Abstract
Objectives: This study aimed to simultaneously observe the expression of mononuclear cells (Mo) and plasma tissue factor (TF) in patients with ischemic cardiocerebrovascular diseases during the stage of acute onset and after the following three weeks and three months for exploration of the clinical implications concerned. Methods: MoTF mRNA and plasma TF antigen (TFAg) from 76 patients with acute myocardial infarction (AMI) together with 46 patients with acute ischemic stroke (AIS) and 61 healthy controls were quantitated respectively through RT-PCR and ELISA. Results: Compared with the results in the control group, the level of MoTFmRNA and plasma TF in the other groups increased simultaneously and dramatically in the acute stage, which showed a good correlation among the groups (P<0.01), especially in AIS group. The quantitative data showed that both MoTF mRNA and plasma TF remained higher than that of the control group (P<0.01 and P<0.05) after three weeks from the acute onset. It was after three months that the content of MoTF mRNA, in spite of its relatively high level (P<0.05), began to decline in AMI and AIS groups. In this stage the level of MoTFmRNA in AIS group was lower than that in the acute onset stage (P<0.05), while the reduction of plasma TF in AMI and AIS groups was not significantly different from that of the control group (P>0.05). However, the reduced level of plasma TF was still different from that in the acute onset stage (P<0.05). Conclusion: The simultaneous increase of the level of peripheral MoTF mRNA and plasma TF in the acute onset stage of ischemic cardiocerebrovascular diseases shows a good correlation and suggests the up-regulation of MoTF mRNA’s expression participates in the maintenance and expansion of thrombotic formation. Dynamic monitoring of MoTF mRNA and plasma TF at different time points after acute onset has important clinical implications for prevention and treatment of arterial thrombotic diseases.
Keywords: Myocardial infarction, brain ischemia, tissue factor (TF), monocytes
Introduction
Previous studies reported that tissue factor (TF), which is released following vascular and endothelial injuries, is the most important source of TF and is necessary for thrombotic formation. With a better understanding about blood-borned TF in recent years, the role of cell-derived TF in the formation of pathologic thrombosis has aroused attention increasingly. Mononuclear cells (Mo), a kind of blood cells, were originally found to express TF and considered as the only generally accepted blood cells that can intrinsically synthesize TF. Cellular activation can induce the release of TF into the surface of cell membranes or plasma and initiate the coagulation cascade [1]. Mo is distributed throughout human’s body, serving as a mobile TF-supply base, and the reason that other cell types in the blood also contain TF is more or less related to [2]. It has been demonstrated that abnormal expression of large amounts of TF in Mo (MoTF) is closely associated with the development and progression of atherosclerosis and thrombotic formation [3]. The association between MoTF activity and acute myocardial infarction (AMI) has also been reported [4]. However, increased TF activity does not necessarily mean that the expression is increased; it may also be due to the decrease of the tissue factor pathway inhibitor (TFPI) or change in other anticoagulants. As previously mentioned [5], TF was increased on Mo surfaces in AMI patients. However, the change on cytomembrane surfaces may also be due to intracellular change of TF distribution. Whether the increase of MoTF mRNA alone can determine the up-regulation of TF expression remains unclear.
In order to explore the pathogenesis of thrombotic diseases and disorders and figure out simple hematological markers for early warning and prognostic prediction of acute thrombotic events, MoTF mRNA levels and plasma TF protein levels from patients with ischemic cardiocerebral diseases were simultaneously observed in acute onset and at different time points after the onset.
Materials and methods
Subjects
Control group
61 clinically healthy subjects (32 male with a mean age of 59.5 (45~72) years) recruited from the health-care staff served as controls.
Inclusion criteria
Healthy subjects without cardiocerebral disease, diabetes, renal disease, hypertension, hyperlipidemia, peripheral vascular disease, hematological disease and tumors (Of the 61 subjects, 12 had a smoking history or a history of quitting smoking for more than three years, and 3 had alcohol consumer history and a history of quitting alcohol for more than a year).
No anticoagulant or lipid-regulating drug was administered within two weeks before examination in any subject included.
Subjects were non-blood related individuals.
