Abstract
Sesamin is naturally occurring lignan from sesame oil with putative antioxidant property. The present study was designed to investigate the protective role of sesamin against carbon tetrachloride induced oxidative liver injury. Male Wistar albino rats (180-200 g) were divided in to 5 groups (n=6). Hepatotoxicity was induced by the administration of CCl4 (0.1 ml/100 g bw., 50% v/v with olive oil) intraperitoneally. Sesamin was administered in two different dose (5 and 10 ml/kg bw) to evaluate the hepatoprotective activity. Sesamin significantly reduced the elevated serum liver marker enzymes (P<0.0001). Reduction of TBARS (P<0.01 and P<0.001) followed by enhancement of GSH., SOD and catalase (P<0.0001) in liver homogenate in sesamin treated groups shows the amelioration of oxidative stress induced by CCl4. Histopathological report also supported the hepatoprotection offered by sesamin. Sesamin effects in both the dose were in comparable to reference standard drug silymarin. From these above findings it has been concluded that sesamin ameliorate the oxidative liver injury in terms of reduction of lipid peroxidation and enhancement of liver antioxidant enzymes.
Keywords: Liver injury, hepatotoxin, oxidative stress, sesame lignan, nutraceuticals
Introduction
Sesamin is most powerful antioxidant lignan obtained from sesame oil [1,2]. A range of pharmacological action of sesamin has been reported by several investigators like anti-inflammatory., antihypertensive., neuroprotective and anticancer effects [3-5]. It is reported that administration of sesamin reduced the serum cholesterol by inhibition of cholesterol biosynthesis in liver [2]. Protective role of sesamin against oxidative liver damage reported by Akimoto et al. [6]. In another study it is reported that sesamin improves hepatic detoxification and act in opposition to oxidative stress [3]. Recent work reported that sesamin prevent endothelial dysfunction of diabetic rats through inhibition of oxidative stress [7].
Liver is the major organ and plays a vital role in human physiology like metabolism of macromolecules and synthesis of useful components [8]. Drugs and chemicals cause liver damage which cause extremely severe abnormalities [9,10]. Generation of free radicals and oxidative stress play a major role liver toxicity induced by chemicals [11,12]. Carbon tetrachloride (CCl4) is one of the chemical, which cause liver damage through lipid peroxidation and oxidative stress [8]. CCl4 is suitable chemical to induce oxidative liver toxicity in experimental animal model and the toxic effects of CCl4 extensively studied by several investigators [9,13]. In recent literatures it is documented that, natural drugs with antioxidant potential can protect the liver from damage caused by CCl4 [10,14,15].
In view of above literature the present study was designed to find out the hepatoprotective role of sesamin against CCl4 induced oxidative liver injury in experimental animal model.
Materials and methods
Drugs and chemicals
Sesamin., CCl4., thiobarbituric acid., 2.,4 dinitrophenyl hydrazine., nito blue tetrazolium and phenazine methosulfate were purchased from Sigma Chemical., USA. Biochemical kits for serum liver marker enzymes were purchased from Transasia Bio-Medicals Limited., Solan. All other reagents and chemicals used in this study were of analytical grade with high purity were purchased from Sigma Aldrich, USA.
Animals
Male Wistar albino rats weighing about 150-200 g were obtained from Institute Animal house and used in the experiments. The protocol was approved by the Institute’s Animal Ethical Committee. Animals were kept in the animal house at an ambient temperature of 25°C and 45-55% relative humidity, with 12 h each of dark and light cycles. Animals were fed pellet diet and water ad-libitum.
Experimental protocol
30 Rats were divided in to 5 groups (n=6) and the duration of the experiment was 14 days. G1 (Normal Control): Rats of this group received 0.5 ml of distilled water/100 g bw/rat/day for 14 days. G2 (Toxic control): Rats of this group received 0.5 ml of distilled water/100 g bw/rat/day for 12 days and on day 13 received a single dose of CCl4 injection intraperitoneally (0.1 ml/100 g bw., 50% v/v with olive oil). G3: Rats of this group received Sesamin 10 mg/kg bw/rat/day for 12 days and on day 13 received a single dose of CCl4 injection intraperitoneally. G4: Rats of this group received Sesamin 20 mg/kg bw/rat/day for 12 days and on day 13 received a single dose of CCl4 injection intraperitoneally. G5: Rats of this group received silymarin 2 mg/kg bw/rat/day for 12 days and on day 13 received a single dose of CCl4 injection intraperitoneally. All the rats of respective groups were treated under fasting condition. At the end of the treatment period, rats were deprived of food overnight and sacrificed on day 15 by light ether anesthesia followed by decapitation after recording the final body weight. Blood was collected from each rat for biochemical estimation and liver was quickly isolated immersed in ice cold saline and weighed. Half of the liver was stored under freezer (-20°C) for estimation of tissue antioxidant parameters and remaining part of the liver was preserved in buffered formalin (10%) for histopathological examination.
