinical outcomes. As a complex disease, the initiation and progression of gastric cancer is strongly influenced by both genetic and environmental factors [3,4]. Therefore, identification of genetic biomarkers that could predict prognosis of gastric cancer patients would greatly benefit the individualized therapy, post-operational treatment and follow-up strategies [5].
DNA repair systems play a pivotal role in maintaining the stability and integrity of the genome, which include nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR) and double-strand break repair (DSBR) [6,7]. Nucleotide excision repair (NER) is a versatile system that monitors and repairs DNA damage caused by both endogenous and exogenous factors, including therapeutic agents [8]. Polymorphisms of core NER genes could change the NER ability by influencing the expression and function of important proteins, thereby altering individual survival of GC patients. Driven by such hypothesis, polymorphisms of several NER genes have previously been studied in relation to the prognosis of gastric cancer patients [9].
In the present study, we performed a study to investigate the role of ERCC1, ERCC2, ERCC5, XPA and XPC polymorphisms from perspective of the whole NER pathway in the prognosis of gastric cancer.
Materials and methods
Study subjects
A total of 410 gastric cancer patients were recruited from the First Affiliated Hospital of Xinxiang Medical University between January 2010 and December 2011. All the GC patients were histopathologically confirmed and classified based on Lauren’s classification. Tumors were staged using the 7th edition of the TNM staging system of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) according to postoperative pathologic examination. Patients who had other malignant tumors and distant metastasis found preoperatively, and underwent preoperative radiotherapy or chemotherapy were excluded from this study.
Each patient agreed to sign an informed consent form for blood sample collection for genotyping. This study was approved by the ethics committee of the First Affiliated Hospital of Xinxiang Medical University and was performed according to the Declaration of Helsinki.
Assessment of treatment outcome
Demographic, clinical, and treatment parameters were obtained from the medical records. Investigated patients received FOLFOX chemotherapy after enrolling into our study until unacceptable toxicity or progressive disease presented.
Prior to chemotherapy, all patients provided their treatment history and underwent a series of medical examinations, including pathological examination or CT scan, complete blood cell counts, platelet counts, and biochemical analysis for liver and renal functions. All tests were repeated every two cycles of treatment. Tumor response was assessed according to WHO Response Evaluation Criteria in Solid Tumors guide and categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients who showed CR and/or PR were considered as good response to chemotherapy, and those with SD and/or PD were considered as poor response to chemotherapy. Progression free survival (PFS) and overall survival (OS) were used as the end of this study. PFS was the time interval between the dates of first treatment and either disease progression or death. OS was calculated from the date of assignment to either the date of death or last clinical follow-up, whichever occurred first. Patients without an event or death at the time of the analysis were censored at the date of the last follow-up by telephone or attending clinics. The follow-up of the gastric cancer patients were completed until December 2014.
DNA extraction and genotyping
Prior to the start of chemotherapy, 2 ml of peripheral blood was taken from each patient for DNA extraction using Qiagen blood mini kit (Qiagen, Germany), according to the manufacturer’s instructions. The extracted DNA samples were stored at -80°C before genotyping. The PCR primers of ERCC1 rs11615 and rs3212986, ERCC2 rs13181 and s1799793, ERCC5 rs17655, XPA rs10817938 and rs2808668, XPC rs1870134 and rs2228000 were designed by Sequenom Assay Design 3.1 software (Sequenom Inc., San Diego, CA). Restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR) was used to analyze genotypes of ERCC1 rs11615 and rs3212986, ERCC2 rs13181 and s1799793, ERCC5 rs17655, XPA rs1800975 and XPC rs2228001. Briefly PCR was carried out in a final volume of 25 μL containing 50 ng genomic DNA template, 1× PCR buffer with 2 mM MgCl2, 0.5 μM of each primer, 50 μM dNTPs and 0.5 U DNA polymerase. For PCR amplification, the standard program was used as follows: one initial denaturation step at 94°C for 7 min, followed by 35 denaturation cycles of 1 min at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C, followed by a final elongation cycle at 72°C for 10 min. Approximately 5% of the samples were repeatedly genotyped, and the results were 100% concordant.
