Abstract
Objective: To explore the critical value and possible influencing factors of fractional exhaled nitric oxide (FeNO) in suspected asthma patients. Methods: 923 suspected asthmatics consecutively referred to our hospital during December 2012 to July 2014 were selected. All cases were carried out FeNO measurement at first; next, spirometry, bronchoprovocation tests or bronchodilation tests were used to confirm or exclude asthma. Receiver operating characteristic curve (ROC) was used to determine the best cut-off value of FeNO for asthma diagnosis. Results: In bronchoprovocation test, 125 cases were diagnosed as asthma, other 283 were non-asthmatics. FeNO levels of asthmatics were significantly higher than non-asthmatics (median, 64.8 ppb vs. 27.9 ppb, P < 0.01). In this group of patients, 64 ppb was the best cut-off value of FeNO to identify asthma with sensitivity of 52.0% and specificity of 94.35%. In bronchodilation test, 185 patients were diagnosed as asthma, other 330 were non-asthmatics. FeNO levels of asthmatics were significantly higher than non-asthmatics (median, 60.6 ppb vs. 29.05 ppb, P < 0.01). In bronchodilation test patients, 41 ppb was the best cut-off value of FeNO to identify asthma with sensitivity of 72.43% and specificity of 74.85%. Influencing factors analysis showed that sex was an independent factor affecting patients’ FeNO level. Conclusion: FeNO was an effective auxiliary diagnosis method for bronchial asthma. 64 ppb and 41 ppb was the best cut-off value of FeNO to identify asthma in bronchoprovocation test or bronchodilation test, respectively. Sex was an independent factor affecting patients’ FeNO level.
Keywords: Fractional exhaled nitric oxide, asthma diagnosis, influencing factors
Introduction
Asthma is a common chronic airway inflammatory disease; it is characterized by chronic inflammation, bronchial hyper-responsiveness (BHR) and usually reversible airway obstruction. According to the report’ Global Burden of Asthma’ from Global Initiative for Asthma (GINA), over 300 million individuals worldwide was affected by asthma, making it one of the most prevalent chronic diseases [1]. Although asthma is rarely fatal, the economic burden resulted from this disease is extensive, including prescription drug costs, health care costs, and productivity losses. Typical symptoms of asthma including cough, chest tightness, dyspnoea and wheezing, which are often triggered by factors such as exercise, allergen or irritant exposure, change of weather, obesity and diet, or viral respiratory infections [2].
The diagnosis of asthma is usually based on patient’s clinical history, symptoms, signs and lung function tests. Bronchoalveolar lavage and endobronchial biopsy are two reliable methods to evaluate inflammation status of airway; However, clinical application of those methods are limited due to their traumatic and costly nature [3,4]. Lung function measurements such as bronchodilation tests and peak flow measurements are used for quantification of the airway obstruction and its reversibility to bronchodilator. For patients who have asthma symptoms but normal lung functions, determination of BHR to bronchoconstrictor agent (such as methacholine and histamine) may help to ascertain the diagnosis of asthma; However, bronchoprovocation tests are time-consuming and carrying a small risk of severe bronchospasm [5]; Besides, they need specific equipment and expertise that are usually only available in a specialized clinical setting.
In the past decades, several biomarkers including exhaled breath condensate, vascular endothelial growth factor, induced sputum, eosinophil cationic protein have been used for asthma diagnosis and control [6]. However, all these tests were lack of sufficient sensitivity and specificity. Fractional exhaled nitric oxide (FeNO) is a small molecule produced by human secrete cells, which is internationally recognized as a marker of airway inflammation [7]. Numerous studies have demonstrated that FeNO was increased in asthmatic patients [8-11]. Meanwhile, FeNO was also associated with airway BHR [12]. Therefore, FeNO was generally considered as a valuable biomarker in asthma diagnosis.
On the other hand, the cut-off value of FeNO for asthma diagnosis is inconsistent due to different study design. In this study, we focused on suspected asthma patients to assess the diagnostic ability and possible influencing factors of FeNO.
Patients and methods
Subjects and study design
This study was approved by ethnic committee of Daping Hospital, Third Military Medical University in 2009, the Ethics committee approved relating screening, treatment and data collection of these patients, all subjects signed written informed consent form. All works were undertaken following the provisions of the Declaration of Helsinki.