AMI group
AMI group included 76 AMI patients (53 males and 23 females) with a mean age of 61.5 (32-90) years, among which 46 (60%) patients were smokers, 39 (51%) patients were alcoholics, 45 (59.2%) patients were hypertensive, 20 (26.3%) patients were diabetic, and 19 (25%) patients had coronary heart disease (CHD).
Acute ischemic stroke AIS group
AIS group included 46 AIS patients (27 males and 19 females) with a mean age of 62.6 (37-84) years, among which 18 (39%) patients were smokers, 16 (35%) patients were alcoholics, 29 (63%) patients were hypertensive, 15 (33%) patients were diabetic, and 6 (13%) patients had coronary heart disease (CHD).
Blood samples of the patient groups were collected from AMI and AIS patients who were admitted and received treatment in the cardiocerebrovascular disease departments, intensive care units and emergency centers of Yangpu District Central Hospital affiliated to Shanghai Tongji University (Shanghai, China) and the affiliated hospital of Xinjiang Medical University (Xinjiang, China) in the same period from October 2006 to June 2011. Acute-onset blood samples signify to the blood samples collected instantly from patients simply after hospital admission without any following treatments, and after-onset blood samples were collected from treated patients after three weeks or three months from the stage of acute onset. Fasting blood samples were collected from the healthy control subjects.
Exclusion criteria
AMI patients who had received coronary artery intervention therapy.
AMI patients who had old MI, re-infarction and post-infarction angina pectoris.
AIS patients who had other nervous system diseases and cerebral hemorrhage. Complicated infectious diseases or other inflammatory clues, complicated heart failure, severe hepatic and renal diseases, new surgical traumas and autoimmune diseases were also excluded.
All participants gave written informed consent and the study protocol was approved by the Ethical Committee of Institution.
Main instruments and reagents
The TF antigen (TFAg) ELISA kit (American Assaypro). RT-PCR primer, Trizol, first-strain cDNA reagent kits, electrophoresis PCR core kit, TF and internal reference ACTIN were provided by Shinegene (China). ELX800enzyme labeling instrument was a product of Bio-Tek instruments Inc. (America). The PCR instrument was MJ150.
Detection of plasma TFAg by ELISA
0.109 mol/L sodium citrate was mixed with the venous blood sample at 1:9 and centrifuged at 3000 rpm for 10 min within 30 min. The isolated plasma was stored at -70°C. TFAg was detected using ELISA (American Assaypro) according to the manufacture’s instruction within 2 months
Detection of MoTF mRNA xpression by RT-PCR
Heparin-based anticoagulation during peripheral Mo collection was performed by the two density gradient centrifugation [6,7] and the expression level of MoTF mRNA was analyzed by RT-PCR. The purity of the isolated circulating Mo was 90~95%, which were identified as positive by non-specific esterase stain and inhibited by sodium fluoride. Cell viability was greater than 90%, which was tested by in vivo trypan blue staining.
To synthesize the first strand of CDNA, RNA was reverse-transcribed in accordance with the manufacture of the RT-PCR reverse transcriptase kit (Takara, Japan). β-actin was used as internal reference. The primers were as followed. TF (255bp), upstream primer: 5’-CACCGACGAGATTGTGAAGG-3’, downstream primer: 5’-CGGAGGCTTAGGAAAGTGTTG-3’; β-actin (268 bp), upstream primer: 5’-ctc cat cct ggcctcgctgt-3’, downstream primer: 5’-gct gtcaccttcaccgttcc-3’. RT-PCR amplification is as follows: 25°C for 10 min, 40°C for 50 min, 90°C for 5 min.
The PCR program is as follows: 94°C for 4 min, 94°C for 30 s, 58°C for 30 s, 72°C for 60 s, totaling 35 cycles. Agarose gel electrophoresis and quantitative analysis were performed according to Molecular Cloning: A Laboratory Manual. The absorbance ratio of the target segment and β-actin bands were defined as the level of MoTFmRNA expression.
Statistical analysis
Data are expressed as x̅±s unless otherwise indicated. Groups were compared using ANOVA with Tukey’s post test. Adjustment for age, gender was performed by covariance analysis ANCOVA on log-transformed data. Only P<0.05 was regarded as statistically significance. Correlation between plasma TF and MoTF was analyzed by Pearson product moment correlation coefficient. All analyses were performed with SPSS13.0.
