Abstract
Objectives: Post-operative stiffness is common after rotator cuff repair, given the difference in susceptibility and severity, the genetic factors may be involved. Interleukin 6 (IL-6) and Matrix metalloproteinases 3 (MMP-3) were previous found as key cytokines in the pathologies of adhesive capsulitis. The present study aims to investigate whether variants within the IL-6 and MMP-3 gene contributed to post-operative stiffness in a Chinese Han population. Methods: A total of 188 patients diagnosed with rotator cuff tears treated with mini-open surgery were enrolled in this study, among which 87 patients were diagnosed as post-operative stiffness and the remaining 101 patients as controls. All subjects were genotyped for IL-6 and MMP-3 SNPs. Results: The rs1800796 of IL-6 and rs679620 of MMP-3 were found significantly associated with increased susceptibility and severity of post-operative stiffness. Conclusion: The rs1800796 SNP of IL-6 and rs650108 SNP of MMP-3 were associated with increased risk of post-operative stiffness susceptibility and severity. This finding can be used in guiding the rehabilitation procedure after rotator cuff surgery, in another word, those with the genetic susceptibility factors should receive a more radical rehabilitation procedure and those without the susceptibility factors can be more conservative.
Keywords: IL-6, MMP-3, SNP, postoperative stiffness
Introduction
Rotator cuff (RC) injuries in the shoulder account for overuse injuries in sports as well as in jobs that require repetitive activity, which accounts for more than 4.5 millions physician visits per year [1-4]. Excessive mechanical loading is considered the major causation factor. When RC tears are symptomatic and nonoperative management fails, they are typically repaired surgically, with over 250 000 RC repair surgeries performed annually in the United States [5]. The RC has limited ability to heal back to its insertion on the humerus [6], as a result, the retear rates after RC repair have been reported to be as high as 70% for large tears (>5 cm) and 20% for small tears (≤3 cm) [7]. Conservative rehabilitation after RC repair is important for a successful outcome, nowadays, the patients are required for a long-term immobilization to decrease the retear rate. The rehabilitation procedure like passive motion exercise is usually delayed as the early motion may influent the tendon healing and increase the retear rate. Unfortunately, an important complication observed during long-term immobilization is the post-operative stiffness, which decreases the surgical effect and quality of life significantly [8-10].
Stiffness is affected by many factors including patient comorbidities such as diabetes mellitus, proneness to scar formation, preoperative shoulder stiffness, surgical technique, and postoperative rehabilitation [11]. For example, surgical repair techniques for RC repair can be divided into open, mini-open, and arthroscopic approaches. Because of advances in skill and technology, arthroscopic repair has emerged as similar effect and less post-operative stiffness, which has gradually replaced the open technique. Though the arthroscopy procedure is minimal invasive, post-operative stiffness is not totally avoided. Controversy still exists regarding the best time to initiate the passive range of motion (ROM) exercise to balance the rate of common stiffness and retear. Importantly, the fact is not all patients face the problem of post-operative stiffness and not all patients face the same severity. It seems the genetic factors may play an important role in it, among which, the single nuclear polymorphism (SNP) may be involved.
Post-operative stiffness, also known as adhesive capsulitis (AC), involves thickened capsule because of chronic inflammatory infiltrate and moderate to extensive subsynovial fibrosis, resulting in obliteration of the normal patulous axillary fold by adhesions and fibrosis of the capsule itself [12,13]. Previous results demonstrate that levels of inflammatory (interleukin 6, IL-6) and fibrogenic cytokines (Matrix metalloproteinases 3, MMP-3) are elevated in the synovium of patients with AC compared with controls. Given the genetic factors like SNP may be involved in the pathologies of post-operative stiffness, the gene polymorphism of IL 6 and MMP 3, which are elevated in AC, may associated with the risk of post-operative stiffness.
