Abstract
Primary extragonadal malignant germ cell tumors (EMGCTs) are rare and characterized by the location in the midline of the body, including mediastinum, CNS, retroperitoneum and coccyx. EMGCTs present with different clinical and biologic characteristics in different tumor locations. Accurately diagnosing MEGCTs would be very difficult by performing on HE staining alone, and requires immunohistochemical verification. This study was to investigate the biological feature of EMGCTs and diagnostic value of immunohistochemical markers OCT3/4, CD117, PLAP, AFP, β-HCG and CD30 in EMGCTs. A retrospective study was performed on 48 patients with EMGCTs. EMGCTs were found to occur predominantly in males, especially for mediastinal MGCTs. The tumor locations included mediastinum, CNS and retroperitoneum. The mediastinum and CNS were the most common sites of EMGCTs. Seminoma/germinomas (64.6%) was the most common histological subtypes of EMGCTs. Chest pain, dyspnea, cough and fever were the most common clinical presentations in mediastinal MGCTs. Headache, visual disturbances, endocrine abnormalities, and signs of increased intracranial pressure were common clinical symptoms in CNS MGCTs. Abdominal mass with or without pain, backache and weight loss were common clinical presentations in retroperitoneal MGCTs. PLAP, CD117 and OCT3/4 were highly expressed in seminomas/gernimomas. CD30, EMA and CK AE1/3 staining were positive in embryonal carcinoma. AFP and β-HCG positive staining are characteristic in yolk sac tumors and choriocarcinoma, respectively. Patients with seminomas/germinomas had a better prognosis than those with NS/G-GCTs. Our finding suggests that the accurate diagnosis of EMGCTs is critical not only for predicting the tumor progression but also for patient management. Immunohistochemical markers have become an important tool in the diagnosis and differential diagnosis of EMGCTs.
Keywords: Extragonada malignant germ cell tumors, immunohistochemistry, diagnosis, prognosis
Introduction
Primary extragonadal malignant germ cell tumors (EMGCTs) are rare and account for only 2~5% of the malignant germ cell tumors (MGCTs). Primary extragonadal germ cell tumors (EGCTs) are characterized by their location on the midline structures of the body from the pineal gland to the coccyx, such as central nervous system (CNS), mediastinum, retroperitoneum and coccyx. The mediastinum is the most common site of EGCTs, accounting for 50~70% of all EGCTs. Mature teratomas are the most common germ cell tumors of the mediastinum, accounting for 70~75% of all mediastinal GCTs. Only 25~30% GCTs in mediastinum are malignant. The CNS and retroperitoneum are also the common sites. Histological, serological and cytogenetic features of EMGCTs are very similar to those in gonadal sites (testis and ovary), but the clinical and biological characteristics of EMGCTs distinct from their testicular and ovarian counterparts. The accurate pathological diagnosis of EMGCTs is critically important for guiding clinical therapy and predicting prognosis. The diagnosis of EMGCTs might not be readily achieved by haematoxylin and eosin (H&E) staining alone and usually requires immunohistochemical confirmation. Because of their distinctive clinical treatment and prognosis, it is imperative for clinicians to make an accurate differential diagnosis between GCTs and others non-GCTs tumors, seminomas and non-seminomatous tumors, as well as germinomas and non-germinomatous tumors [1-4].
The diagnosis of gonadal germ cell tumors usually requires immunohistochemical verification. PLAP, CD117, AFP and β-HCG are routine immunohistochemical markers for diagnosis and differential diagnosis of gonadal GCTs. In this study, we investigated the biological feature of EMGCTs, and evaluated the recently described GCT markers, including OCT3/4, CD117, CD30, PLAP, AFP and β-HCG together with CK and EMA in the diagnosis and differential diagnosis of EMGCTs [5,6]. Here, we aimed to investigate whether there was a shared pattern of expression in EMGCTs. To our knowledge, it is the first comprehensive study to evaluate the clinical significance of above markers in EMGCTs detection.