Results
Comparison of the clinicopathological data between the patient and control groups
The ratio of smoking, CHD and diabetes was significantly higher in AMI and AIS groups than that in the control group (P<0.05). Significantly lower HDL-C level and significantly higher LDL-C level were observed in AIS group compared with that in the control group (1.03±0.26 vs. 1.43±0.31, P<0.05; 3.36±1.42 vs. 2.15±0.85, P<0.05). In addition, blood glucose in both AMI and AIS groups was significantly higher than that in the control group (7.50±3.3 and 6.83±3.0 vs. 4.76±0.79, P<0.01 and P<0.05). There was no significant difference in blood lipid level between AMI group and the control group.
Dynamic observation of MoTF mRNA and plasma TFAg levels at different time points in AMI and AIS groups
As shown in Table 1 and Figure 1, the expression of peripheral MoTF mRNA and plasma TF during the acute onset period was significantly higher in AMI and AIS patients than that in the control group, especially in AIS group (P<0.01). However, there was no significant difference in the level of peripheral MoTF mRNA and plasma TF between AMI and AIS groups (P>0.05). Whether during the acute onset stage or at subsequent dynamic observation points, MoTF mRNA and plasma TF increased or decreased simultaneously in both AMI and AIS groups.
Table 1.
Comparison of MoTF and plasma levels between the patient and control groups (x̅±s)
| Item | Control (n=61) | AMI | AIS | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Acute onset (n=76) | 3 w (n=69) | 3 mon (n=62) | Acute onset (n=46) | 3 w (n=41) | 3 mon (n=36) | |||
| MOTF mRNA | 0.254±0.038 | 0.297±0.056** | 0.294±0.049** | 0.286±0.048* | 0.313±0.093** | 0.311±0.090** | 0.282±0.041*,▲ | |
| Plasma TFAg | 197.18±94.91 | 249.50±106.45* | 241.70±108.23* | 209.80±99.74Δ | 279.70±109.50** | 250.60±108.11* | 211.46±105.14Δ | |
Note: compared with control;
P<0.05;
P<0.01.
Compared with AIS acute onset;
P<0.05.
Compared with AMI and AIS acute onset;
P<0.05.
Figure 1.
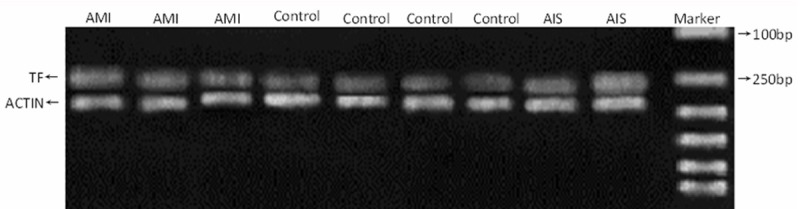
Electrophoresis of MoTFmRNA expression in different groups (Marker from up to below: 100, 250, 500, 750, 1000 and 2000 bp).
Analysis on the characteristics of TF change in AMI and AIS groups
Compared with the control group, simultaneous increase of MoTF mRNA and plasma TF was observed in 54 of the 76 patients with AMI group during the acute onset period; increase in MoTF mRNA level alone in three patients; and increase in plasma TF alone in four patients. In the 46 AIS patients, simultaneous increase in MoTF mRNA and plasma TF was observed in 31 patients; increase in MoTF mRNA level alone in three patients; and increase in plasma TF alone in four patients.
Compared with the acute onset period, no obviously change of TF level was observed in AMI and AIS groups at 3 weeks after onset. Both MoTF mRNA and plasma TF levels decreased at 3 months after onset in both AMI and AIS groups, while plasma TF decrease was more pronounced. However, TF level remained at a relatively high level in 9 AMI patients and 4 AIS patients. MoTF mRNA restored relatively slowly in both groups, and there remained a significant difference as compare with the control group (P<0.05). In two patients who developed recurrent unstable angina pectoris in the post-infarction early stage, MoTF mRNA and plasma TF levels remained at a high level at 3 months, and were significantly higher than those in patients without recurrent angina pectoris.