Estimation of biochemical parameters
Alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), total protein and bilirubin levels were estimated from the serum by using standard kits. Ten percent liver homogenate was prepared by homogenizing the liver tissue by using 0.3 m phosphate buffer. Thiobarbituric acid reactive substance (TBARS) [16], reduced glutathione (GSH) [17], superoxide dismutase (SOD) [18], catalase (CAT) [19] and protein [20] levels were estimated from the liver homogenate by using spectrophotometric determination.
Histopathological studies
The livers were excised quickly and fixed in 10% formalin and stained with haemotoxylin and eosin and then observed under microscope for degeneration, fatty changes or necrotic changes as evidence of hepatotoxicity.
Statistical analysis
All values are expressed as mean ± SEM for 6 animals in each group. Data for various biochemical parameters were analyzed using analysis of variance (ANOVA) (GraphPad Version 3.06., La Jolla., CA., USA). Significance is set at P<0.05.
Results
There was no mortality in any of the groups during treatment period. SGPT, SGOT, ALP, total bilirubin and total protein levels were estimated in serum. The results were presented in Table 1.
Table 1.
Level of ALT, AST, ALP, total bilirubin and total protein
| Groups | ALT U/L | AST U/L | ALP U/L | TOTAL BILIRUBIN mg/dL | TOTAL PROTEIN mg/dL |
|---|---|---|---|---|---|
| G1 | 29.67±0.84 | 66.3±3.7 | 81.5±1.1 | 1.3±0.06 | 8.3±0.46 |
| G2 | 177.3±3.9# | 290.5±28.4# | 338.0±15.1# | 2.7±0.16# | 5.1±0.37# |
| G3 | 38.03±0.29*** | 73.6±4.5*** | 107.7±6.2*** | 1.3±0.12*** | 7.5±0.13*** |
| G4 | 66.0±6.8*** | 63.6±4.9*** | 71.5±6.0*** | 1.8±0.21*** | 7.4±0.02*** |
| G5 | 48.5±4.5*** | 64.8±9.9*** | 68.0±6.6*** | 1.5±0.11*** | 7.7±0.06*** |
All values expressed as mean ± SEM; Oneway Anova followed by Newman-Keuls Multiple Comparison Test.
P<0.0001 vs G1;
P<0.0001 vs G2.
G1 - normal control rats were treated with vehicle (distilled water 0.5 ml/100 g bwp.o.). G2 - toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p. G3 - rats were treated with sesamin 10 mg/kg body weight p.o. G4 - rats were treated with sesamin 20 mg/kg body weight p.o. G5 - rats were treated with silymarin 2 mg/100 g body weight p.o.
Serum level of SGPT
There is significant increase in the level of SGPT in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.0001). Significant decreased level of serum SGPT in sesamin (G3, G4) and silymarin (G5) treated rats when compared with CCl4 treated rats (G2) (P<0.0001).
Serum level of SGOT
There is significant increase in the level of SGOT in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.0001). Significant decreased level of serum SGOT in sesamin (G3., G4) and silymarin (G5) treated rats when compared with CCl4 treated rats (G2) (P<0.0001).
Serum level of ALP
There is significant increase in the level of ALP in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.0001). Significant decreased level of serum ALP in sesamin (G3, G4) and silymarin (G5) treated rats when compared with CCl4 treated rats (G2) (P<0.0001).
Serum level of total bilirubin
There is significant increase in the level of total bilirubin in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.0001). Significant decreased level of serum total bilirubin in sesamin (G3, G4) and silymarin (G5) treated rats when compared with CCl4 treated rats (G2) (P<0.0001).
Serum level of total protein
There is significant decrease in the level of total protein in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.0001). Significant increased level of serum total protein in sesamin (G3, G4) and silymarin (G5) treated rats when compared with CCl4 treated rats (G2) (P<0.0001).
Thiobarbituric acid reactive substance (TBARS)., reduced glutathione (GSH)., Superoxide dismutase (SOD) and Catalase (CAT) levels were estimated in liver homogenate. The results were present in Table 2.
Table 2.