Materials and methods
Patient data
A total of 48 cases diagnosed with EMGCTs were collected from Renji Hospital, School of Medicine, Shanghai Jiaotong University between 1990 and 2014. 16 cases of mediastinum MGCTs, 27 cases of CNS MGCTs, 5 cases of retroperitoneum MGCT were included in this study. There were 37 male and 11 female patients (male: female ratio 3.4:1, male predominance) with a median age of 19.3 years (ranging from 10 days to 46 years).
16 cases of mediastinum MGCTs were comprised of 7 seminoma (S), 3 yolk sac tumor (YST), 1 malignant teratoma (MT), 2 immature teratoma (IT), and 3 mixed GCTs (1 YST+ IT, 1 S+IT, 1 S+MT). 27 cases of CNS GCTs was comprised of 22 germinomas (G), 1 embryonal carcinoma (EC), 1 immature teratoma, 1 choriocarcinoma (CC), and 2 mixed GCTs (1 G+EC, 1 G+YST). 5 cases of retroperitoneum MGCTs were comprised of 2 MT, 2 seminoma, 1 YST. None of the patients with primary EGCTs had a previous history of gonadal GCTs. The study was approved by the ethics committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University. The written informed consents from patients for using their tissue specimens were also obtained.
Follow-up study
Forty-eight patients were followed up by telephone or mail and only 42 patients had completed follow-up results. After discharge from the hospital, all patients in this study were evaluated every 6-months for the first 5 years and 12-month intervals thereafter, as well as their clinical history, physical examination, laboratory analysis, or CT scan. Median follow-up period was 6.5 years, ranging from 4 months to 17 years. The date of death or last follow-up was defined as the endpoint.
Immunohistochemistry and staining evaluation
Surgical specimens were fixed in 10% formalin and embedded in paraffin. 4 μm sections were cut and stained with H&E. Additional 4 μm sections were deparaffinized with xylene and rehydrated in a graded series of ethanol. The deparaffinised sections were then incubated with 3% H2O2 to inhibit the endogenous peroxidase, followed by microwave-treated or trypsin digestion for antigen retrieval before incubation with different primary antibodies, using a two-step polymer method (EnVision TM). The sections were incubated in a humid chamber at 4°C overnight after adding primary antibodies and the Table 1 showed the details of primary antibodies used in our study. Subsequently, second antibodies were added after PBS rinse. The sections were incubated at room temperature for 30 minutes, and then colored with DAB for 15 minutes, and finally light counterstained with hematoxylin. Positive control staining were prepared using placental tissue, testicular neoplasm and gastrointestinal stromal tumor, respectively. Negative controls were performed using blocking serum in place of primary antibody. Immunohistochemical expression was graded using a semi-quantitative scoring system based on the proportion of positive cells over total cells (percent positivity) ranging from 0 to 100% where 0% was negative expression, <50% was weak expression, ≥50% was strong expression [7].
Table 1.
Summary of immunohistochemical antibodies
| Antibody | Type | Pre-treated | Dilution | Location | Detail |
|---|---|---|---|---|---|
| PLAP | mouse anti-human | heated | WS | cytoplasm | DaKo (GM719102) |
| OCT3/4 | goat anti-IgG | heated | 1:50 | nucleus | SantaCruz (c-20, sc-8629) |
| CD117 | rabbit anti-human | heated | WS | cytomembrane | DaKo (A450202) |
| HCG | rabbit anti-human | heated | WS | cytoplasm | DaKo (GA023102) |
| AFP | rabbit anti-human | None | WS | cytoplasm | DaKo (GA000804) |
| CK AE1/3 | mouse anti-human | typsin digestion | 1:50 | cytoplasm | DaKo (GM082110) |
| EMA | mouse anti-human | WS | Cytomembrane/cytoplasm | DaKo (GM061329) | |
| CD30 | mouse anti-human | heated | WS | cytomembrane | DaKo (GM075102) |
WS: working solution; goat anti-mouse, rabbit anti-goat, goat anti-rabbit.
