Abstract
Endometrioid-type endometrial carcinoma (EEC) developing on the ground of endometrial hyperplasia (EH) is amongst the most commonly observed type of cancer in the world. Folate receptor α (FRα) is a vitamin molecule that has a role in cell proliferation. The fact that FRα, which is known to be needed extremely by the cells of malignancies that proliferate rapidly, is present in limited amounts in normal tissues while it is overexpressed in malignant cells of the same tissues makes folate a candidate for target molecular therapy. In our study, FRα expression in 214 cases, with 95 diagnosed within EEC and 119 with EH, was studied immunohistochemically. FRα expression in EEC was found significantly high compared to EH and normal endometrium (P<0.01). Similarly, FRα expression in EH cases with complex atypia were significantly high compared to other hyperplasia subgroups (P<0.01). The findings of our results make us think that FRα overexpression may play a role in the EEC carcinogenesis and carcinoma progression from EH. Furthermore, we suggest that it can be helpful in the treatment of EEC and/or transition from hyperplasia stage to EEC as a molecular therapy targeting receptors labeled with antibody-based props containing FRα. Finally, we suggest that FRα may be used, based on the expression intensity, as a supplemental option to determine the patients that shall be directed to radical therapy amongst patients with complex atypical EH.
Keywords: Endometrial carcinoma, endometrial hyperplasia, folate receptor α, target therapy
Introduction
Endometrial carcinomas are the most common malignancy in the female genital system. The majority of these adenocarcinomas consist of endometrioid-type (type 1) endometrium carcinoma (EEC) that occurs as a result of unmet excess estrogen and develops on the ground of endometrial hyperplasia (EH) [1].
Having a role in cellular methylation in structures such as lipids, proteins, and DNA as well as being a basic cofactor in the synthesis of purine and prymidine, folate, also known as vitamin B9, is a molecule highly needed by rapidly proliferating cells [2,3]. Folate receptor (FR), whose main task is to transfer folate vitamin through the cell membrane, also play a role in cellular proliferation [3]. Folate receptor α (FRα) is the most important subunit of FR and the alpha isoform has been shown to be selectively overexpressed in cancer types like breast and ovarian cancer compared to normal breast and ovarian epithelial cells [4,5]. It was determined that FRα exhibits a limited expression on the apical surfaces of the epithelial cells of normal lung, breast, thyroid, parathyroid, and kidney tissues [6-8]. For their uptake of folate, normal cells rely almost exclusively on the reduced folate carrier, whereas many carcinomas and myeloid leukemia cells overexpress a high-affinity FR on their surfaces, perhaps reflecting their increased need for folate to support rapid cell division [6,9]. In recent years, studies were made reporting that FRα may play a role in cancer development and may be used in potential anti-cancer therapies [10-17]. While importance and the details of the relationship between FRα overexpression and the deteriorated cellular proliferation have not been sufficiently cleared yet, high FRα expressions at various rates have been shown in previous studies in some carcinomas such as ovarian, non-small cell lung, breast, and colon [6,7,16-21]. Moreover, there are also studies suggesting that FRα expression is related to the survey and tumor stages and may be a prognostic indicator [6,13,21,22].
Although FRα status has been considered from some aspects [15,18,23,24], data on FRα expression in endometrium cancer is limited. In this study, we investigated the FR levels in EC and its precursor EH and the potential difference between these two entities, we aimed to present the effect of FRα on tumorgenesis in endometrium carcinoma and its potential to become a target molecule for this cancer.
Material method
Clinicopathologic data
The study consisted of 95 patients diagnosed with EEC, 58 patients diagnosed with EH without simple atypia, 21 patients diagnosed with EH without complex atypia, 18 patients diagnosed with EH with simple atypia, and 22 patients diagnosed with EH with complex atypia in Medeniyet University Goztepe Training and Research Hospital, Istanbul, Turkey between the dates January 2007 and June 2014. Thirty normal endometrium tissues at secretory and proliferative phase were used as control.
Age and menopausal status of the patients together with nuclear grade taken as per the FIGO grading system in carcinoma cases, estrogen (ER) and progesterone (PR) hormone receptor status were used as study parameters. The study protocol was approved by the local ethical committee.