During December 2012 to July 2014, all suspected asthma patients consecutively referred to the outpatient clinic of our hospital were included in this study. Patient was considered as suspected asthma based up on their symptoms (recurrent wheezing, dyspnoea, chest tightness and/or cough, duration over 6 months), physical examination results and history of atopy. Patients with serious cardiovascular system diseases or other diseases (such as emphysema, pneumothorax, pulmonary fibrosis and lung cancer etc) that can damage lung function were excluded from this study. Other exclusion criteria including: (1) Vigorous exercise in 1 hour before FeNO measurement; (2) Smoking or drinking or used bronchodilators in 4 hours before FeNO measurement; (3) Had clear respiratory infection in 7 days before FeNO measurement; (4) Used systemic steroids in 2 days before FeNO measurement; (5) Used inhaled corticosteroids or had allergic rhinitis attack in 4 weeks before FeNO measurement; (6) Chest imaging showed there were pulmonary infections or tumors or other abnormalities. Finally, 923 patients were included in this study, in which 426 were male, 497 were female. All cases were carried out FeNO measurement at first; next, spirometry was performed to check patients’ baseline lung functions; after then, bronchoprovocation test or bronchodilation test was used to confirm or exclude asthma. In this study, the diagnosis of asthma was made based up on the following: bronchoprovocation test result was positive or bronchodilation test result was positive. Patients or their legal guardians gave written informed consent for this study.
FeNO measurement
FeNO was measured by a Nano Coulomb nitric oxide analyzer (Shangwo Biotechnology Co., Ltd., Jiangsu, China) according to the American Thoracic Society (ATS) guidelines and expressed as parts per billion (ppb). Patients were tested at resting state. After inhalation of ambient air through a nitric oxide scrubber to total lung capacity, testers then exhaled against expiratory resistance to exclude nasal air. The exhaled platform time duration was more than 2 seconds with a 2-min analysis period. Repeated exhalations (two values that agree within 5% or 3 that agree within 10%) were performed at a constant flow rate of 50 mL/s.
Determination of bronchoprovocation test
Baseline spirometry was performed by JAEGER spirometer (Erich Jaeger GmbH, Friedberg, Germany) according to ATS guidelines. Bronchoprovocation test was made for patients whose baseline forced expiratory volume in the first second (FEV1) was more than 80% of predicted. Methacholine (Sigma, MO, USA) was administrated by quantitative aerosol inhalation method to induce BHR. Methacholine was diluted into 25 mg/ml and 50 mg/ml and placed in a nebulizer. 0.9% saline was inhaled at first, followed by 25 mg/ml, 25 mg/ml, 25 mg/ml, 50 mg/ml, and 50 mg/ml methacholine, respectively. Lung function was measured two minutes after each inhalation. Nebulization was stopped when FEV1 decreased by 20% of baseline value or methacholine reached maximum dose. After then, salbutamol was inhaled to repair patients’ lung functions to baseline level. Provocation test result was judged as positive when FEV1 drop ≥ 20% of baseline value.
Determination of bronchodilation test
Baseline spirometry was performed by JAEGER spirometer (Erich Jaeger GmbH, Friedberg, Germany) according to ATS guidelines. Bronchodilation test was made for patients whose baseline FEV1 was less than 70% of predicted. Patients were asked to inhale 400 μg albuterol sulfate inhalation aerosol (GSK, Middlesex, UK). After 15-20 minutes rest, spirometry was repeated. Bronchodilation test result was considered as positive if patient’s FEV1 after albuterol sulfate inhalation was 15% greater than baseline value and the absolute value of FEV1 was increased more than 200 ml.
Statistical analysis
All data were analyzed by SPSS 19.0 software (SPSS Inc., IL, USA) and presented as N (%) or median (range). Categorical variables were analyzed by chi square test. Non-normal distribution variables were analyzed by Mann-Whitney U method. ROC curve analysis was performed by Medcalc 9.2 (MedCalc Software, Mariakerke, Belgium).
Bronchoprovocation test
408 suspected asthma patients were suitable for bronchoprovocation test, in which 125 were diagnosed as asthma, other 283 were non-asthmatics. When compared with asthma patients, the sex ratio and smoking history of non-asthmatics patients showed no significant difference (P > 0.05). However, age of non-asthmatics patients was significantly higher than that of asthma patients (median, 46 yeas vs. 41 years, P < 0.05); FeNO level of non-asthmatics patients was significantly lower than that of asthma patients (median, 27.9 ppb vs. 64.8 ppb, P < 0.01) (Table 1).