In addition, a total of 20 primary mediastinal and intracranial tumors, including thymoma (8), lymphoma (5), neuroendocrine neoplasm (2) and pituitary adenoma (5), were retrieved to serve as controls.
Statistics analysis
Statistical analyses were performed using SPSS for Windows (version 17.0). The chi-square test or Fisher exact tests were used for categorical and ordinal variables. P values of <0.05 were considered statistically significant, and all reported P values are two-sided.
Results
Clinical characteristics of the patients
The duration of symptoms before diagnosis ranged from 5 days to 5 years, with a mean time of 10 months. 7 patients (20.6%) were younger than 10 years, 9 patients (26.5%) were older than 20 years, and 18 patients (52.9%) were between 10 and 20 years. There were many different clinical characteristics according to age, genders and histological types in different locations of EGCTs. CNS GCTs occurred predominantly in children and adolescents, accounting for 81.5% (22/27) of the cases before the age of 20 years. The peak incidence of CNS GCT was between 10 and 20 years of age. 12 of 16 mediastinum MGCTs (75%) occurred in adults between the ages 20 and 35 years. In retroperitoneal MGCTs, 3 patients (60%) were younger than 10 years (age of 10 days, 30 days and 7 years respectively), 2 patients (40%) were older than 20 years (age of 27 years and 35 years respectively).
EMGCTs are more common in males, especially in mediastinal MGCTs (all male patients). Clinical presentations of EMGCTs were dependent on the location and size of the tumor in the extragonadal sites of the midline of the body. The size of CNS MGCT (diameter 0.5~5.5 cm) were smaller than that of mediastinal MGCTs (diameter 6~21 cm) and retroperitoneal MGCTs (diameter 7~16 cm). Symptoms of CNS MGCTs were various at diagnosis, ranging from endocrine abnormalities, headache, visual disturbances to signs of increased intracranial pressure. Patients with mediastinal MGCTs and retroperitoneal MGCTs would not present clinical symptoms until their tumors have developed into larger size. Representative symptoms included chest pain, dyspnea, cough, fever, abdominal mass with/without pain, backache and weight loss. Seminoma/germinoma was the most common histological type in the EMGCTs, accounting for 81.5% in CNS EGCTs and 43.8% in mediastinum MGCTs. The 2 newborn patients (40%) were diagnosed as malignant teratomas (both female) (Table 2; Figure 1A-D).
Table 2.
Clinical features of 48 cases of primary extragonadal malignant germ cell tumors
| CNS | mediastinum | retroperitoneum | |
|---|---|---|---|
| sex | |||
| male | 18 | 16 | 3 |
| female | 9 | 0 | 2 |
| M/F | 2:1 | All M | 1.5:1 |
| Age | |||
| <10 | 2 | 0 | 3 |
| 10~20 | 20 | 4 | 0 |
| 21~30 | 4 | 6 | 1 |
| >30 | 1 | 6 | 1 |
| size (diate cm) | 0.5~5.5 | 6~21 | 7~16 |
| Histological type | |||
| S/G | 22 | 7 | 2 |
| IT/MT | 1 | 3 | 2 |
| YST | 0 | 3 | 1 |
| EC | 1 | 0 | 0 |
| CC | 1 | 0 | 0 |
| MGCTs | 2 | 3 | 0 |
G: germinoma; IT: immature teratoma; MT: malignant teratoma; YST: yolk sac tumor; EC: embryonal carcinoma; CC: choriocarcinoma; MGCTs: mixed germ cell tumors; M/F: male/female.
Figure 1.