Tissue microarray construction (TMA)
Cylindrical samples of 4-mm diameter were taken by comparing tissues in paraffin blocks and hemotoxylin-eosin cross-sections. This process was carried out using manual tissue microarrayer (Quick Ray; Unitma Co. Ltd., Seoul, Korea). The obtained tumor tissue samples were mapped and re-blocked and made ready for immunohistochemical study.
Immunohistochemistry and scoring
Sections of 4 μm thickness were cut from 224 THK tissues. Using specific primary antibodies, Dewaxed and rehydrated tissue sections were immunostained by the streptavidin-biotin peroxidase complex (SAB) method. Immunohistochemical staining for FRα, ER and PR receptor ER and PR receptor ER and PR receptor was done on step sections of TMA blocks. The slides were deparaffinized by 2 xylene rinses, followed by 2 rinses with 100% ethanol. Antigen retrieval was performed by heating the slides in a pressure cooker filled with either 7.5 mM sodium citrate with a pH of 6.0. After 5 minutes of casein blocking for nonspecific binding, the tissue sections were incubated for 25 minutes with primary antibodies for the detection of anti- FRα antibody (Leica Biosystems, NE, UK; BN 3.2, mouse monoclonal antibody, 1:100), ER receptor antibody (DAKO, ID5, mouse monoclonal antibody, ready to use) and PR receptor antibody (DAKO, PgR 636, mouse monoclonal antibody, ready to use). This step was followed by detection using the Bond Polymer Refine kit for 25 minutes on the Bond Max Autostainer (Leica Biosystems), visualization with diaminobenzidine chromogen, and counterstaining with hematoxylin. Breast and endometrium studies made previously were taken as basis in (FR) scoring [15,25]. In short, regarding staining intensity, observation of no staining was evaluated as 0; membranous staining limited only to gland lumen and observed at ×200 magnification as +1; staining observed at ×100 magnification as +2; and membranous staining easily observed at even ×40 magnification as +3. For staining rate, glandular cell staining over 25% was evaluated as positive. In statistical evaluation score 0 and 1 were classified as “low” expression and score 2 and 3 were classified as “high” expression. ER and PR immunoreactivity was scored as mild =1, medium =2 and severe =3 in intensity; and with regards to the ratio as follows: 0% as 0 points, 1-10% as 1 point, 11-33% as 2 points, 34-66% as 3 points, and 67-100% as 4 points. Ones with the ratio and intensity multiplication points of 0-1 were evaluated as negative (score 2), ones between 2-5 were low positive, and ones between 6-12 as high positive.
Statistical analysis
Pearson Chi-Square test was used for the comparison of the data from Yates Continuity Correction and Fisher Freeman Halton (Monte Carlo) tests. For consistency assessments between immunohistochemical variables, Spearman’s correlation analysis was used. Significant was evaluated at the level of P<0.05. Statistical analyses were carried out using the program NCSS (Number Cruncher Statistical System) 2007&PASS (Power Analysis and Sample Size) 2008 Statistical Software (NCSS LLC, Kaysville, Utah, USA).
Results
FRα expression was present in 81.1% of the EEC cases and was determined to have high expression in 50.5%. In endometrium hyperplasia, these ratios were 29.4% and 6.7%, respectively. All of the control tissues and 70.6% of EHs were negative (Figure 1A, 1B). FRα level in EEC was significantly high in comparison to EH and normal endometrium (P<0.01). Similarly, FRα expression in EH cases with complex atypia were significantly higher when compared to other hyperplasia subgroups (P<0.01). When examined in two main groups as EEC and EH, observation of moderate (++) (Figure 1E) and severe (+++) (Figure 1F) FRα expression in EECs was determined to be significantly higher than the EHs and normal endometrial tissues (P<0.001). A statistical significance was also determined (P<0.01) when the FRα expression is assessed as low and high. Observation of high FRα expression in EECs was determined to be significantly higher than the EHs and normal endometrial tissues (Table 1).
Figure 1.