Statistical analysis
Frequencies were used to describe the distribution of categorical variables and median and interquartile ranges were used for continuous variables. Logistic regression was used to assess the influence of genetic polymorphisms in the response to chemotherapy, and the results were determined by odds ratios (ORs) and their 95% confidence interval (CIs). Cox proportional hazards model was carried out to calculate hazard ratio (HR) and the 95% confidence interval (CI) of each genotype to estimate its effect on survival with adjustment for confounding factors by different genotype groups. Significant variables in multivariate models were further analyzed by multivariate Cox proportional hazards regression models to identify the independent prognostic value. Statistical analysis was performed by using SPSS (16.0) statistical software (SPSS, Chicago, IL, USA). Two-tailed P values <0.05 were considered statistically significant.
Results
A total of 410 gastric cancer patients were enrolled in the present study, including 134 females and 276 males (Table 1). The age of gastric cancer ranges from 29 to 78 years, and the mean age is 63.7±11.4 years. Of the included 410 patients, 143 (34.88%) patients had a habit of tobacco smoking, 158 (38.54%) had a habit of alcohol drinking, 253 (61.71%), 253 (61.71%) showed diffuse type of tumor, 220 (53.66%) showed III-IV stage, 226 (55.12%) had tumor size ≥5 cm, 242 (59.02%) showed metastasis, and 269 (65.61%) showed response to chemotherapy.
Table 1.
Characteristics of investigated gastric cancer patients
| Variables | Number | % |
|---|---|---|
| Age, years | ||
| <60 | 158 | 38.54 |
| ≥60 | 252 | 61.46 |
| Gender | ||
| Female | 134 | 32.68 |
| Male | 276 | 67.32 |
| Tobacco smoking | ||
| Never | 267 | 65.12 |
| Current or former | 143 | 34.88 |
| Alcohol drinking | ||
| Never | 252 | 61.46 |
| Current or former | 158 | 38.54 |
| Lauren’s type | ||
| Intestinal | 157 | 38.29 |
| Diffuse | 253 | 61.71 |
| TNM stage | ||
| I-II | 190 | 46.34 |
| III-IV | 220 | 53.66 |
| Tumor size (cm) | ||
| <5 cm | 184 | 44.88 |
| ≥5 cm | 226 | 55.12 |
| Metastasis | ||
| No | 168 | 40.98 |
| Yes | 242 | 59.02 |
| Response to chemotherapy | ||
| No | 141 | 34.39 |
| Yes | 269 | 65.61 |
The results of the relation between gene polymorphisms of NER pathway and response to chemotherapy in gastric cancer were summarized in Table 2. Of the seven investigated SNPs in the study, carriers of ERCC1 rs3212986 TT genotype showed significantly favorable survival than wide-type GG genotype in multivariate analysis (OR=6.38, 95% CI=2.54-19.03), and patients with variant CC genotype of ERCC2 rs13181 exhibited better response to chemotherapy than those with AA genotype (OR=2.21, 95% CI=1.17-4.25). However, polymorphisms in ERCC1 rs11615, ERCC2 rs1799793, ERCC5 rs17655, XPA rs1800975 and XPC rs2228001 had no statistical association with response to chemotherapy in gastric cancer patients.
Table 2.