Accordingly, this study aims to determine whether the IL-6 SNPs (rs10499563, rs1800796) and MMP-3 SNPs (rs679620, rs591058 and rs650108) were associated with post-operative stiffness in Chinese Han Populations.
Patients and methods
The study was approved by the ethics committee of the Qilu Hospital of Shandong University, and informed consent was obtained from patients and control participants.
Study population
A total of 188 patients diagnosed with rotator cuff tears treated with mini-open surgery in Qilu Hospital of Shandong University were recruited in this study. All subjects included in this study were Chinese Han Population. Patients were recruited among those suffered from persistently painful cuff tear and undergo a mini-open rotator cuff repair in Qilu hospital. And patients were excluded with radiographic glenohumeral arthritis, concomitant glenohumeral injuries, an irreparable tendon injury, post-operative infection, and performed revision cuff repair. Patients who needed concomitant procedures such as distal clavicular excision and biceps tenotomy and/or tenodesis were retained.
Mini-open repair technique
Each patient was treated with mini-open repair technique by the same operator. All patients were operated under general anesthesia in the lateral decubitus position with the arm held in a 3-point shoulder distraction device. The shoulder was prepped and draped in the usual sterile fashion. Standard posterior and anterior portals were used for the glenohumeral joint exploration for subacromial decompression. The anterolateral approach was enlarged to 3-5 cm parallel to the deltoid fibers to minimize damage to the deltoid. The tear was gain visualized, and the footprint was prepared for reinsertion of the tendon. The supraspinatus was repaired by use of 1-3 bioabsorbable suture anchors armed with 2 pairs of nonabsorbable No. 2 sutures. Side-to-side FiberWire sutures were used if necessary. The tendon was sutured to the bone via the modified Mason-Allen technique with the combination of a U-shaped mattress suture and single suture on top of the mattress suture.
Postoperative rehabilitation protocols
All patients were immobilized with the abduction brace at 30° for 6 weeks. Shrugging of shoulders, active elbow flexion/extension, active forearm supination/pronation, and active hand and wrist motion were encouraged immediately after surgery. Controlled early passive motion exercise consisting of forward flexion, abduction, and external rotation was conducted twice a day from 3 weeks after the operation. Muscle strengthening was usually initiated at 9 to 12 weeks postoperatively, and all sports activities were permitted from 6 months after the operation.
Clinical evaluations and outcomes
Passive range of motion was recorded with a goniometer for flexion, external rotation with the arm at the side, and external rotation with the shoulder abducted 90°. Internal rotation was recorded by the maximal height achieved with the thumb when attempting to reach behind the back. Subjects were followed at 3 months postoperatively for a complete clinical examination. The definition of the post-operative stiffness was the limitation of passive motion in at least 2 directions (abduction and forward flexion <100°, external rotation <20°, or internal rotation <L3) in this study.
Genotyping
DNA samples were extracted from whole-blood samples with the Chelex-100 method according to the manufacturer’s instructions [14]. The alleles of the IL 6 and MMP 3 SNPs were genotyped by the 5’-nuclease TaqMan assay. The primers and probes were designed and synthesized by Sigma (Sigma-Proligo, The Woodlands, TX). A laboratory personnel blinded to the case-control status performed the assessment of genotyping results. All assays were carried out in 96-well arrays in triplicate for quality control.
Statistical analysis
The Statistical Package for Social Sciences software (SPSS, Inc., Chicago, IL, USA), version 16.0 for Windows were used for statistical analysis. The demographic and clinical data were presented as Mean ± SD and compared between groups by the Student’s t-tests. The genotype and allelic frequencies were evaluated by Hardy-Weinberg equilibrium and compared by the Chi-square test. The association between the SNPs and post-operative stiffness was assessed with allele level comparison, with dominant model comparison, with recessive model comparison, and with the extreme genotype comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The patients were grouped according to the ROM evaluation at 3 months post-operative. The definition of the post-operative stiffness was the limitation of passive motion in at least 2 directions (abduction and forward flexion <100°, external rotation <20°, or internal rotation <L3) in this study. As a result, among the 188 patients, 87 patients was diagnosed as post-operative stiffness and the remaining 101 patients defined as controls. Demographic data of the population studied and the number of individuals in each group were shown in Table 1. There were no significant differences between groups in terms of age, gender, dominant arm, diabetes/hypertension/thyroid disease status, smoking, and pre-operation stiffness status. However, the patients with post-operative stiffness appeared to suffer from larger cuff tear than the patients with no post-operative stiffness (P=0.043).