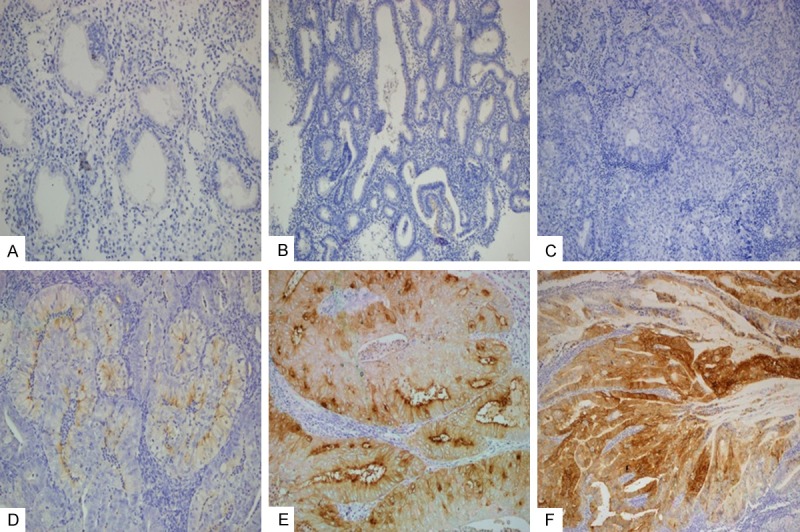
FRα expression status in endometrial tissues. Negativity in secretory endometrium (A) and simple EH (B). Negative (C), +1/weak (D), +2/moderate (E) and +3/strong FRα immunreactivity in EEC.
Table 1.
Evaluation of FRα expression in EEC and EH main groups
| EEC (n=95) | EH (n=119) | Secretuar/Proliferative endometrial tissue (n=30) | P | ||
|---|---|---|---|---|---|
|
|
|||||
| n (%) | n (%) | n (%) | |||
| FRα | Negative | 18 (18.9%) | 84 (70.6%) | 30 (100.0%) | 0.001** |
| + | 29 (30.5%) | 27 (22.7%) | 0 (0.0%) | ||
| ++ | 27 (28.4%) | 8 (6.7%) | 0 (0.0%) | ||
| +++ | 21 (22.1%) | 0 (0.0%) | 0 (0.0%) | ||
| FRα | Low | 47 (49.5%) | 111 (93.3%) | 30 (100.0%) | 0.001** |
| High | 48 (50.5%) | 8 (6.7%) | 0 (0.0%) | ||
Fisher Freeman Halton Test; *p<0.05;
p<0.01.
When the EH subgroups are individually compared, observation of severe (+++) FRα expression in EECs was determined to be significantly higher than all the EH subgroups and normal endometrial tissues (P<0.001). Observation of moderate (++) FRα expression in EECs and EHs with complex atypia was determined to be significantly higher than the other EHs subtypes and normal endometrial tissues (P<0.001). A statistical significance was also determined for observation of FRα expression based on low and high positivity (P<0.01). Observation of high FRα in EHs with complex atypia was determined to be higher than EHs without complex atypia, EHs without simple atypia and normal endometrium group. The findings are summarized in Table 2.
Table 2.
Evaluation of folate receptor α (FRα) expression in EEC, atypical-complex EH and simple without atipical EH
| n (%) | EEC (n=95) | Atypical-Complex EH (n=61) | Simple w/o atipical EH (n=58) | Normal endometrium (n=30) | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| n (%) | n (%) | n (%) | n (%) | |||
| FRα | Negative | 18 (18.9%) | 37 (60.7%) | 47 (81.0%) | 30 (100.0%) | 0.001** |
| + | 29 (30.5%) | 17 (27.9%) | 10 (17.2%) | 0 (0.0) | ||
| ++ | 27 (28.4%) | 7 (11.5%) | 1 (1.7%) | 0 (0.0%) | ||
| +++ | 21 (22.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| FRα | Low | 47 (49.5%) | 54 (88.5%) | 57 (98.3%) | 30 (100.0%) | 0.001** |
| High | 48 (50.5%) | 7 (11.5%) | 1 (1.7%) | 0 (0.0%) | ||
Fisher Freeman Halton Test; *p<0.05;
p<0.01.
While ER receptor expression did not exhibit a statistically significant relationship with age, PR expression increased with age (P<0.05). The proportion that the cases with high FRα expression were in patients 50 years old or more was significantly high (P<0.05). While ER and PR expressions did not exhibit a statistically significant difference based on menopause (P>0.05), observation of high FRα expression in menopausal patients was significantly higher than non-menopausal patients (P<0.05). Although no correlation between the EEC grades and the FRα, ER and PR expressions were observed, the EEC cases with high FRα expression tended to have high grades. In EEC, the negativity and low expression of ER and PR were significantly higher than the EHs and normal endometrial tissues and high expressions were significantly low. Similarly, in EHs with complex atypia, the negativity and low expression of ER and PR were significantly higher than the EHs without simple atypia and normal endometrial tissues and high expressions were significantly low (P<0.05). When their consistency and correlations were compared, no statistical significance was observed between the FRα, ER and PR expressions (P<0.05). The results are shown in Table 3.