Association between gene polymorphisms in NER pathway and response to chemotherapy
| Gene | Total | % | CR+PR | % | SD+PD | % | OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| ERCC1 rs11615 | ||||||||
| TT | 162 | 39.5 | 101 | 37.5 | 61 | 43.3 | 1.0 (Ref.) | - |
| TC | 187 | 45.6 | 124 | 46.1 | 63 | 44.7 | 1.19 (0.75-1.89) | 0.44 |
| CC | 61 | 14.9 | 44 | 16.4 | 17 | 12.1 | 1.56 (0.79-3.18) | 0.17 |
| ERCC1 rs3212986 | ||||||||
| GG | 187 | 45.6 | 104 | 38.7 | 83 | 58.9 | 1.0 (Ref.) | - |
| GT | 169 | 41.2 | 117 | 43.5 | 52 | 36.9 | 1.80 (1.14-2.85) | 0.008 |
| TT | 54 | 13.2 | 48 | 17.8 | 6 | 4.3 | 6.38 (2.54-19.03) | <0.05 |
| ERCC2 rs13181 | ||||||||
| AA | 158 | 38.5 | 89 | 33.1 | 69 | 48.9 | 1.0 (Ref.) | - |
| AC | 175 | 42.7 | 123 | 45.7 | 52 | 36.9 | 1.83 (1.14-2.96) | 0.01 |
| CC | 77 | 18.8 | 57 | 21.2 | 20 | 14.2 | 2.21 (1.17-4.25) | 0.01 |
| ERCC2 rs1799793 | ||||||||
| GG | 174 | 42.4 | 108 | 40.1 | 66 | 46.8 | 1.0 (Ref.) | - |
| GA | 188 | 45.9 | 123 | 45.7 | 65 | 46.1 | 1.16 (0.74-1.82) | 0.51 |
| AA | 48 | 11.7 | 37 | 13.8 | 11 | 7.8 | 2.06 (0.94-4.77) | 0.06 |
| ERCC5 rs17655 | ||||||||
| CC | 183 | 44.6 | 104 | 38.7 | 79 | 56.0 | 1.0 (Ref.) | - |
| CG | 174 | 42.4 | 116 | 43.1 | 58 | 41.1 | 1.52 (0.97-2.39) | 0.06 |
| GG | 73 | 17.8 | 49 | 18.2 | 24 | 17.0 | 1.55 (0.85-2.88) | 0.13 |
| XPA rs1800975 | ||||||||
| AA | 194 | 47.3 | 120 | 44.6 | 74 | 52.5 | 1.0 (Ref.) | - |
| AG | 165 | 40.2 | 113 | 42.0 | 52 | 36.9 | 1.34 (0.85-2.13) | 0.19 |
| GG | 51 | 12.4 | 36 | 13.4 | 15 | 10.6 | 1.48 (0.73-3.11) | 0.25 |
| XPC rs2228001 | ||||||||
| AA | 215 | 52.4 | 131 | 48.7 | 84 | 59.6 | 1.0 (Ref.) | - |
| AC | 128 | 31.2 | 90 | 33.5 | 38 | 27.0 | 1.52 (0.93-2.50) | 0.08 |
| CC | 67 | 16.3 | 48 | 17.8 | 19 | 13.5 | 1.62 (0.86-3.12) | 0.11 |
At the end of follow-up (December 2014), 171 patients showed progression of disease and 97 patients died. The five-years overall survival was 76.34%. After adjustment, patients with variant TT genotype of ERCC1 rs3212986 exhibited longer PFS and OS than those who had GG genotype (for PFS, HR=0.37, 95% CI=0.17-0.75; for OS, HR=0.36, 95% CI=0.13-0.87) (Table 3). For ERCC2 rs13181 polymorphism, carriers with CC genotype demonstrated significantly increased hazards of progression of disease and death in multivariate model (for PFS, HR=0.48, 95% CI=0.26-0.88; for OS, HR=0.44, 95% CI=0.20-0.91).
Table 3.