Table 1.
Basic information for bronchoprovocation test patients
| Parameters | Bronchoprovocation test | |
|---|---|---|
|
|
||
| Asthma (N=125) | Non-asthma (N=283) | |
| Sex | ||
| Male | 53 (42.4%) | 122 (43.1%) |
| Female | 72 (57.6%) | 161 (56.9%) |
| Smoking history | ||
| Yes | 31 (24.8%) | 71 (25.1%) |
| No | 94 (75.2%) | 212 (74.9%) |
| Age, years | 41 (17-75) | 46 (13-82)* |
| FeNO, ppb | 64.8 (8.9-315.1) | 27.9 (9.0-169.2)** |
Note: Asthma vs. Non-asthma;
P < 0.05;
P < 0.01.
Categorical variables were expressed as an N (%); continuous variables were expressed as median (range).
Besides, We also used binary logistic regression model to evaluate the influence of age and FeNO in diagnosing asthma in bronchoprovocation test (bronchoprovocation test result was taken as dependent variable, age and FeNO were taken as covariates, results were not shown). Results showed that FeNO level was the only independent influencing factor for asthma diagnosis.
ROC curve was used to determine the performance of FeNO predicting asthma in bronchoprovocation test suitable patients. Results showed that the best cut-off value, area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FeNO predicting asthma was 64 ppb, 0.758, 52.0%, 94.35%,80.24% and 72.75%, respectively (Figure 1).
Figure 1.
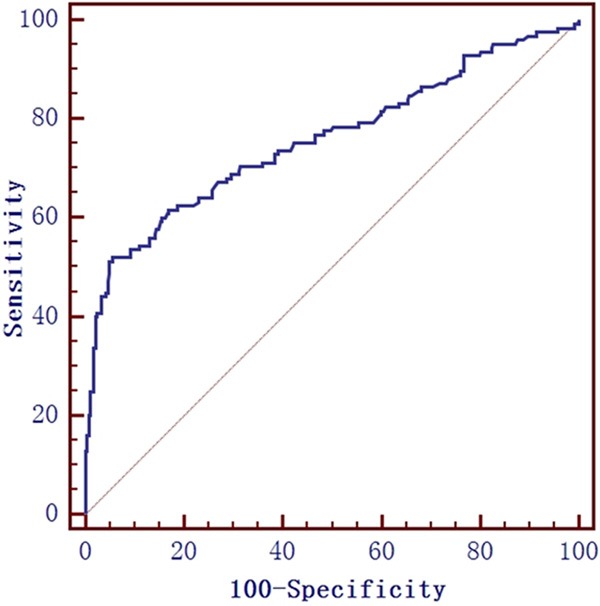
The ROC curve of FeNO measurement for predicting asthma in bronchoprovocation test patients. The best cut-off value , area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FeNO predicting asthma was 64 ppb, 0.758, 52.0%, 94.35%, 80.24% and 72.75%, respectively.
Bronchodilation test
515 patients were suitable for bronchodilation test, in which 185 patients were diagnosed as asthma, other 330 were non-asthmatics. When compared with asthma patients, the sex ratio, smoking history and age of non-asthmatics patients showed no significant difference (P > 0.05). FeNO level of non-asthmatics patients was significantly lower than asthma patients (median, 29.05 ppb vs. 60.6 ppb, P < 0.01) (Table 2). ROC curve analysis demonstrated that, in bronchodilation test patients, the best cut-off value, AUC, sensitivity, specificity, PPV and NPV of FeNO predicting asthma was 41 ppb, 0.78, 72.43%, 74.85%, 61.75% and 82.89%, respectively (Figure 2).
Table 2.
Basic information for bronchodilation test patients
| Parameters | Bronchodilation test | |
|---|---|---|
|
| ||
| Asthma (N=185) | Non-asthma (N=330) | |
| Sex | ||
| Male | 90 (48.6%) | 161 (48.8%) |
| Female | 95 (51.4%) | 169 (51.2%) |
| Smoking history | ||
| Yes | 54 (41.2%) | 105 (31.8%) |
| No | 131 (58.8%) | 225 (68.2%) |
| Age, years | 45 (15-89) | 48 (9-85) |
| FeNO, ppb | 60.6 (10.7-735.5) | 29.05 (6.0-380.5)** |
Note: Asthma vs. Non-asthma; *P < 0.05;
P < 0.01.