Table 3.
Comparison between clinic-diagnostic parameters and FRα, ER-PR expressions
| Folate receptor α | ER | PR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Low | High | p value | Negative | Low | High | p value | Negative | Low | High | p value | |
| Age | |||||||||||
| < 50 | 83 (86.5) | 13 (13.5) | 0.001** | 11 (11.5) | 9 (9.4) | 76 (79.2) | 0.016* | 16 (16.7) | 10 (10.4) | 70 (72.9) | 0.048* |
| ≥ 50 | 75 (63.6) | 43 (36.4) | 19 (16.1) | 26 (22.0) | 73 (61.9) | 30 (25.4) | 21 (17.8) | 67 (56.7) | |||
| Menopose | |||||||||||
| - | 78 (83.0) | 16 (17.0) | 0.011* | 11 (11.7) | 14 (14.9) | 69 (73.4) | 0.548 | 14 (14.9) | 18 (19.1) | 62 (66.0) | 0.047* |
| + | 80 (66.7) | 40 (33.3) | 19 (15.8) | 21 (17.5) | 80 (66.7) | 32 (26.7) | 13 (10.8) | 75 (62.5) | |||
| Grade in EECs (n=101) | |||||||||||
| 1 | 24 (61.5) | 15 (38.5) | 0.051 | 8 (20.5) | 10 (25.6) | 21 (53.8) | 0.943 | 17 (43.6) | 5 (12.8) | 17 (43.6) | 0.484 |
| 2 | 17 (48.6) | 18 (51.4) | 8 (22.9) | 9 (25.7) | 18 (51.4) | 10 (28.6) | 8 (22.9) | 17 (48.6) | |||
| 3 | 6 (28.6) | 15 (71.4) | 5 (23.8) | 7 (33.3) | 9 (42.9) | 7 (33.3) | 6 (28.6) | 8 (38.1) | |||
| EEC | 47 (49.5) | 48 (50.0) | 0.001** | 21 (22.1) | 26 (27.4) | 48 (50.5) | 0.001** | 34 (35.8) | 19 (20.0) | 42 (44.2) | 0.001** |
| EH | 111 (93.3) | 8 (6.7) | 9 (7.6) | 9 (7.6) | 101 (84.9) | 12 (10.1) | 12 (10.1) | 95 (79.8) | |||
| EEC | 47 (49.5) | 48 (50.5) | 0.001** | 21 (22.1) | 26 (27.4) | 48 (50.5) | 0.001** | 34 (35.8) | 19 (20.0) | 42 (44.2) | 0.001** |
| Atypical&Complex EH | 54 (88.5) | 7 (11.5) | 0.004** | 7 (11.5) | 4 (6.6) | 50 (82.0) | 0.026* | 9 (14.8) | 7 (11.5) | 45 (73.8) | 0.243 |
| Simple w/o atipical EH | 57 (98.3) | 1 (1.7) | 0.001** | 2 (3.4) | 5 (8.6) | 51 (87.9) | 0.001** | 3 (5.2) | 5 (8.6) | 50 (86.2) | 0.001** |
Fisher Freeman Halton Test;
p<0.05;
p<0.01.
Discussion
Current study is based on the evaluation of the FRα level immunohistochemically in EEC and its precursor EH using normal endometrium controls and thus presenting the possible difference of the FRα expression level between normal endometrial tissue, hyperplasia and carcinoma. We aimed to exhibit the possible contribution of FRα to the diagnosis and treatment of endometrial lesions by presenting FRα staining patterns between different endometrium tissues and lesions. The literature about FRα expression in endometrial malignancies and comparative analyses between the EHs and EECs in wide series is lacking.