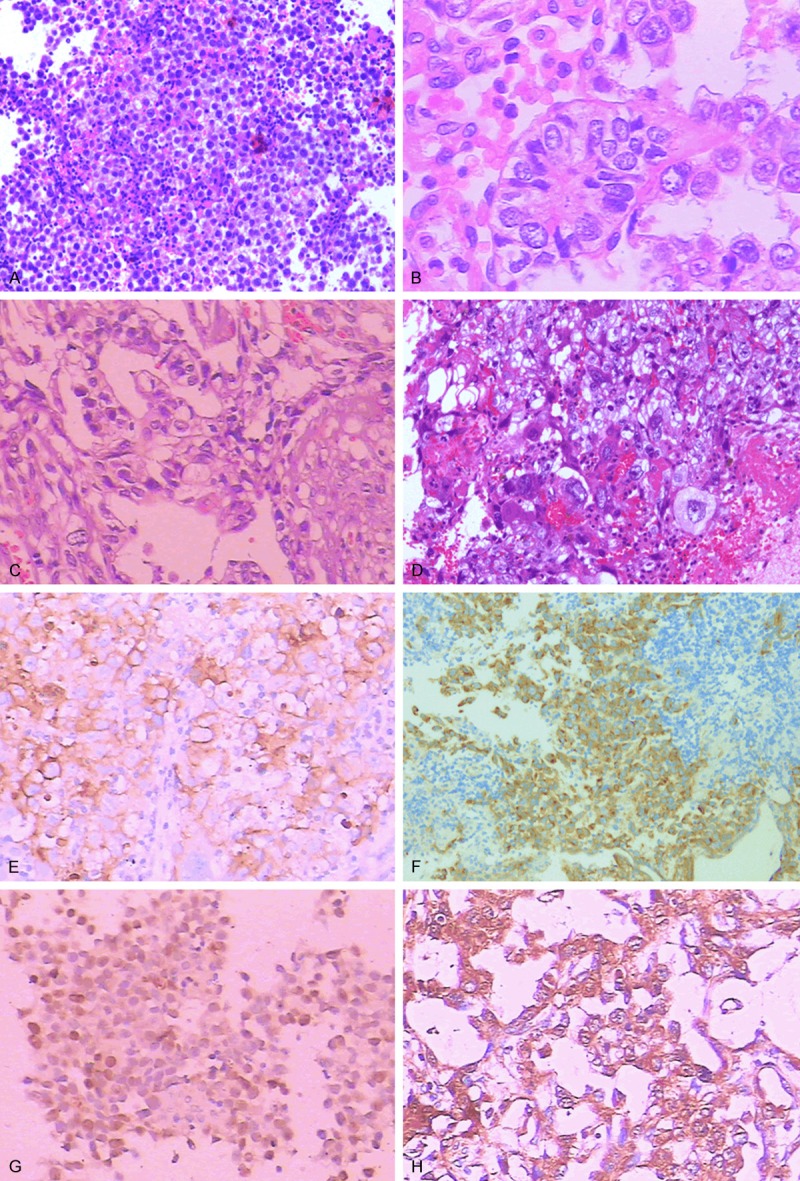
Histological features: A: Germinomas (CNS) are recognized for tumor cells with abundant clear cytoplasm, sheet growth pattern, lymphocytic infiltrating along fibrovascular stroma (HE×200). B: Yolk sac tumors (mediastinum) are composed of clear, columnar epithelial cells arranged in sheets, cords and tubules structures. “Schiller-Duval bodies” is a hallmark of YST (HE×400). C: Embryonal carcinomas (CNS) are characterized by tumors cells organized in sheets, cord, or gland-like structures (HE×200). D: Choriocarcinomas (CNS) are composed of two characteristic cell types: cytotrophoblastic cells and syncytiotrophoblastic giant cells (HE×200). E: Immunostaining for PLAP in serminoma (retroperitoneum) (×200). F: Immunohistochemistry for CD117 showing positive staining in the tumor cells of the germinoma (CNS) (×200). G: Immunohistochemistry for OCT3/4 showing positive staining in the tumor cells of the germinoma (CNS) (×200). H: Immunostaining for AFP in yolk sac tumor (mediastinum) (×200).
Immunohistochemical results (Table 3)
Table 3.