Level of TBARS, GSH, SOD and Catalase (CAT) in liver homogenate
| Groups | TBARS nmol/g wet wt | GSH µg/g wet wt | SOD IU/mg protein | CAT IU/mg protein |
|---|---|---|---|---|
| G1 | 13.8±2.8 | 360.2±1.4 | 3.7±0.59 | 10.8±0.44 |
| G2 | 29.4±3.6a | 47.1±4.4b | 0.34±0.01b | 1.7±0.71b |
| G3 | 18.8±5.9* | 175.0±10.9*** | 5.1±1.4*** | 9.6±1.9*** |
| G4 | 10.2±0.7** | 129.0±13.4*** | 3.1±0.68*** | 11.1±0.18*** |
| G5 | 7.7±1.7** | 185.6±15.6*** | 3.5±0.86*** | 10.8±0.09*** |
All values expressed as mean ± SEM; Oneway Anova followed by Newman-Keuls Multiple Comparison Test.
P<0.01 vs G1;
P<0.0001 vs G1;
P<0.01 vs G2;
P<0.001 vs G2;
P<0.0001 vs G2.
G1 - normal control rats were treated with vehicle (distilled water 0.5 ml/100 g bwp.o.). G2 - toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p. G3 - rats were treated with sesamin 10 mg/kg body weight p.o. G4 - rats were treated with sesamin 20 mg/kg body weight p.o. G5 - rats were treated with silymarin 2 mg/100 g body weight p.o.
Thiobarbituric acid reactive substance (TBARS)
There is significant increase in the level of TBARS in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.01). Significant decrease in the level of TBARS in sesamin (G3) (P<0.01), G4 and silymarin (G5) treated rats when compared with CCl4 treated rats (G2) (P<0.0001).
Reduced glutathione (GSH)., Superoxide dismutase (SOD) and Catalase (CAT)
There is significant decrease in the level of GSH., SOD and CAT in CCl4 treated rats (G2) when compared with control rats (G1) (P<0.0001). Significant increase in the level of GSH, SOD and CAT in sesamin (G3) (P<0.01), G4 (P<0.001)) and silymarin (G5) (P<0.001)) treated rats when compared with CCl4 treated rats (G2).
Histopathology
Rats treated with vehicle (G1) shows normal architecture of hepatocytes and central vein. Rats treated with CCl4 (G2) shows extensive necrosis on hepatocytes with enlarged central vein, massive fatty changes, ballooning degeneration and vacuolization. Rats treated with Sesamin (G3 and G4) and silymarin (G5) shows normal architecture of hepatocytes and central vein. Sesamin treated with 20 mg/kg dose shows better protection when compared with low dose (10 mg/kg). The results were presented in Figure 1.
Figure 1.
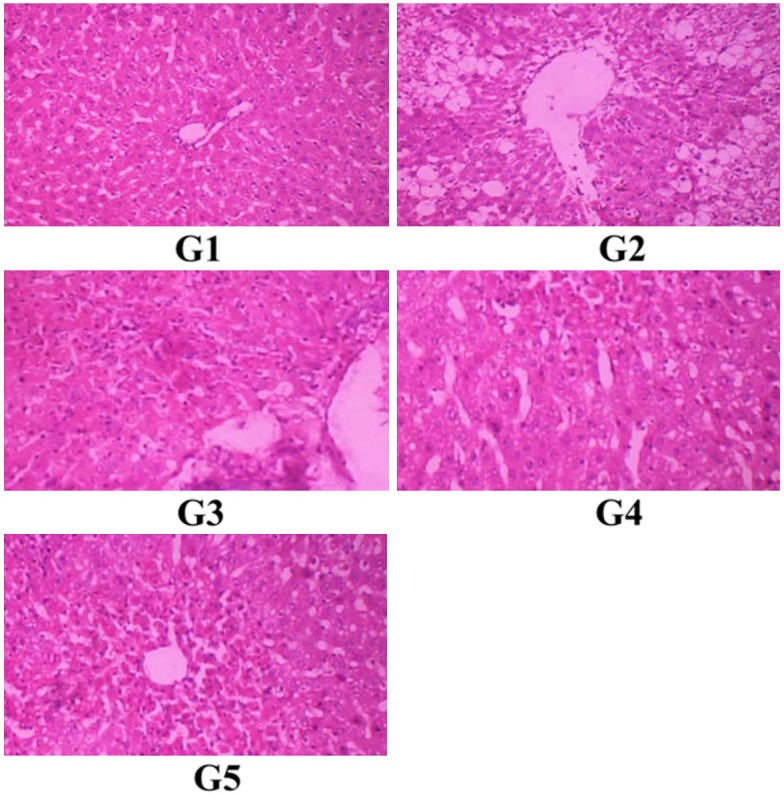
Histopathology of liver. G1. Normal control rats were treated with vehicle (distilled water 0.5 ml/100 g bwp.o.). G2. Toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p. G3. Rats were treated with sesamin 10 mg/kg body weight p.o. G4. Rats were treated with sesamin 20 mg/kg body weight p.o. G5. Rats were treated with silymarin 2 mg/100 g body weight p.o.