Categorical variables were expressed as an N (%); continuous variables were expressed as median (range).
Figure 2.
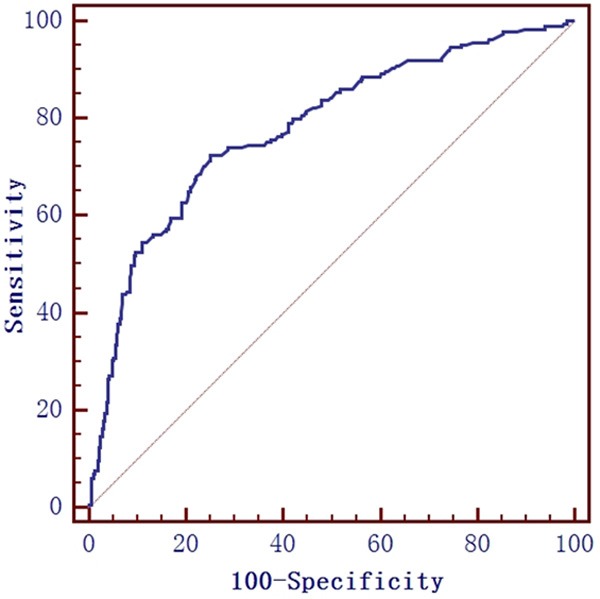
The ROC curve of FeNO measurement for predicting asthma in bronchodilation test patients. The best cut-off value, AUC, sensitivity, specificity, PPV and NPV of FeNO predicting asthma was 41 ppb, 0.78, 72.43%, 74.85%, 61.75% and 82.89%, respectively.
Influencing factors of FeNO
In this study, we only explored the influences of sex, age and smoking history on FeNO measurement. Results demonstrated that male patients had greater FeNO level than female (median, 37.85 ppb vs.31.5 ppb, P < 0.01). While, age (< 60 years vs. ≥ 60 years) and smoking history (positive vs. negative) didn’t show significant inter-group difference (P > 0.05) (Table 3).
Table 3.
Influencing factors of FeNO in suspected asthma patients
| Influencing factors | FeNO (ppb) | P value |
|---|---|---|
| Sex | ||
| Male | 37.85 (6.0-380.5) | 0 |
| Female | 31.5 (8.9-735.5) | |
| Age, years | ||
| < 60 | 35.1 (6.0-735.5) | 0.476 |
| ≥60 | 32.85 (8.9-254.7) | |
| Smoking history | ||
| Positive | 34.2 (10.0-276.8) | 0.976 |
| Negative | 34.9 (6.0-735.5) | |
In light of the aforementioned results, smoking history had no influence on FeNO level in suspected asthma patients; however, due to the fact that only little female patients had smoking history; therefore, we further explored the influences of smoking on FeNO from the perspective of sex.
In male, positive smoking history patients had significant lower FeNO level than negative (median, 34.2 ppb vs. 43.6 ppb, P = 0.001); However, in female, the FeNO level was almost the same between positive and negative smoking history patients (median, 34.2 ppb vs. 31.5 ppb, P = 0.558) (See Table 4).
Table 4.
The influence of smoking history on FeNO from the perspective of sex
| Sex | Smoking history | N (%) | FeNO (ppb) | P value |
|---|---|---|---|---|
| Male | Positive | 238 (55.9%) | 34.2 (10.6-276.8) | 0.001 |
| Negative | 188 (44.1%) | 43.6 (6.0-380.5) | ||
| Female | Positive | 23 (4.6%) | 34.2 (10.0-151.3) | 0.558 |
| Negative | 474 (95.4%) | 31.5 (8.9-735.5) |
Discussion
Asthma is one of the most prevalent chronic diseases worldwide. According to the report’ Global Burden of Asthma’ from Global Initiative for Asthma (GINA), it is estimated that around 300 million people in the world currently have asthma, and there may be an additional 100 million asthmatics by 2025. When compared with countries that have a high incidence of asthma, such as Scotland (18.4%) and Jersey (17.6%), the prevalence of clinical asthma in China is relatively low (2.1%); however, the burden of asthma in our country is very extensive. Firstly, there are almost 30 million asthmatics in China and this number will increase markedly during the next decade due to urbanization, environmental pollution and lifestyle changing; Secondly, China has the highest asthma case fatality rates in the world, and this rate in rural areas is about double of urban areas; Thirdly, lots of asthmatics are underdiagnosis (due to lack of objective diagnosis tests) and undertreatment (due to lack of access to asthma medications). Therefore, convenient and useful diagnosis tests or biomarkers are desperately needed in China, especially in the vast rural areas where medical resources are limited.