Association between gene polymorphisms in NER pathway and overall survival of gastric cancer
| Gene | Total | % | Progression N=171 | % | Adjusted HR (95% CI) | P value | Death N=97 | % | Overall survival time (month) | Log-rank test | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERCC1 rs11615 | ||||||||||||
| TT | 162 | 39.5 | 73 | 42.7 | 1.0 (Ref.) | 43 | 44.3 | 31.4 | 1.0 (Ref.) | - | ||
| TC | 187 | 45.6 | 75 | 43.9 | 0.82 (0.52-1.28) | 0.35 | 41 | 42.3 | 33.5 | 0.78 (0.46-1.31) | 0.31 | |
| CC | 61 | 14.9 | 23 | 13.5 | 0.74 (0.38-1.40) | 0.32 | 13 | 13.4 | 34.1 | 0.27 | 0.75 (0.34-1.58) | 0.42 |
| ERCC1 rs3212986 | ||||||||||||
| GG | 187 | 45.6 | 91 | 53.2 | 1.0 (Ref.) | 55 | 56.7 | 27.8 | 1.0 (Ref.) | - | ||
| GT | 169 | 41.2 | 66 | 38.6 | 0.68 (0.43-1.05) | 0.07 | 35 | 36.1 | 34.7 | 0.63 (0.37-1.05) | 0.06 | |
| TT | 54 | 13.2 | 14 | 8.2 | 0.37 (0.17-0.75) | 0.00 | 7 | 7.2 | 37.5 | <0.05 | 0.36 (0.13-0.87) | 0.01 |
| ERCC2 rs13181 | ||||||||||||
| AA | 158 | 38.5 | 77 | 45.0 | 1.0 (Ref.) | 47 | 48.5 | 28.5 | 1.0 (Ref.) | - | ||
| AC | 175 | 42.7 | 70 | 40.9 | 0.70 (0.44-1.11) | 0.11 | 38 | 39.2 | 34.6 | 0.66 (0.39-1.11) | 0.09 | |
| CC | 77 | 18.8 | 24 | 14.0 | 0.48 (0.26-0.88) | 0.01 | 12 | 12.4 | 37.2 | <0.05 | 0.44 (0.20-0.91) | 0.02 |
| ERCC2 rs1799793 | ||||||||||||
| GG | 174 | 42.4 | 78 | 45.6 | 1.0 (Ref.) | 45 | 46.4 | 32.7 | 1.0 (Ref.) | - | ||
| GA | 188 | 45.9 | 75 | 43.9 | 0.82 (0.53-1.27) | 0.34 | 42 | 43.3 | 33.6 | 0.82 (0.49-1.38) | 0.43 | |
| AA | 48 | 11.7 | 18 | 10.5 | 0.74 (0.36-1.49) | 0.36 | 10 | 10.3 | 34.2 | 0.14 | 0.75 (0.31-1.71) | 0.47 |
| ERCC5 rs17655 | ||||||||||||
| CC | 183 | 44.6 | 79 | 46.2 | 1.0 (Ref.) | 47 | 48.5 | 32.5 | 1.0 (Ref.) | - | ||
| CG | 174 | 42.4 | 70 | 40.9 | 0.74 (0.36-1.49) | 0.36 | 39 | 40.2 | 32.9 | 0.84 (0.50-1.40) | 0.47 | |
| GG | 73 | 17.8 | 22 | 12.9 | 0.89 (0.57-1.38) | 0.57 | 11 | 11.3 | 35.2 | 0.28 | 0.51 (0.23-1.09) | 0.07 |
| XPA rs1800975 | ||||||||||||
| AA | 194 | 47.3 | 86 | 50.3 | 1.0 (Ref.) | 49 | 50.5 | 33.1 | 1.0 (Ref.) | - | ||
| AG | 165 | 40.2 | 66 | 38.6 | 0.84 (0.54-1.30) | 0.41 | 37 | 38.1 | 33.6 | 0.86 (0.51-1.43) | 0.53 | |
| GG | 51 | 12.4 | 19 | 11.1 | 0.75 (0.37-1.47) | 0.36 | 11 | 11.3 | 34.5 | 0.25 | 0.81 (0.35-1.78) | 0.59 |
| XPC rs2228001 | ||||||||||||
| AA | 215 | 52.4 | 94 | 55.0 | 1.0 (Ref.) | 55 | 56.7 | 32.8 | 1.0 (Ref.) | - | ||
| AC | 128 | 31.2 | 52 | 30.4 | 0.88 (0.55-1.41) | 0.57 | 29 | 29.9 | 33.7 | 0.85 (0.49-1.47) | 0.54 | |
| CC | 67 | 16.3 | 25 | 14.6 | 0.77 (0.42-1.39) | 0.35 | 13 | 13.4 | 34.5 | 0.19 | 0.70 (0.33-1.43) | 0.3 |
By Log-rank test and Kaplan-Meier method, patients with variant TT genotype of ERCC1 rs3212986 and CC genotype of ERCC2 rs13181 had significantly longer overall survival time when compared with wide-type genotype (P<0.05) (Figures 1, 2). However, variants of ERCC1 rs11615, ERCC2 rs1799793, ERCC5 rs17655, XPA rs1800975 and XPC rs2228001 could not predict the PFS and OS of gastric cancer (P>0.05).