Table 1.
Summary of the basic characteristics of the groups
| Post-operative stiffness | Control | P value | ||
|---|---|---|---|---|
| No. of patients | 87 | 101 | ||
| Age (year) | 62.4±5.7 | 60.7±5.3 | 0.627 | |
| Gender | male | 39 | 49 | 0.661 |
| female | 48 | 52 | ||
| Dominant arm | right | 83 | 95 | 0.754 |
| left | 4 | 6 | ||
| Comorbidities (n) | Diabetes | 15 | 14 | 0.549 |
| Hypertension | 26 | 38 | 0.544 | |
| Thyroid disease | 5 | 7 | 0.775 | |
| Smoking (n) | Yes | 45 | 47 | 0.559 |
| No | 42 | 54 | ||
| Tear size | Small | 45 | 66 | 0.043 |
| Medium | 25 | 27 | ||
| Large | 17 | 8 | ||
| Pre-operative stiffness | Yes | 26 | 35 | 0.534 |
| No | 61 | 66 | ||
Association of IL-6 and MMP-3 polymorphisms with post-operative stiffness susceptibility
As expected, the distribution of the genotypes of SNPs of IL-6 and MMP-3 gene conformed to the Hardy-Weinberg equilibrium and the genotyping success rate was 100%. Tables 2 and 3 listed the genotyped and allele distributions of the 5 SNPs for the cases and controls.
Table 2.
Genotype and allele distributions of the 2 SNPs of IL-6 for the cases and controls
| Group | rs10499563 | ||||||
|
| |||||||
| TT | CC | TC + CC | TT + TC | CC | T (%) | C (%) | |
|
| |||||||
| Control | 66 | 3 | 35 | 98 | 3 | 81.2 | 18.8 |
| Case | 53 | 4 | 34 | 83 | 4 | 78.2 | 21.8 |
| OR (95% CI) | / | 1.660 (0.356 to 7.748) | 1.210 (0.668 to 2.192) | / | 1.574 (0.342 to 7.239) | / | 1.204 (0.968 to 1.498) |
| P | / | 0.700 | 0.547 | / | 0.706 | / | 0.107 |
|
| |||||||
| Group | rs1800796 | ||||||
|
| |||||||
| CC | GG | CG + GG | CC + CG | GG | C (%) | G (%) | |
|
| |||||||
| Control | 61 | 5 | 40 | 96 | 5 | 77.7 | 22.3 |
| Case | 38 | 11 | 49 | 76 | 11 | 65.5 | 34.5 |
| OR (95% CI) | / | 3.532 (1.138 to 10.960) | 1.966 (1.099 to 3.519) | / | 2.779 (0.926 to 8.343) | / | 1.835 (1.506 to 2.237) |
| P | / | 0.030 | 0.028 | / | 0.070 | / | <0.0001 |
Table 3.