The expression of immunohischemical markers in 48 primary extragonadal malignant germ cell tumors
| Histology | PLAP | CD117 | OCT3/4 | HCG | AFP | CD30 | CK AE1/3 | EMA |
|---|---|---|---|---|---|---|---|---|
| S/G | + | + | + | - | - | - | +/- | -/+ |
| YST | -/+ | -/+ | - | - | + | - | + | + |
| EC | +/- | - | + | - | - | + | + | + |
| MT/IT | - | - | - | - | - | - | + | + |
| CC | + | - | - | + | - | - | + | + |
| S+IM/MT | + | + | + | - | - | - | + | + |
| G+EC | + | + | + | - | - | + | + | + |
| G+YST | + | + | + | - | + | - | +/- | +/- |
| YST+IM | -/+ | -/+ | - | - | + | - | + | + |
S: seminoma; G: germinoma; YST: yolk sac tumor; EC: embryonal carcinoma; MT: malignant teratoma; IT: immature teratoma; CC: choriocarcinoma.
The immunohistochemical results are summarized in Table 3. PLAP was mainly located in the cell membrane and cytoplasm. It was detected in 25 of 31 (80.6%) germinomas/seminomas, and 3 of 3 (100%) germinomas/seminomas component in mixed MGCTs. CD117 was detected in 29 of 31 (93.5%) germinomas/seminomas, and 3 of 3 (100%) germinomas/seminomas component in mixed MGCTs (Figure 1E, 1F). OCT3/4 was expressed in all germinomas/seminomas (31/31), embryonal carcinoma (1/1), and germinomas/seminomas component (4/4) and embryonal carcinoma component (1/1) in mixed GCTs, while yolk sac tumor, teratoma and choriocarcinoma were consistently negative (Figure 1G). None of 6 patients with teratoma (immature and malignant), 4 patients with yolk sac tumor and one patient with choriocarcinoma had a positive expression of PLAP, CD117 and OCT3/4. β-HCG, CK AE1/3 staining and EMA staining were seen in choriocarcinoma (1/1) and scattered syncytiotrophoblats (1/1). AFP staining, CK AE1/3 staining, EMA staining and local PLAP staining were seen in yolk sac tumor (4/4) and the tumor containing YST (2/2) (Figure 1H). CD30 and CK AE1/3 staining, EMA staining was positive in embryonal carcinoma and embryonal carcinoma component of mixed GCT.
All control cases of thymoma, lymphoma, gliblastoma, neuroendocrine neoplasm, pituitary adenoma, and meniangioma were immunonegative for PLAP, CD117 and OCT3/4.
Follow-up data after surgery were available for 42 patients and 6 patients were out of follow up. 4 patients with seminomas/germinomas had died and the 5-year overall survival rate of patients with seminomas/germinomas was 93.3%. One patient with embryonal carcinoma and one patient with choriocarcinoma in CNS died 12 months and 6 months after tumors resection, respectively. Immature and malignant teratomas also had favorable prognosis with a 5-year survival rates of 80%. The overall 3-year and 5-year survival rates of the patients with malignant NS/G-GCTs were 50% and 28.6% respectively.
Discussions
Germ cell tumor most frequently occurs in the gonads (ovary and testis), but rarely occurs in the extragonadal sites of the midline of the body including mediastinum, CNS, retroperitoneum and sacrococcygeal region. More than 90% of EGCTs occur in adult man between 20 and 35 years, such as mediastinal GCTs. The mediastinum is the most common site of primary EGCTs, and comprising 50~70% of all primary EGCTs, followed by CNS, retroperitoneum and sacrococcygeal region [8]. However, there are many differences in the clinical characteristics according to age, sex and histological type in different locations of EGCTs. The incidence of primary CNS GCTs account for all primary CNS tumors is higher in Far East Asian countries, such as Japan, China and South Korea, than that in Western countries. CNS GCTs occur predominantly in children and adolescents with approximately 90% of the cases before the age of 20 years, of which the peak incidence is between 10 and 20 years of age [9]. The patients with teratoma in retroperitoneum are usually younger than 1 year [10].