Figure 1.
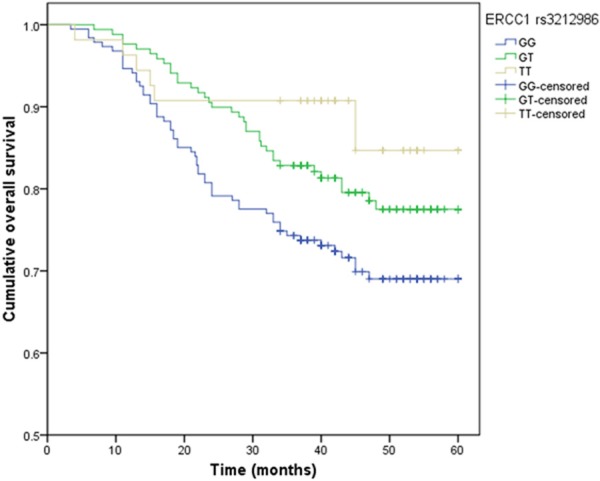
Kaplan-Meier survival curves by the genotypes of ERCC1 rs3212986 polymorphism in gastric cancer patients.
Figure 2.
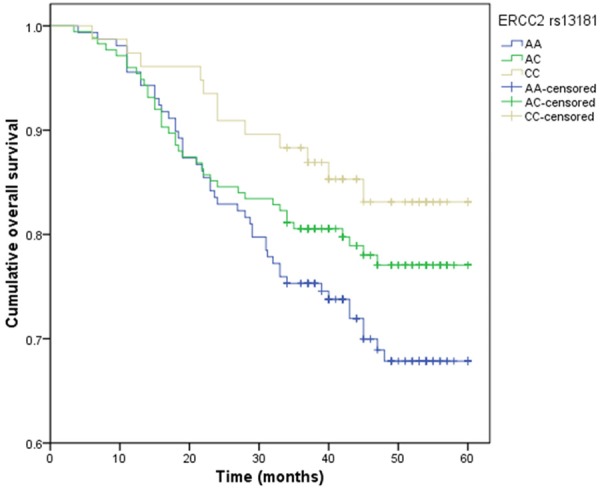
Kaplan-Meier survival curves by the genotypes of ERCC2 rs13181 polymorphism in gastric cancer patients.
Discussion
In the present study, we investigated the influence of SNPs in DNA repair mechanisms on treatment response and survival in gastric cancer patients treated with FOLFOX chemotherapy. We found that TT genotype of ERCC1 rs3212986 and CC genotype of ERCC2 rs13181 were associated with better response to chemotherapy and longer PFS and OS of gastric cancer patients when compared with wide-type genotype.