Correlations between Mo TF mRNA and plasma TFAg in AMI, AIS and control groups
Pearson correlation coefficient analysis showed that the correlation coefficient (r) between Mo TFmRNA and plasma TFAg was 0.565 in the control group, 0.680 in AMI group, and 0.568 in AIS group (P<0.05), and there was no significant difference between the groups (P>0.05).
Discussion
Tissue factor is an initiator of the TF pathway, playing a key role in initiating the coagulation process and pathologic thrombotic formation in vivo, especially in the development of atherosclerosis and CHD. It used to believe that blood cells and vascular endothelial cells (VEC) can directly contact with the circulating blood and do not express TF under normal physiological conditions. As there is no TF in the circulating blood, coagulation could not be initiated directly. Only when tissue cells are exposed due to vascular damage or some cytokines stimulation, the secretion of TF by VEC and mononuclear cells was increased which initiated the coagulation process and thrombotic formation [1,8,9].
However, it is currently believed that TF exists not only in extravascular tissue cells but in the circulating blood. TF produced by Mo is the main source of hematogenous TF [1.9]. MoTF can be released to the cell membrane surface or plasma upon activation, thus initiating the coagulation process independently [10]. Although TF in the blood may come from Mo or VEC, the former are more sensitive to proinflammatory mediators than the latter. In addition, Mo can up-regulate VEC TF. TF in atherosclerotic plaques is synthesized by foam cells, while foam cells were evolved from Mo. As MoTF gene expression is regulated at the transcription level, the expression level of MoTF mRNA can reflect TF gene transcription level. In addition, simultaneous detection of plasma TF at different time points can further indicate the change of TF gene expression so as to mirror the true situation of blood coagulation. For this reason, using Mo as the target point at the cellular and molecular levels can not only help elucidate the mechanism of pathological hemostasis and pathological thrombosis but help the prevention and treatment of arterial thrombotic diseases.
The results of the present study showed the following characteristics: circulating MoTF mRNA and plasma TFAg levels increased simultaneously during the acute onset period in both AMI and AIS groups. Correlation analysis showed that the expression of MoTFmRNA was closely associated with plasma TFAg change under either physiological or pathological conditions, showing a good correlation between them. At 3 weeks after onset, plasma TF level remained at a relatively high level; and at 3 months both MoTFmRNA and TFAg showed a simultaneous decrease. It is noteworthy that MoTF increased more significantly than TF either during the acute onset period or 3 weeks after onset in both AMI and AIS patients, while the 3-month follow-up showed that plasma TF decreased more significantly than MoTF, which was significantly different from the situation during the acute stage. It was reported in the literature [11-13] that in AMI group both plasma TFAg and TFact increased significantly during the acute onset period. Some other studies [14] reported that increased TF expression is the main cause of thrombotic formation in atherosclerosis, and that TF gene is associated with the occurrence of AIS. Experimental studies [15] demonstrated that TF-FVIIa could not only trigger the occurrence of thrombotic events but alter vascular remodeling via the promoting effect of migration suggesting that AMI and AIS induced by atherosclerotic plaque rupture is associated with TF initiating the coagulation process. Bilgen et al [16] reported that Mo had a stronger ability tp produce TF in circulating blood of patients with unstable angina pectoris, which may increase the production of thrombin and promote thrombotic formation, thus increasing the instability of atherosclerotic plaques. It was TF expression and increased promoting activity of peripheral Mo that led to the high coagulation state or even thrombosis in microvessels in patients with acute coronary artery syndrome. Some other studies [5] also found that both MoTFact and TFAg were increased in AMI patients. He et al [6] demonstrated that Ang II could induce MoTF expression, and that it acted on MoTF activity and antigen by regulating the mRNA level. The result of the present study is consistent with previous reports in terms of both plasma TF and MoTF expressions. The highlight of the present study is that we further confirmed that these changes depended on the upregulation of MoTFmRNA expression. We also address that although acute ischemic cardiocerebrovascular events are mainly and closely attributed to the initial stage of local thrombotic formation triggered by TF release from atherosclerotic plaques, the up-regulation of TF expression in peripheral Mo not only participates in the maintenance of thrombosis but enhance the procoagulatory activity in the circulating blood, which may be an important reason why thrombosis and vascular re-obstruction are more likely to occur after success of the initial treatment, suggesting that MoTF expression may be an important factor causing acute thrombosis. Is it because of excessive accumulation of Mo on the vascular wall that causes MoTF to remain at a high level during convalescence, or because the circulating blood has a stronger ability to produce Mo during the arterial events so that the coagulatory activity is increased and thrombosis is formed, resulting in increased instability of atherosclerotic plaques? The question needs to be further discussed. Data of the present study also show that plasma TF, MoTF level in AIS patients was significantly higher than that in AMI. Other experimental studies [17] also demonstrated that high TF expression was mainly detected in tissues and organs with rich blood supply such as the lung, brain and embryonic tissue. The reason that blood lipid levels varied greatly in AIS patients may be due to increased LDL-C level in these patients, because plasma TF is more likely to increase in patients with relatively high LDL-C levels [18,19]. An overview of the above results suggests that there may be some differences in the mechanism underlying the occurrence of cardocerebrovascular events such as differences in cytokines, inflammatory stimulations or TFPI distribution and expression, all of which may affect the regulation of MoTF [8,20]. Of course, this postulation needs to be verified in further studies.