FeNO is a biomarker of airway inflammation, which may potentially a valuable aid in asthma diagnosis. Almost twenty years ago, Alving et al. firstly found that the amount of nitric oxide in exhaled air of asthmatics was increased [8]. Since then, plenty of studies have assessed the relationship between FeNO and asthma. Lane et al. discovered that FeNO was associated with the epithelial expression of iNOS within the airways but not with other NOSs [13]. Steerenberg et al. examined 450 children aged 7-12 years to explore the relationship between FeNO, impairment of lung function, BHR, and blood eosinophilia; They found BHR and the number of blood eosinophils per ml were positively associated with FeNO levels in atopic children but not in non-atopic children [14]. Lex et al. investigated 27 children with moderate to severe persistent asthma; they found that FeNO has significant correlations with both eosinophils in sputum and bronchoalveolar lavage [15].
In this study, we found that, no matter in bronchoprovocation tests (median, 64.8 ppb vs. 27.9 ppb) or in bronchodilation tests patients (median, 60.6 ppb vs. 29.05 ppb), the levels of FeNO in asthma patients were significantly higher than that of non-asthmatics, which was in accordance with a study performed by Sato et al. In their study, they focused on 71 consecutive out-patients with chronic cough and examined patients’ FeNO, pulmonary function, serum IgE, methacholine airway responsiveness and induced sputum. They found that the level of FeNO was significantly higher in patients with asthma compared to other diseases including chronic obstructive pulmonary disease (COPD) and eosinophilic pneumonia without asthma, and the best cut-off value for FeNO to diagnosis asthma was 38.8 ppb with sensitivity of 79.2% and specificity of 91.3% [16]. Although, it was almost agreed that FeNO in asthma patients were higher than that of non-asthmatics; the cut-off value of FeNO to distinguish asthma vary a lot due to different study design. Smith et al. investigated 47 consecutive patients with symptoms suggestive of asthma; they took 20 ppb as the cut-off value of FeNO to diagnosis asthma with sensitivity of 88% and specificity of 79% [17]. Schleich et al. assessed the ability of FeNO to identify BHR in 174 suspected asthma patients whose FEV1 ≥ 70% predicted and no demonstrated reversibility to β2-agonist. They found that the best cut-off value of FeNO to identify BHR was 34 ppb with sensitivity of 35% and specificity of 95% [11]. Our results demonstrated that the best cut-off value of FeNO to diagnosis asthma was 64 ppb with sensitivity of 52.0% and specificity of 94.35% in bronchoprovocation test patients, and 41 ppb with sensitivity of 72.43% and specificity of 74.85% in bronchodilation test patients. Interestingly, the best cut-off value to distinguish asthmatics from non-asthmatics in bronchoprovocation test was much higher than that of in bronchodilation test. One possible explains for this phenomenon was that the asthma phenotypes proportions in those two group patients were different.
Lots of demographic and biological factors, including sex, eight, age, cigarette smoking, atopy, IgE levels, may cause variation in FeNO levels. In this study, we explored the influence of sex, smoking history and age on FeNO levels in suspicious asthma patients. Our results showed that male patients had higher level of FeNO than female (median, 37.85 ppb vs. 31.5 ppb, P < 0.05), suggesting sex was a significant influencing factor of FeNO in suspected asthma patients. This result was consistent with other studies [18,19]. Researchers have found cigarette smoking can reduce FeNO levels and the degree of reduction seems to depend on the daily cigarette consumption [20]. In our study, we found patients with or without smoking history has no influence on FeNO levels; However, for male, those patients with smoking history had significant lower FeNO level than those without (median, 34.2 ppb vs.43.6 ppb, P = 0.001); The FeNO of female patients with or without smoking history didn’t display inter-group difference. The probable reason for this was that, in China, the daily cigarette consumption of female was normally less than male; Therefore, cigarette smoking play more important role in male populations. The influence of age in FeNO measurement was indeterminate. Olin et al. showed that age was independently and positively associated with FeNO, individuals aged > 64 years had 40% higher FeNO levels than those aged 35-44 years [21]; While, just like our results, the study performed by Olivieri et al. showed that age and FeNO had no correlation [18].