EGCTs are also more common in male compared with the gonadal GCTs, especially for EMGCTs. There is an overwhelming male predominance in malignant GCTs of mediastinum, while in a mild female predominance or a nearly equivalent gender ratio was observed in benign teratoma of mediastinum. In our report, all patients of mediastinal MGCTs were men. This result was in accordance with previous studies which also supported the overall male predominance in malignant GCTs of mediastinum. The male: female ratio was reported to range from 1.5 to 3:1 in CNS GCTs [1,11].
Symptoms of EMGCTs were dependent on the location and size of the tumor. Patients with mediastinal and retroperitoneal MGCTs would not present until their tumors have developed into large sizes. The clinical presentations of mediastinal include chest pain, dyspnea, cough, hemoptysis, and fever et al. while abdominal mass with/without pain, backache and weight loss were usually found in retroperitoneal MGCTs. However, some asymptomatic cases were diagnosed as mediastinal MGCTs accidently by routine physical examination in our study. In addition, almost all cases (≥90%) occurred in the anterior mediastinum [10,11]. The tumor size of CNS MGCT is much smaller than that of mediastinal and retroperitoneal MGCTs. The clinical features including signs of increased intracranial pressure, visual changes and endocrine abnormalities are similar to that of other CNS tumors, such as glioblastoma, ependymoma, medulloblastoma, or pineoblastomas, which result from tumor invasion or compression of in adjacent tissue. The sellar and suprasellar region, pineal gland, hypothalamus, and third ventricle are the most common sites of intracranial MGCTs [12].
Pathological classifications of primary EGCTs by the WHO contain seminoma/germinoma, teratoma (mature, immature and malignant), yolk sac tumor, embryonal carcinoma, choriocarcinoma and mixed germ cell tumor (containing more than one germ cell component). Malignant EGCTs can be divided into two broad groups: seminoma/germinoma and non-seminomatous/non-germinomatous germ cell tumor (NGGCT). 70~75% of mediastinal GCTs are mature teratomas and only 25~30% GCTs in mediastinum are malignant. Yolk sac tumor and seminoma are the most common NSGCT of the mediastinum. In CNS GCTs, 65% of GCTs are germinomas, followed by teratomas (18%), embryonal carcinomas (5%), yolk sac tumor (5%), choriocarcinomas (3%), and mixed GCTs. Teratomas are also common histological types in EGCTS of retroperitoneum and sacrococcygeal region [1,2,8,9].
Compared with gonadal sites GCTs, accurately diagnosing MEGCTs by HE staining alone would be relatively more difficult, because of their nonspecific clinical symptoms and imaging characteristics. It was widely accepted that immunohistochemical staining occupies an critical role in accurate histological diagnosis. Although most tumor specimens from CNS lesions are so limited in size that it is difficult to obtain them, additional immunohistochemical staining was routinely required for confirming diagnosis. PLAP and CD117 are commonly employed as a routine diagnostic marker for seminoma/germinoma [13,14]. Our study demonstrated that the expression of PLAP and CD117 were respectively detected in 80.6% and 93.5% of the seminomas/germinomas, which agreed with several previous reports [14,15]. However, it has been recently suggested that PLAP and CD117 may have their own shortcomings. Because of its surface membrane and diffuse cytoplasmic staining, the staining background of PLAP may be so heavy that immunohistochemical evaluation will be a little challenging. With regard to CD117, it has been reported to express in various non-GCTs tissues, including gastrointestinal stromal tumors and melanomas, suggesting that it may not be a highly specific marker. Recently, Hattab et al. have found that OCT3/4 could act as a highly specific and sensitive immunohistochemical marker for detecting seminomas/germinomas [15]. In this study, OCT3/4 was expressed in 100% seminomas/germinomas cases, while OCT3/4, together with PLAP and CD117, were found to be negative in yolk sac tumors. Interestingly, the distinctively positive expression of AFP was detected in yolk sac tumors, suggesting its potential value in distinguishing these tumors from seminomas/germinomas and embryonal carcinomas. Our study also found that embryonal carcinomas showed positive staining for OCT3/4, CK AE1/3, EMA and CD30, but negative staining for PLAP and CD117. In contrast, we found choriocacinomas showed immunopositivity for βHCG, CK AE1/3 and PLAP, but negative staining for CD117, OCT3/4 and AFP. These findings were consistent with a recent work by Sugiyama et al [16].