With respect to the associated between TF level and diseased conversion, it may be related to the significant increase in TF during the acute onset stage and multiple high risk factors of the patients. In AIS group, three patients were older than 70 years and all had hypertension, and two of them also had diabetes. There is one AIS patient whose level of MoTF and plasma TF were both higher than that of the other patients either during the acute stage or 3 weeks after onset. His TF level after three months from the acute onset was still higher than that of the control subjects. In another case: plasma TF level of one male patient in AMI group after three weeks and three months from the acute onset was lower than that during the acute stage, but the percentage of decrease was only 8% and 28% respectively. It should be noted that this patient had hypertension, diabetes and a long history of smoking. In other case of two old female AMI patients, both TF and MoTF levels during the acute stage were significantly higher than those of the other patients. They developed recurrent unstable angina pectoris in the post-infarction early stage. TF and MoTF remained at a high level at the 3-month follow-up visits. They had a history of essential hypertension for 15 and 28 years respectively, in addition to hypercholesterolemia. Steffel et al. [21] showed that plasma TF was elevated in patients with hypertension, diabetes, abnormal lipidemia, smoking and acute coronary artery syndrome who had high risk of developing cardiovascular diseases. Based on these findings, it could be concluded that TF level could indirectly reflect the unstable state of thrombosis.
In summary, the present study suggested that MoTF mRNA plays a key role in acute arterial thrombosis and event maintenance and has a good correlation with plasma TF. Simultaneous observation on the variation of the level of MoTF and plasma TF at different time points after acute onset of cardiocerebrovascular disease has important clinical implications in the prevention and treatment in the field of arterial thrombotic diseases.
Disclosure of conflict of interest
None.
References
- 1.Osterud B, Olsen JO, Bjørklid E. What is blood borne tissue factor? Thromb Res. 2009;124:640–41. doi: 10.1016/j.thromres.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Jiang DJ, Cao Y, Xin HY, Li XH, Luo ZQ, Li YJ. Asymmetric dimethylarginine induces tissue factor expression in monocytes Via NF- kappaB-dependent pathway: Role in acute coronary syndromes. Atherosclerosis. 2009;205:554–60. doi: 10.1016/j.atherosclerosis.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, Leukocytes and platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008;28:947–53. doi: 10.1161/ATVBAHA.107.161471. [DOI] [PubMed] [Google Scholar]
- 4.Ott I, Andrassy M, Zieglgänsberger D, Geith S, Schömig A, Neumann FJ. Regulation of monocyte procoaguloant activity in acute myocardial infarction: role of tissue factor and tissue factor pathway inhibitor-1. Blood. 2001;97:3721–26. doi: 10.1182/blood.v97.12.3721. [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Song KS, Park YS, Yun YS, Shim WH. Changes of plasma tissue factor and tissue factor pathway inhibitor antigen levels and induction of tissue factor expression on monocytes in coronary artery disease. Cardiology. 2000;93:31–6. doi: 10.1159/000006999. [DOI] [PubMed] [Google Scholar]