Discussion
Several chemicals and drugs are involving in the development of toxicity. In the present research CCl4 was selected as chemical to induce hepatotoxicity in experimental animals. Carbon tetrachloride (CCl4) is effective hepatotoxin which is used in preclinical laboratory to induce liver damage in animals [21-23]. Cytochrome P450 an enzyme is act on CCl4 and converts CCl4 in to active trichloromethylradical (CCl3) which is further react with O2 to generate more free radicals. The generated free radical initiates the lipid peroxidation to cause cell necrosis and subsequent cell death [8,10,24,25].
In the present research administration of CCl4 significantly altered the serum marker enzymes of liver. Similar findings also observed by other investigators in this model [26-28]. During liver damage serum marker enzymes like SGPT, SGOT and ALP were released in to blood stream from hepatocytes [29]. The elevated level of these enzymes along with total bilirubin and diminished level of total protein are indicative of cellular leakage and loss of functional integrity of cell membranes in liver [26,30]. Increase in the normal upper limits in the measured serum transaminases of CCl4 administered group was a biochemical indication of liver injury. The biochemical parameters has been reverted significantly by the administration of Sesamin in two different dose (10, 20 mg/kg) and the Sesamin effect almost comparable to rats treated by silymarin. So, the protective role of Sesamin may be via stabilization of plasma membrane and protection of liver tissue from necrotic damage caused by CCl4.
Oxidative stress plays a major role in the development of hepatotoxicity. Generation of trichloromethyl radical from CCl4 during metabolism is an indication for generation of oxidative stress via lipid peroxidation [31]. The level of lipid peroxide is a measure ofmembrane damage and alteration in structure and function of cellular membranes [28]. The elevated level of lipid peroxidation in the form of TBARS is marker enzyme for oxidative stress in liver homogenate of CCl4 treated animals is consistent with this hypothesis.
Endogenous antioxidants like GSH, SOD and CAT plays a major role to fight against free radicals. Depletion of GSH, SOD and CAT in CCl4 treated rats is confirmed the loss of antioxidant mechanism in animals against oxidative stress. Loss of antioxidant enzymes may cause accumulation of highly reactive free radicals leading to further damage. CCl4 leads to generation of peroxy and superoxide radicals which are associated with the inactivation of these antioxidant enzymes [27]. Oral administration of Sesamin in two different dose (10 and 20 mg/kg) and silymarin significantly decreased the TBARS and enhanced the levels of GSH., SOD and CAT is indicating the protective role of Sesamin against CCl4 induced oxidative stress. Several investigators reported the efficacy of Sesame lignans against CCl4 and ethanol-induced liver toxicity [32]. Our study also confirmed the same via its putative antioxidant property.
The protective role further supported by histopathological study. CCl4 treated rat shows extensive necrosis on hepatocytes with enlarged central vein, massive fatty changes., ballooning degeneration and vacuolization. Treatment with Sesamin is reverted these histopathological changes to normal level indicating its hepatoprotective activity.
In conclusion, the present findings observed in this study revealed that, Sesamin is natural antioxidant lignin possess significant antioxidant activity against CCl4 induced hepatotoxicity via antioxidant mechanism. However, further research is required to find out the other possible mechanism of hepatoprotection to conform the Sesamin as hepatoprotective molecule.
Disclosure of conflict of interest
None.