Surgical resection is the cornerstone of treatment for EMGCTs and improved adjuvant radiotherapy and chemotherapy have ensured favorable recurrence control and survival probability [3,17-19]. It is well-established that histological subtype of EMGCTs is a decisive factor influencing therapeutic strategies and patient prognosis. In this study, seminomas/germinomas were highly sensitive to radiation therapy, with a five-year survival rate of 93.3%. Mixed GCTs, yolk sac tumors, embryonal carcinomas, and choriocarcinomas are less radiosensitive than pure seminomas/germinomas and the prognosis of patients with NS/G-GCTs prognosis was poor with five-year survival rates of 28.6%. Matsutani et al reported that 5-years survival rates of pure germinomas, mixed GCTs and immature and malignant teratomas in CNS were 95.4%, 57.1% and 70.7%, respectively, and the 1-year survival rates for embryonal carcinomas, yolk sac tumors and choriocarcinomas in CNS were 80%, 33.3% and 0, respectively [9]. Bokemeyer et al reported that 5-years overall survival rates for seminomas and nonseminoma GCTs in mediastinum and retroperitoneum were 88%, 49% and 88%, 63%, respectively [20].
In summary, EMGCTs are rare and usually arise in midline structures, including mediastinum, CNS, retroperitoneum and coccyx. The histological classification includes seminoma/germinoma and NS/G-GCTs (including immature and malignant teratomas, embryonal carcinomas, yolk sac tumors, choriocarcinomas, and mix GCTs). Seminomas/germinomas have a better prognosis than malignant NS/G-GCTs. An accurate diagnosis of EMGCTs is critical not only for predicting the tumor progression but also for patient management. Immunohistochemistry has become an important tool in the diagnosis and distinguishing the different histological types of extragonadal germ cell tumors.
Acknowledgements
This work was partly supported by the funding of Science and Technology Commission of Shanghai Municipality (NO. 134119a9502), 12DZ2260600.
Disclosure of conflict of interest
None.
References
- 1.Takeda S, Miyoshi S, Ohta M, Minami M, Masaoka A, Matsuda H. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer. 2003;97:367–376. doi: 10.1002/cncr.11068. [DOI] [PubMed] [Google Scholar]
- 2.Thakkar JP, Chew L, Villano JL. Primary CNS germ cell tumors: current epidemiology and update on treatment. Med Oncol. 2013;30:496–515. doi: 10.1007/s12032-013-0496-9. [DOI] [PubMed] [Google Scholar]
- 3.Fukui N, Kohno Y, Ishioka J, Fukuda H, Kageyama Y, Higashi Y. Treatment outcome of patients with extragonadal nonseminomatous germ cell tumors: the Saitama Cancer Certer experience. Int J Clin Oncol. 2013;18:731–734. doi: 10.1007/s10147-012-0436-2. [DOI] [PubMed] [Google Scholar]
- 4.Grammatikopoulou I, Kontomanolis EN, Chatzaki E, Chouridou E, Pavlidis P, Papadopoulos EM, Lambropoulou M. Immature malignant sacrococcygeal teratoma: case report and review of the litrerature. Clin Exp Obstet Gynecol. 2013;40:437–439. [PubMed] [Google Scholar]
- 5.Iczkowski KA, Butler SL, Shanks JH, Hossain D, Schall A, Meiers I, Zhou M, Torkko KC, Kim SJ, MacLennan GT. Trials of new germ cell immunohistochemical stains in 93 extragonadal and metastatic germ cell tumors. Hum Pathol. 2008;39:275–281. doi: 10.1016/j.humpath.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Biermann K, Klingmuller D, Koch A, Pietsch T, Schorle H, Buttner R, Zhou H. Diagnostic value of markers M2A, OCT3/4, AP-2gamma, PLAP and c-KIT in the detection of extragonadal seminomas. Histopathology. 2006;49:290–297. doi: 10.1111/j.1365-2559.2006.02496.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- 8.Albany C, Einhorn LH. Extragonadal germ cell tumors: clinical presentation and management. Curr Opin Oncol. 2013;25:261–265. doi: 10.1097/CCO.0b013e32835f085d. [DOI] [PubMed] [Google Scholar]