It was shown that the FRα was expressed in normal progenitor cells and had a regulating effect on malign transformation [18]. Observation of high FRα expression with varying rates in some tumors in spite of its limited expression in normal and precancerous cells brings to mind that FRα may play a key role in carcinogenesis and might become a possible target in the treatment [6,7,18,24]. It has been reported that FRα is a part of the malign transformation in breast carcinoma and increases proliferation in carcinoma cells [5,25]. However, its expression in normal breast tissue epithelia was limited or non-existent [5]. Wu et al. showed that FRα expression did not disappear in serous ovarian and endometrioid malignancies although malign transformation from cervix glandular epithelia and the transformation from ovarian germinal epithelia to serous or mucinous benign neoplasia were related to FRα down-regulation [18]. Similarly, Shia et al. reported that FRα expression was significantly high in collateral carcinomas and metastasis compared to normal mucosa and adenomas and that they were related to poor surveys in univariance analyses [16]. Christoph et al. reported that FRα was frequently expressed in advanced non-small cell lung carcinomas [26]. Cagle et al. determined high FRα expression in lung adenocarcinoma and squamous-cell carcinomas and suggested that FR-target therapy might be a good indicator especially in lung carcinomas unresponsive to therapy [27]. Toffoli et al. and Kalli et al. found that FRα expression was related to bad prognostic factors and aggressive progress in ovarian carcinoma and that FRα overexpression was significantly high in high-grade ovarian cancers compared to low-grade ones [13,28]. Similarly, strong FRα expression in breast carcinoma was found to be correlated with poor clinical progress [2,22]. Regarding the studies on endometrial carcinomas, Wu et al. reported that malign transformation was related to de nova expression of FRα in endometrial glandular cells [18]. Brown Jones et al. reported that high FRα has a significant relationship with poor prognostic factors such as late-stage and high-grade in endometrium carcinomas [15] and Allard et al. reported high FRα immunoexpression at a rate of 12% in EEC, 33% in the serous type, and 25% in the clear-cell type and no correlation was observed in the same study, in which radical resection materials were used, between FRα and age, grade, stage, deep myometrial invasion and lymph node metastasis [23]. In a study carried out recently, O’Shannessy et al. showed that FRα was expressed negatively or weak apical positively in some of the normal endometrium tissues and strongly expressed in some of the complex EHs and EECs with atypia [24].
Although it is still unclear whether FRα overexpression encountered in some cancers is a passive condition caused by rapid proliferation of tumor cells or arises since it’s a direct factor on pathogenesis [4,6,12,18,20], we suggest that FRα overexpression may play a role in EEC carcinogenesis and carcinoma progress from EH as we have found FRα expression is increased significantly in EECs in comparison to EHs and complex and atypia EHs when compared to simple hyperplasia. Furthermore, as established in some similar studies [6,11], the non-existence of FRα expression in normal tissues brings to mind that folate might be used as a potential molecule in endometrium cancer therapy and receptor-targeted molecular therapies. Importantly, folate receptor-targeted therapies suppress metastasis and inhibit primary tumor growth in animal tumor models. And the recent data show that FRα is a target of immune system in breast and ovarian cancer patients [12,31]. Moreover, for cancers with strong overexpression of folate receptor, targeting this receptor therapeutically shown to be beneficial [11,12,32,33]. Recent studies reported promising results with experimental folate receptor labeled combination chemotherapies in ovarian and lung cancers with high FRα expression [34-36].
Moreover, there are applications as a serum marker and imaging in tumors such as lung, ovary and endometrium where FRα is overexpressed [37,38] and studies suggesting a correlation between FRα expression and monitoring and follow-up of the disease and the response to therapy [29,39,40].
Although down-regulation or up-regulation has been reported between sex steroids in some studies and it has been shown that FRα regulation may be achieved through anti-estrogen therapy such as tamoxifen and ICI 182780 [2,29,30] we did not observe any correlation between the sex steroids, ER-PR receptor expression and FRα expression in both EHs and EECs which is consistent with the results of the study on breast carcinomas by Hartman et al [22].
As a result, although hormonal therapy and various chemotherapeutics are used in the treatment of endometrioid-type carcinomas, target treatment is the treatment of choice in all tumor types. Significantly higher FRα expression encountered in endometiroid-type endometrium carcinomas in comparison to hyperplasia and normal endometrium brings to mind that FRα might have a role in the development of carcinoma along with other etiologic factors and folate may be used in the treatment of EEC and/or in the prevention of transformation to EEC from hyperplasia stage. Furthermore, the non-existence of FRα in normal endometrium glands, but its high expression in ECC may be interpreted as target therapies like paxlitaxel and anti-EGFR labeled with probes based on antibodies containing folate may be useful in the treatment of EEC. FRα might be used as an indicator in the wide spectrum of normal cyclic endometrium-simple hyperplasia-malign endometrial carcinoma transition and might become a supplemental marker to determine the patients that shall be directed to radical therapy amongst patients with complex atypical EH.