- 6.He M, He X, Xie Q. Angiotensin II induces the expression of tissue factor and its mechanism in human monocytes. Thromb Res. 2006;117:579–90. doi: 10.1016/j.thromres.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Osterud B. Tissue factor expression in blood cells. Thromb Res. 2010;125(Suppl 1):S31–4. doi: 10.1016/j.thromres.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Basavaraj MG, Gruber FX, Sovershaev M, Appelbom HI, Osterud B, Petersen LC, Hansen JB. The role of TFPI in regulation of TF-induced thrombogenicty on the surface of human monocytes. Thromb Res. 2010;126:418–25. doi: 10.1016/j.thromres.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Halim AG, Hamidon BB, Cheong SK, Raymond AA. The prognostic value of tissue factor levels in acute ischaemic stroke. Singapore Med J. 2006;47:400–3. [PubMed] [Google Scholar]
- 10.Osterud B, Bjorklid E. Tissue factor in blood cells and endothelial cells. Front Biosci (Elite Ed) 2012;4:289–99. doi: 10.2741/e376. [DOI] [PubMed] [Google Scholar]
- 11.Steppich BA, Braun SL, Stein A, Demetz G, Groha P, Schömig A, von Beckerath N, Kastrati A, Ott I. Plasma TF activity predicts cardiovascular mortality in patiens with acute myocardial infarction. Thromb J. 2009;7:11. doi: 10.1186/1477-9560-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osterud B. Tissue factor/TFPI and blood cells. Thromb Res. 2012;129:274–78. doi: 10.1016/j.thromres.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Campo G, Valgimigli M, Ferraresi P, Malagutti P, Baroni M, Arcozzi C, Gemmati D, Percoco G, Parrinello G, Ferrari R, Bernardi F. Tissue factor and coagulation factor VII levels during acute myocardial infarction: association with genotype and adverse events. Arterioscler Thromb Vasc Biol. 2006;26:2800–6. doi: 10.1161/01.ATV.0000247249.82030.94. [DOI] [PubMed] [Google Scholar]
- 14.DE Gaetano M, Quacquaruccio G, Pezzini A, Latella MC, DI Castelnuovo A, Del Zotto E, Padovani A, Lichy C, Grond-Ginsbach C, Gattone M, Giannuzzi P, Nowak M, Dorn J, Trevisan M, Donati MB, Iacoviello L. Tissue factor gene polymorphisms and naplotypes and the risk of ischemic vascular events: Four studies and meta-analysis. J Thromb Haemost. 2009;7:1465–71. doi: 10.1111/j.1538-7836.2009.03541.x. [DOI] [PubMed] [Google Scholar]
- 15.Demetz G, Seitz I, Stein A, Steppich B, Groha P, Brandl R, Schömig A, Ott I. Tissue factor-factor VIIa complex induces cytokine expression in coronary artery smooth muscle cells. Atherosclerosis. 2010;2212:466–71. doi: 10.1016/j.atherosclerosis.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Bilgen D, Sonmez H, Ekmekei H. The relationship of TFPI, LP (a), and oxidized LDL antibody Levels in patients with coronary artery disease. Clin Biochem. 2005;38:92–6. doi: 10.1016/j.clinbiochem.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 18.Sambola A, Osende J, Hathcoc J, Degen M, Nemerso Y, Fuster V, Crandall J, Badimon JJ. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation. 2003;107:973–77. doi: 10.1161/01.cir.0000050621.67499.7d. [DOI] [PubMed] [Google Scholar]
- 19.Basavaraj MG, Sovershaev MA, Egorina EM, Gruber FX, Bogdanov VY, Fallon JT, Østerud B, Mathiesen EB, Hansen JB. Circulating monocytes mirror the imbalance in TF and TFPI expression in carotid atherosclerotic plaques with lipid-rich and calcified morphology. Thromb Res. 2012;129:e134–41. doi: 10.1016/j.thromres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Basavaraj MG, Osterud B, Hansen JB. Influence of different anticoagulants on monocyte procoagulant functions and monocyte-platelet aggregates formation. J Thromb Haemost. 2012;10:1698–702. doi: 10.1111/j.1538-7836.2012.04821.x. [DOI] [PubMed] [Google Scholar]
- 21.Steffel J, Luscher TF, Tanner FC. Tissue Factor in Cardiovascular diseases molecular mechanisms and clinical implications. Circulation. 2006;113:722–31. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]