NER process are involved in many steps of damage recognition, damage demarcation and unwinding, damage incision, and new strand ligation [10,11]. Multiple genes are participated into this pathway and in charge of different functions [12]. ERCC1 and ERCC5 participate in the DNA damage incision, ERCC2 are involved in the damage unwinding process, and XPA and XPC are involved in the DNA damage recognition. Polymorphism of these genes in the NER process could change alternative splicing patterns in altering the regulation of the gene’s transcription and thereby modulating function of specific factors of NER pathway. The alteration in the NER capacity may change frequencies of DNA mutation due to unrepaired damaged DNA. Therefore, it is biologically plausible that polymorphisms in NER genes may influence clinical outcomes of gastric cancer patients [13].
Many previous studies have reported the role of DNA repair gene polymorphisms in the clinical outcome of several kinds of cancers, such as non-small cell lung cancer (NSCLC), gastric cancer, colorectal cancer and osteosarcoma [14-18]. Sullivan et al. conducted a cohort study with 161 NSCLC patients to investigate the association of 17 SNPs of eight genes in NER pathways with clinical outcome of NSCLC, and they found that ERCC1, ERCC2 and ERCC3 gene polymorphisms were associated with response to chemotherapy and survival of NSCLC [14]. Another study in Chinese population found that patients carrying the ERCC1 rs11615 AA genotype and the XPF rs2276465 GG genotype were associated with longer survival time of gastric cancer [15]. One study conducted a study with 66 osteosarcoma patients, and found that carriers of ERCC2 rs1799793 polymorphism had longer even-free survival of osteosarcoma [18]. The above-mentioned studies showed that polymorphisms in NER genes may influence clinical outcomes of several kinds of cancers.
Since a complicated and multi-step process, NER pathway factors may play an important role in functioning jointly to alter clinical outcome of gastric cancer. Several previous studies have shown the association between DNA repaired genes and prognosis of gastric cancer, but the results are inconsistent [15,16,19-21]. Li et al. conducted a study to investigate the ERCC1 and XPF gene polymorphisms in the prognosis of gastric cancer [15]. They found that GA and AA genotypes of ERCC1 11615 were associated with higher increased risk of death from gastric cancer, and no association between ERCC1 rs3212986 polymorphism and prognosis of gastric cancer [19]. Another study in Chinese population found that TT genotype of ERCC1 rs11615 was associated with a better response to chemotherapy and longer overall survival of gastric cancer, and no associated between ERCC1 rs3212986 polymorphism and cancer prognosis [20]. However, Li et al. reported an opposite results, and they found that ERCC1 rs11615 gene polymorphisms was associated with poorer overall survival of gastric cancer [21]. In our study, we found that TT genotype of ERCC1 rs3212986 and CC genotype of ERCC2 rs13181 were associated with better response to chemotherapy and longer PFS and OS of gastric cancer patients. The results of above mentioned studies are inconsistent, the main reasons might be the differences in studies populations, study design and sample size as well as by chance. Although some of the above-mentioned mechanisms might explained the observed significant associations of certain NER polymorphisms and GC survival, further molecular researches are still needed to reveal the underlying mechanism.
In conclusion, our finding suggests that ERCC1 rs3212986 and ERCC2 rs13181 gene polymorphism could influence the response to chemotherapy and clinical outcome of gastric cancer. In the future, these SNPs could contribute to identification of patients, more likely to achieve favorable response to chemotherapy. Translation of these pharmacogenetic predictors into clinical practice could lead to improved gastric cancer treatment planning and treatment outcome.
Disclosure of conflict of interest
None.