Genotype and allele distributions of the 3 SNPs of MMP-3 for the cases and controls
| Group | rs591058 | ||||||
|
| |||||||
| CC | TT | CT + TT | CC + CT | TT | C (%) | T (%) | |
|
| |||||||
| Control | 49 | 8 | 52 | 93 | 8 | 70.3 | 29.7 |
| Case | 49 | 7 | 38 | 80 | 7 | 74.1 | 25.9 |
| OR (95% CI) | / | 0.875 (0.294 to 2.600) | 0.731 (0.411 to 1.300) | / | 1.017 (0.353 to 2.929) | / | 0.827 (0.680 to 1.007) |
| P | / | 1.000 | 0.308 | / | 1.000 | / | 0.065 |
|
| |||||||
| Group | rs650108 | ||||||
|
| |||||||
| AA | GG | AG + GG | AA + AG | GG | A (%) | G (%) | |
|
| |||||||
| Control | 40 | 15 | 61 | 86 | 15 | 62.4 | 37.6 |
| Case | 23 | 22 | 64 | 65 | 22 | 50.6 | 49.4 |
| OR (95% CI) | / | 2.551 (1.109 to 5.868) | 1.825 (0.980 to 3.397) | / | 1.941 (0.934 to 4.032) | / | 1.62 (1.356 to 1.936) |
| P | / | 0.037 | 0.064 | / | 0.097 | / | < 0.0001 |
|
| |||||||
| Group | rs679620 | ||||||
|
| |||||||
| GG | AA | GA + AA | GG + AG | AA | G (%) | A (%) | |
|
| |||||||
| Control | 51 | 13 | 50 | 88 | 13 | 68.8 | 31.2 |
| Case | 30 | 25 | 57 | 62 | 25 | 52.9 | 47.1 |
| OR (95% CI) | / | 3.269 (1.457 to 7.334) | 1.938 (1.075 to 3.495) | / | 2.730 (1.296 to 5.750) | / | 1.963 (1.636 to 2.357) |
| P | / | 0.005 | 0.038 | / | 0.010 | / | <0.0001 |
For the analysis of the IL-6 SNPs, no statistical association was found for the rs10499563 SNP no matter which genetic model was used. For the rs1800796 SNP, the increased G allele was found significantly associated with increased susceptibility of post-operative stiffness with the allele level comparison (P<0.0001), with dominant model comparison (P=0.028), and with the extreme genotype comparison (P=0.030). But no statistical difference was found with the recessive model comparison (P=0.070), which may be a result of the relatively small population.
For the analysis of the MMP-3 SNPs, the increased A allele in rs679620 SNP was found significantly associated with increased incidence rate of post-operative stiffness, no matter with the allele level comparison (P<0.0001), with dominant model comparison (P=0.038), with recessive model comparison (P=0.010), or with the extreme genotype comparison (P=0.005). For the rs650108 SNP of MMP-3, the significant association was only found with the extreme genotype comparison (OR=2.551, 95% CI 1.109- 5.868, P=0.037) and the allele level comparison (OR=1.62, 95% CI 1.356- 1.936, P<0.0001). And no statistical association was found for the rs591058 SNP no matter which genetic model was used.
Association of IL-6 and MMP-3 Polymorphisms with post-operative stiffness severity
As only the rs1800796 SNP of IL-6 and rs679620 SNP of MMP-3 were found to be associated with the risk of post-operative stiffness, these two SNPs were chose to be analyzed in association with post-operative stiffness severity. And the forward flexion was selected as the definition of the severity of stiffness. One way ANOVA analysis found that there was a significant decreased forward flexion degree in correction with the minor allele of the SNP rs1800796 (Figure 1A; P<0.0001) and SNP rs679620 (Figure 1B; P<0.0001).
Figure 1.
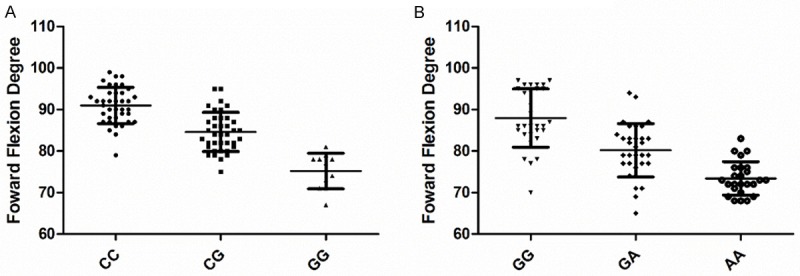
Association of IL-6 and MMP-3 Polymorphisms with post-operative stiffness severity. (A: rs1800796 SNP of IL-6; B: rs679620 SNP of MMP-3).