Ackonwledgements
This study was supported by Research Fund of Istanbul Medeniyet University (Project Number: TSA-2013-401).
Disclosure of conflict of interest
None.
References
- 1.Kurman RJ, Ellenson LH, Ronnett BM. In: Blaustein’s Pathology of the Female Genital Tract. Kurman RJ, editor. New York, NY: 2010. pp. 394–400. [Google Scholar]
- 2.Zhang Z, Wang J, Tacha DE, Li P, Bremer RE, Chen H, Wei B, Xiao X, Da J, Skinner K, Hicks DG, Bu H, Tang P. Folate receptor alpha associated with triple-negative breast cancer and poor prognosis. Arch Pathol Lab Med. 2014;138:890–895. doi: 10.5858/arpa.2013-0309-OA. [DOI] [PubMed] [Google Scholar]
- 3.Corona G, Giannini F, Fabris M, Toffoli G, Boiocchi M. Role of folate receptor and reduced folate carrier in the transport of 5-methyltertahydrofolic acid in human ovarian carcinoma cells. Int J Cancer. 1998;75:125–33. doi: 10.1002/(sici)1097-0215(19980105)75:1<125::aid-ijc19>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Elnakat H, Ratnam M. Role of folate receptor genes in reproduction and related cancers. Front Biosci. 2006;11:506–19. doi: 10.2741/1815. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann LH, Keeney GL, Lingle WL, Christianson TJH, Varghese B, Hillman D, Oberg AL, Low PS. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–42. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 6.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines: physiologic and clinical implications. Cancer. 1994;73:2432–43. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401. [PubMed] [Google Scholar]
- 8.O’Shannessy DJ, Somers EB, Albone E, Cheng X, Park YC, Tomkowicz BE, Hamuro Y, Kohl TO, Forsyth TM, Smale R, Fu YS, Nicolaides NC. Characterization of the human folate receptor alpha via novel antibody-based probes. Oncotarget. 2011;2:1227–43. doi: 10.18632/oncotarget.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–9. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 10.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130:129–32. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 11.Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv Drug Deliv Rev. 2004;56:1055–8. doi: 10.1016/j.addr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Sega E, Leamon CP, Low PS. Folate receptor-targeted immunotherapy of cancer: mechanism and therapeutic potential. Adv Drug Deliv Rev. 2004;56:1161–1176. doi: 10.1016/j.addr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 15.Brown Jones M, Neuper C, Clayton A, Mariani A, Konecny G, Thomas MB, Keeney G, Hartmann L, Podratz KC. Rationale for folate receptor alpha targeted therapy in “high risk” endometrial carcinomas. Int J Cancer. 2008;123:1699–703. doi: 10.1002/ijc.23686. [DOI] [PubMed] [Google Scholar]
- 16.Shia J, Klimstra DS, Nitzkorski JR, Low PS, Gonen M, Landmann R, Weiser MR, Franklin WA, Prendergast FG, Murphy L, Tang LH, Temple L, Guillem JG, Wong WD, Paty PB. Immunohistochemical expression of folate receptor alpha in colorectal carcinoma: patterns and biological significance. Hum Pathol. 2008;39:498–505. doi: 10.1016/j.humpath.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 17.O’Shannessy DJ, Yu G, Smale R, Fu YS, Singhal S, Thiel RP, Somers EB, Vachani A. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–25. doi: 10.18632/oncotarget.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev. 1999;8:775–82. [PubMed] [Google Scholar]
- 19.Bueno R, Appasani K, Mercer H, Lester S, Sugarbaker D. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–33. doi: 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- 20.Markert S, Lassmann S, Gabriel B, Klar M, Werner M, Gitsch G, Kratz F, Hasenburg A. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res. 2008;28:3567–72. [PubMed] [Google Scholar]