FeNO may be an effective auxiliary diagnosis method for bronchial asthma. In bronchoprovocation test patients, 64 ppb was the best cut-off value of FeNO to identify asthma with sensitivity of 52.0% and specificity of 94.35%. In bronchodilation test patients, 41 ppb was the best cut-off value of FeNO to identify asthma with sensitivity of 72.43% and specificity of 74.85%. Sex was an independent factor affecting patients’ FeNO level; while, smoking history played more important role on male patients’ FeNO measurement.
Disclosure of conflict of interest
None.
References
- 1.GBo A. Global Initiative for Asthma (GINA) 2012 [Google Scholar]
- 2.GSfAMa P. Global Initiative for Asthma (GINA) 2012 [Google Scholar]
- 3.Connett GJ. Bronchoalveolar lavage. Paediatr Respir Rev. 2000;1:52–56. doi: 10.1053/prrv.2000.0007. [DOI] [PubMed] [Google Scholar]
- 4.Gordon IO, Husain AN, Charbeneau J, Krishnan JA, Hogarth DK. Endobronchial biopsy: a guide for asthma therapy selection in the era of bronchial thermoplasty. J Asthma. 2013;50:634–641. doi: 10.3109/02770903.2013.794239. [DOI] [PubMed] [Google Scholar]
- 5.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 6.Leung TF, Ko FW, Wong GW. Recent advances in asthma biomarker research. Ther Adv Respir Dis. 2013;7:297–308. doi: 10.1177/1753465813496863. [DOI] [PubMed] [Google Scholar]
- 7.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 8.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6:1368–1370. [PubMed] [Google Scholar]
- 9.Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, Skiepko R, Szmitkowski M. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naive asthma patients. J Investig Allergol Clin Immunol. 2006;16:239–246. [PubMed] [Google Scholar]
- 10.Schneider A, Tilemann L, Schermer T, Gindner L, Laux G, Szecsenyi J, Meyer FJ. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement--results of a prospective diagnostic study: FENO < or =16 ppb better than FENO < or =12 ppb to rule out mild and moderate to severe asthma [added] . Respir Res. 2009;10:15. doi: 10.1186/1465-9921-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schleich FN, Asandei R, Manise M, Sele J, Seidel L, Louis R. Is FENO50 useful diagnostic tool in suspected asthma? Int J Clin Pract. 2012;66:158–165. doi: 10.1111/j.1742-1241.2011.02840.x. [DOI] [PubMed] [Google Scholar]
- 12.Tossa P, Paris C, Zmirou-Navier D, Demange V, Acouetey DS, Michaely JP, Bohadana A. Increase in exhaled nitric oxide is associated with bronchial hyperresponsiveness among apprentices. Am J Respir Crit Care Med. 2010;182:738–744. doi: 10.1164/rccm.200903-0415OC. [DOI] [PubMed] [Google Scholar]
- 13.Lane C, Knight D, Burgess S, Franklin P, Horak F, Legg J, Moeller A, Stick S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59:757–760. doi: 10.1136/thx.2003.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steerenberg PA, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, Opperhuizen A, Brunekreef B, van Amsterdam JG. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003;58:242–245. doi: 10.1136/thorax.58.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006;174:1286–1291. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]
- 16.Sato S, Saito J, Sato Y, Ishii T, Xintao W, Tanino Y, Ishida T, Munakata M. Clinical usefulness of fractional exhaled nitric oxide for diagnosing prolonged cough. Respir Med. 2008;102:1452–1459. doi: 10.1016/j.rmed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004;169:473–478. doi: 10.1164/rccm.200310-1376OC. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri M, Talamini G, Corradi M, Perbellini L, Mutti A, Tantucci C, Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir Res. 2006;7:94. doi: 10.1186/1465-9921-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor DR, Mandhane P, Greene JM, Hancox RJ, Filsell S, McLachlan CR, Williamson AJ, Cowan JO, Smith AD, Sears MR. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res. 2007;8:82. doi: 10.1186/1465-9921-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson MG, Zetterstrom O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343:146–147. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- 21.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130:1319–1325. doi: 10.1378/chest.130.5.1319. [DOI] [PubMed] [Google Scholar]