- 9.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 10.Paradies G, Zullino F, Orofino A, Leggio S. Rare extragonadal teratomas in children: complete tumor excision as a reliable and essential procedure for significant survival. Clinical experience and review of the literature. Ann Ital Chir. 2014;85:56–68. [PubMed] [Google Scholar]
- 11.Wada Y, Yokoyama T, Yamamoto H, Hanaoka M, Kawakami S, Koizumi T. Primary nonseminomatous germ cell tumor in the posterior mediastinum. Respirol Case Rep. 2014;2:45–47. doi: 10.1002/rcr2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur H, Singh D, Peereboom DM. Primary central nervous system germ cell tumors. Curr Treat Options Oncol. 2003;4:491–498. doi: 10.1007/s11864-003-0049-0. [DOI] [PubMed] [Google Scholar]
- 13.Hoei-Hansen CE, Sehested A, Juhler M, Lau YF, Skakkebaek NE, Laursen H, Rajpert-de Meyts E. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: expression of pluripotency and cell differentiation markers. J Pathol. 2006;209:25–33. doi: 10.1002/path.1948. [DOI] [PubMed] [Google Scholar]
- 14.Takeshima H, Kuratsu J. A review of soluble c-kit (s-kit) as a novel tumor marker and possible molecular target for the treatment of CNS germinoma. Surg Neurol. 2003;60:321–4. doi: 10.1016/s0090-3019(03)00430-0. discussion 324-5. [DOI] [PubMed] [Google Scholar]
- 15.Hattab EM, Tu PH, Wilson JD, Cheng L. OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am J Surg Pathol. 2005;29:368–371. doi: 10.1097/01.pas.0000149709.19958.a7. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama K, Arita K, Tominaga A, Hanaya R, Taniguchi E, Okamura T, Itoh Y, Yamasaki F, Kurisu K. Morphologic features of human chorionic gonadotropin- or alpha-fetoprotein-producing germ cell tumors of the central nervous system: histological heterogeneity and surgical meaning. Brain Tumor Pathol. 2001;18:115–122. doi: 10.1007/BF02479424. [DOI] [PubMed] [Google Scholar]
- 17.Frazier AL, Hale JP, Rodriguez-Galindo C, Dang H, Olson T, Murray MJ, Amatruda JF, Thornton C, Arul GS, Billmire D, Shaikh F, Pashankar F, Stoneham S, Krailo M, Nicholson JC. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United kingdom and United States. J. Clin. Oncol. 2015;33:195–201. doi: 10.1200/JCO.2014.58.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa K, Toita T, Nakamura K, Uno T, Onishi H, Itami J, Shikama N, Saeki N, Yoshii Y, Murayama S. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer. 2003;98:369–376. doi: 10.1002/cncr.11495. [DOI] [PubMed] [Google Scholar]
- 19.De Latour B, Fadel E, Mercier O, Mussot S, Fabre D, Fizazi K, Dartevelle P. Surgical outcomes in patients with primary mediastinal non-seminomatous germ cell tumours and elevated post-chemotherapy serum tumour markers. Eur J Cardiothorac Surg. 2012;42:66–71. doi: 10.1093/ejcts/ezr252. [DOI] [PubMed] [Google Scholar]
- 20.Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, Gerl A, Fossa SD, Beyer J, Pont J, Kanz L, Einhorn L, Hartmann JT. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J. Clin. Oncol. 2002;20:1864–1873. doi: 10.1200/JCO.2002.07.062. [DOI] [PubMed] [Google Scholar]