- 21.O’Shannessy DJ, Somers EB, Maltzman J, Smale R, Fu YS. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus. 2012;28:1–22. doi: 10.1186/2193-1801-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann LC, Keeney GL, Lingle WL, Christianson TJ, Varghese B, Hillman D, Oberg AL, Low PS. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–42. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 23.Allard JE, Risinger JI, Morrison C, Young G, Rose GS, Fowler J, Berchuck A, Maxwell GL. Overexpression of folate binding protein is associated with shortened progression-free survival in uterine adenocarcinomas. Gynecol Oncol. 2007;107:52–7. doi: 10.1016/j.ygyno.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 24.O’Shannessy DJ, Somers EB, Smale R, Fu YS. Expression of Folate Receptor-a (FRA) in Gynecologic Malignancies and its Relationship to the Tumor Type. Int J Gynecol Pathol. 2013;32:258–68. doi: 10.1097/PGP.0b013e3182774562. [DOI] [PubMed] [Google Scholar]
- 25.Jhaveri MS, Rait AS, Chung KN, Trepel JB, Chang EH. Antisense oligonucleotides targeted to the human alpha folate receptor inhibit breast cancer cell growth and sensitize the cells to doxorubicin treatment. Mol Cancer Ther. 2004;3:1505–12. [PubMed] [Google Scholar]
- 26.Christoph DC, Reyna-Asuncion B, Hassan B, Tran C, Maltzman JD, O’Shannessy DJ, Gauler TC, Wohlschlaeger J, Schuler M, Eberhardt WE, Hirsch FR. Assessment of folate receptor-a and epidermal growth factor receptor expression in pemetrexed-treated none small-cell lung cancer patients. Clin Lung Cancer. 2014;15:320–30. doi: 10.1016/j.cllc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Cagle PT, Zhai QJ, Murphy L, Low PS. Folate receptor in adenocarcinoma and squamous cell carcinoma of the lung: potential target for folate-linked therapeutic agents. Arch Pathol Lab Med. 2013;137:241–4. doi: 10.5858/arpa.2012-0176-OA. [DOI] [PubMed] [Google Scholar]
- 28.Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, Hartmann LC. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619–26. doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. 2003;63:2820–8. [PubMed] [Google Scholar]
- 30.O’Donnell AJ, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-a mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer. 2005;12:851–66. doi: 10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- 31.Knutson K, Krco C, Erskine C, Goodman K, Kelemen L, Wettstein P, Low PS, Hartmann L, Kalli K. T-cell immunity to the folate receptor a is prevalent in women with breast or ovarian cancer. J. Clin. Oncol. 2006;24:1–9. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother. 2002;51:153–62. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leamon CP, Reddy JA, Vlahov IR, Kleindl PJ, Vetzel M, Westrick E. Synthesis and biological evaluation of EC140: A novel folate-targeted vinca alkaloid conjugate. Bioconjug Chem. 2006;17:1226–32. doi: 10.1021/bc060145g. [DOI] [PubMed] [Google Scholar]
- 34.Dosio F, Milla P, Cattel L. EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Curr Opin Investig Drugs. 2010;11:1424–33. [PubMed] [Google Scholar]
- 35.Pribble P, Edelman MJ. EC145: a novel targeted agent for adenocarcinoma of the lung. Expert Opin Investig Drugs. 2012;21:755–761. doi: 10.1517/13543784.2012.671294. [DOI] [PubMed] [Google Scholar]
- 36.Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, Vander Els N, Phillips MD, Schweizer C, Weil SC, Larson SM, Old LJ. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–95. doi: 10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 37.Mathias CJ, Wang S, Lee RJ, Waters DJ, Low PS, Green MA. Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of gallium-67-deferoxamine-folate. J Nucl Med. 1996;37:1003–8. [PubMed] [Google Scholar]
- 38.Wang S, Luo J, Lantrip DA, Waters DJ, Mathias CJ, Green MA, Fuchs PC, Low PS. Design and synthesis of [111 In] DTPA-folate for use as a tumor-targeted radiopharmaceutical. Bioconj Chem. 1997;8:673–9. doi: 10.1021/bc9701297. [DOI] [PubMed] [Google Scholar]
- 39.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–84. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Luhrs C, Raskin CA, Durbin R, Wu B, Sadasivan E, Rothenberg SF. Transfection of a glycosylated phosphatidylinositol-anchored folate-binding protein complementary DNA provides cells with the ability to survive in low folate medium. J Clin Invest. 1992;90:840–7. doi: 10.1172/JCI115959. [DOI] [PMC free article] [PubMed] [Google Scholar]