Discussion
Nowadays, genetic factors have been related to various disease pathologies. But no study has been performed with regard to the genetic factors of post-operative stiffness, whit the fact of the difference in risk of stiffness susceptibility and severity. With the present study, we demonstrated the rs1800796 of IL-6 and rs679620 of MMP-3 were associated with increased risk of post-operative stiffness susceptibility and severity.
Post-operative stiffness, also known as adhesive capsulitis, is a quite common disease happened after all surgeries, not only after rotator cuff repair. Previous studies demonstrated the pathogenesis of adhesive capsulitis is caused by the evolution of a controlled inflammatory response to an unknown injurious stimulus into an aberrant fibrotic process.
Recently, histological studies demonstrated a matrix of type I and type III collagen populated by fibroblasts and myofibroblasts in the capsular tissue [15,16]. High levels of MMP and even higher levels of TIMP have been found in AC patients [17]. Another study also showed the serum levels of MMP-1, MMP-2, MMP-3, TIMP-1, and TIMP-2, which are the key cytokines in remodeling of the extracellular matrix (ECM), may be associated with the pathologies of adhesive capsulitis [18]. On the other hand, studies have found increased levels of growth factor and cytokines [17,19] and the presence of chronic inflammatory cells [20] in biopsy specimens of capsular tissue from patients with adhesive capsulitis. A consistent finding is that of vascular hyperplasia in the capsular tissue [17,20,21]. Ryu et al have also demonstrated an increased expression of vascular endothelial growth factor and angiogenesis in diabetic patients with frozen shoulder [21]. The altered levels of inflammatory cytokines may act as a persistent fibrogenic stimulus, causing capsular fibrosis and the development of AC [22]. Importantly, the IL-6 and MMP-3 genes, which are known as the key cytokines in inflammatory and fibrogenic response, were found elevated expressed in the synovium of patients with adhesive capsulitis.
As far as we know, this is the first study investigating the genetic factors of the post-operative stiffness. And we demonstrated here that the rs1800796 SNP of IL-6 and rs650108 SNP of MMP-3 were associated with increased risk of post-operative stiffness susceptibility and severity. This finding can be used in guiding the rehabilitation procedure after rotator cuff surgery, in another word, those with the genetic susceptibility factors should receive a more radical rehabilitation procedure and those without the susceptibility factors can be more conservative. However, there was some limitation in this study. Firstly is the relative small population number. And a case control study is not of high level of evidence for supporting the conclusion. Second, no functional analysis of the rs1800796 SNP of IL-6 and rs650108 SNP of MMP-3 were performed to understand the mechanism of the association with post-operative stiffness. Moreover, besides the SNPs explored in this study, there may have more other polymorphisms in developing stiffness, a genome-wide association study with a large sample size is a better choice for it.
Disclosure of conflict of interest
None.