References
- 1.Stadtlander CT, Waderbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195–08. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
- 2.Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, Zhang L, Tang J, Chen J, Wei K, Huang S, Wang J, Yu L, Zhao D, Song G, Chen J, Shen Y, Yang X, Gu X, Jin F, Li Q, Li Y, Ge H, Zhu F, Dong J, Guo G, Wu M, Du L, Sun X, He Y, Coleman MP, Baade P, Chen W, Yu XQ. Cancer survival in China, 2003-2005: A population-based study. Int J Cancer. 2014;2014:29227. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 3.Zabaleta J. Multifactorial etiology of gastric cancer. Methods Mol Biol. 2012;863:411–35. doi: 10.1007/978-1-61779-612-8_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Takano Y, Zheng HC. The pathobiological features of gastrointestinal cancers (Review) Oncol Lett. 2012;3:961–9. doi: 10.3892/ol.2012.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–56. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 6.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–30. [PubMed] [Google Scholar]
- 7.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 8.de Laat WL, Jaspers NG, Hoeijmakers JH. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–85. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, He C, Xing C, Yuan Y. Nucleotide excision repair related gene polymorphisms and genetic susceptibility, chemotherapeutic sensitivity and prognosis of gastric cancer. Mutat Res Fundam Mol Mech Mutagen. 2014;765:11–21. doi: 10.1016/j.mrfmmm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kamileri I, Karakasilioti I, Garinis GA. Nucleotide excision repair: new tricks with old bricks. Trends Genet. 2012;28:566–73. doi: 10.1016/j.tig.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Ng JM, Vermeulen W, van der Horst GT, Bergink S, Sugasawa K, Vrieling H, Hoeijmakers JH. A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 2003;17:1630–45. doi: 10.1101/gad.260003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer. 2001;1:22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- 13.Hamajima N, Naito M, Kondo T, Goto Y. Genetic factors involved in the development of Helicobacter pylori-related gastric cancer. Cancer Sci. 2006;97:1129–38. doi: 10.1111/j.1349-7006.2006.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan I, Salazar J, Majem M, Pallarés C, Del Río E, Páez D, Baiget M, Barnadas A. Pharmacogenetics of the DNA repair pathways in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Lett. 2014;353:160–6. doi: 10.1016/j.canlet.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZH, Wang L, Luo LP. Association of DNA repair gene polymorphisms with response to chemotherapy and prognosis of gastric cancer. Genet Mol Res. 2014;13:7484–91. doi: 10.4238/2014.September.12.15. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Liu ZY, Li CB, Gao S, Ding LH, Wu XL, Wang ZY. Genetic polymorphisms of DNA repair pathways influence the response to chemotherapy and overall survival of gastric cancer. Tumour Biol. 2015;36:3017–23. doi: 10.1007/s13277-014-2936-3. [DOI] [PubMed] [Google Scholar]
- 17.Sun K, Gong A, Liang P. Predictive impact of genetic polymorphisms in DNA repair genes on susceptibility and therapeutic outcomes to colorectal cancer patients. Tumour Biol. 2015;36:1549–59. doi: 10.1007/s13277-014-2721-3. [DOI] [PubMed] [Google Scholar]
- 18.Goričar K, Kovač V, Jazbec J, Zakotnik B, Lamovec J, Dolžan V. Genetic variability of DNA repair mechanisms and glutathione-S-transferase genes influences treatment outcome in osteosarcoma. Cancer Epidemiol. 2015;39:182–8. doi: 10.1016/j.canep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Liu Z, Liu H, Wang LE, Tan D, Ajani JA, Wei QY. ERCC1 and ERCC2 variants predict survival in gastric cancer patients. PLoS One. 2013;8:e71994. doi: 10.1371/journal.pone.0071994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu ZM, Luo TH, Nie MM, Fang GE, Ma LY, Xue XC, Wei G, Ke CW, Bi JW. Influence of ERCC1 and ERCC4 polymorphisms on response to prognosis in gastric cancer treated with FOLFOX-based chemotherapy. Tumour Biol. 2014;35:2941–8. doi: 10.1007/s13277-013-1378-7. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zuo X, Lv X, Kong F, Xu W, Yang S. Association of DNA repair gene polymorphisms with response to chemotherapy and prognosis of gastric cancer in a Chinese population. Tumour Biol. 2014;35:7569–74. doi: 10.1007/s13277-014-1959-0. [DOI] [PubMed] [Google Scholar]