References
- 1.Camargo PR, Haik MN, Ludewig PM, Filho RB, Mattiello-Rosa SM, Salvini TF. Effects of strengthening and stretching exercises applied during working hours on pain and physical impairment in workers with subacromial impingement syndrome. Physiother Theory Pract. 2009;25:463–475. doi: 10.3109/09593980802662145. [DOI] [PubMed] [Google Scholar]
- 2.Cools AM, Declercq G, Cagnie B, Cambier D, Witvrouw E. Internal impingement in the tennis player: rehabilitation guidelines. Br J Sports Med. 2008;42:165–171. doi: 10.1136/bjsm.2007.036830. [DOI] [PubMed] [Google Scholar]
- 3.Martins LV, Marziale MH. Assessment of proprioceptive exercises in the treatment of rotator cuff disorders in nursing professionals: a randomized controlled clinical trial. Rev Bras Fisioter. 2012;16:502–509. doi: 10.1590/s1413-35552012005000057. [DOI] [PubMed] [Google Scholar]
- 4.Marcondes FB, de Jesus JF, Bryk FF, de Vasconcelos RA, Fukuda TY. Posterior shoulder tightness and rotator cuff strength assessments in painful shoulders of amateur tennis players. Braz J Phys Ther. 2013;17:185–194. doi: 10.1590/S1413-35552012005000079. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 6.Gulotta LV, Rodeo SA. Growth factors for rotator cuff repair. Clin Sports Med. 2009;28:13–23. doi: 10.1016/j.csm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835–841. doi: 10.1177/0363546509359679. [DOI] [PubMed] [Google Scholar]
- 8.Brislin KJ, Field LD, Savoie FH 3rd. Complications after arthroscopic rotator cuff repair. Arthroscopy. 2007;23:124–128. doi: 10.1016/j.arthro.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Koo SS, Burkhart SS. Rehabilitation following arthroscopic rotator cuff repair. Clin Sports Med. 2010;29:203–211. vii. doi: 10.1016/j.csm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Tauro JC. Stiffness and rotator cuff tears: incidence, arthroscopic findings, and treatment results. Arthroscopy. 2006;22:581–586. doi: 10.1016/j.arthro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Denard PJ, Ladermann A, Burkhart SS. Prevention and management of stiffness after arthroscopic rotator cuff repair: systematic review and implications for rotator cuff healing. Arthroscopy. 2011;27:842–848. doi: 10.1016/j.arthro.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Neviaser AS, Hannafin JA. Adhesive capsulitis: a review of current treatment. Am J Sports Med. 2010;38:2346–2356. doi: 10.1177/0363546509348048. [DOI] [PubMed] [Google Scholar]
- 13.Neviaser AS, Neviaser RJ. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 2011;19:536–542. doi: 10.5435/00124635-201109000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Walsh PS, Metzger DA, Hiquchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 15.Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surq Br. 1995;77:677–83. [PubMed] [Google Scholar]
- 16.Lundberg BJ. The frozen shoulder. Clinical and radiographical observations. The effect of manipulation under general anesthesia. Structure and glycosaminoglycan content of the joint capsule. Local bone metabolism. Acta orthop Scand Suppl. 1969;119:1–59. [PubMed] [Google Scholar]
- 17.Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. JBone Joint Surg Br. 2000;82:768–773. doi: 10.1302/0301-620x.82b5.9888. [DOI] [PubMed] [Google Scholar]
- 18.Lubis AM, Lubis VK. Matrix metalloproteinase, tissue inhibitor of metalloproteinase and transforming growth factor-beta 1 in frozen shoulder, and their changes as response to intensive stretching and supervised neglect exercise. J Orthop Sci. 2013;18:519–527. doi: 10.1007/s00776-013-0387-0. [DOI] [PubMed] [Google Scholar]
- 19.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997;15:427–436. doi: 10.1002/jor.1100150316. [DOI] [PubMed] [Google Scholar]
- 20.Hand GC, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007;89:928–932. doi: 10.1302/0301-620X.89B7.19097. [DOI] [PubMed] [Google Scholar]
- 21.Ryu JD, Kirpalani PA, Kim JM, Nam KH, Han CW, Han SH. Expression of vascular endothelial growth factor and angiogenesis in the diabetic frozen shoulder. J Shoulder Elbow Surg. 2006;15:679–685. doi: 10.1016/j.jse.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Kabbabe B, Ramkumar S, Richardson M. Cytogenetic analysis of the pathology of frozen shoulder. Int J Shoulder Surg. 2010;4:75–78. doi: 10.4103/0973-6042.76966. [DOI] [PMC free article] [PubMed] [Google Scholar]