References
- 1.Philip JK, Joseph ZB, Jestina BK, Joseph BA. Nutraceutical Importance of Sesame Seed and Oil: A Review of the Contribution of their Lignans. Sierra Leone J Biomedical Research. 2010;2:4–16. [Google Scholar]
- 2.Mohamed Saleem TS, Chetty M, Kavimani S. Nutraceutical value of sesame oil - An update. Int J Res Pharm Sci. 2013;3:650–656. [Google Scholar]
- 3.Cheng FC, Jinn TR, Hou RC, Tzen JT. Neuroprotective Effects of Sesamin and Sesamolin on Gerbil Brain in Cerebral Ischemia. Int J Biomed Sci. 2006;2:284–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Chung B, Lee JJ, Kim J, Jeoung D, Lee H, Ha K, Kwon Y, Kim Y, Choe J. Angiogenic activity of sesamin through the activation of multiple signal pathways. Biochem Biophys Res Commun. 2010;391:254–60. doi: 10.1016/j.bbrc.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Lee HJ, Park KH, Park H, Choi H, Lim S, Lee MK. Modulatory effects of sesamin on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. Neuropharmacology. 2012;62:2219–2226. doi: 10.1016/j.neuropharm.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Akimoto K, Kitagawa Y, Akamatsu T, Hirose N, Sugano M, Shimzu S, Yamada H. Protective effects of sesamin against liver damage caused by alcohol or carbon tetrachloride in rodents. Ann Nutr Metab. 1993;37:218–224. doi: 10.1159/000177771. [DOI] [PubMed] [Google Scholar]
- 7.Baluchnejadmojarad T, Roghani M, Nadoushan MJ, Mahdavi MV, Kalalian-Moghaddam H, Roghani-Dehkordi F, Dariani S, Raoufi S. The sesame lignan sesamin attenuates vascular dysfunction in streptozotocin diabetic rats: Involvement of nitric oxide and oxidative stress. Eur J Pharmacol. 2013;698:316–321. doi: 10.1016/j.ejphar.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Moreira PR, Maioli MA, Medeiros HCD, Guelfi M, Pereira FTV, Mingatto FE. Protective effect of bixinon carbon tetrachloride induced hepatotoxicity in rats. Biological Research. 2014;47:49. doi: 10.1186/0717-6287-47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Dbass AM, Al-Daihan SK, Bhat RS. Garicus blazei Murill as an efficient he patoprotective and antioxidant agent against CCl4-induced liver injury in rats. Saudi J Biol Sci. 2012;19:303–309. doi: 10.1016/j.sjbs.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andritoiu CV, Andritoiu V, Cuciureanu M, Nica-Badea D, Bibire N, Popa M. Effect of apitherapy products against carbon tetrachloride induced toxicity in Wistar rats. Rom J Morphol Embryol. 2014;55:835–847. [PubMed] [Google Scholar]
- 11.Muhtaseb MS, El Talwar D, Duncan AJ, O’reilly D, Mckee RF, Anderson JH, Foulisa FIG. Free radical activity and lipid soluble anti-oxidant vitamin status in patients with longterm ileal pouch-anal anastomosis. Colorectal Dis. 2008;11:67–72. doi: 10.1111/j.1463-1318.2008.01517.x. [DOI] [PubMed] [Google Scholar]
- 12.Appiah I, Milovanovic S, Radojicic A, Nikolic-Kokic A, Orescanin-Dusic Z, Slavic M, Trbojevic S, Skrbic R, Spasic MB, Blagojevic D. Hydrogen peroxide affects contractile activity and anti-oxidant enzymes in rat uterus. Br J Pharmacol. 2009;158:1932–1941. doi: 10.1111/j.1476-5381.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azeem AK, Mathew M, Nair C, Dilip C. Hepatoprotective effect of Averrhoea carambola fruit extract on carbon tetrachloride induced hepatotoxicity in mice. Asian Pacific J Trop Med. 2010:610–613. [Google Scholar]
- 14.Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Sanmugapriya E, Venkataraman S. Studies on hepatoprotective and antioxidant actions of Strychnos potatorum Linn. seeds on CCl4-induced acute hepatic injury in experimental rats. J Ethnopharmacol. 2006;105:154–160. doi: 10.1016/j.jep.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Okhawa H, Oohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Bichem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophy. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 19.Aebi H, Bergmeyer HU. Methods of enzymatic analysis. 2nd edition. Verlag: Chemic Academic Press, Inc.; 1974. pp. 673–685. [Google Scholar]
- 20.Bradford MM. A rabid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;772:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Girish C, Konar BC, Jayanthi S, Rao KR, Rajesh B, Pradhan SC. Hepatoprotective activity of six polyherbal formulation in CCl4 -induced liver toxicity in mice. Indian J Exp Biol. 2009;47:257–263. [PubMed] [Google Scholar]
- 22.Bagban IM, Roy SP, Chaudhary A, Das SK, Gohil KJ, Bhandari KK. Hepatoprotective activity of the methanolic extract of Fagonia indica Burm in carbon tetra chloride induced hepatotoxicity in albino rats. Asian Pacific J Trop Med. 2012:S1457–S1460. [Google Scholar]
- 23.Brai BIC, Adisa RA, Odetola AA. Hepatoprotective properties of aqueous leaf extracts of Persea Americana Mill (Lauraceae) Avocado against CCL4 induced damage in rats. Afr J Tradit Complement Altern Med. 2014;11:237–244. doi: 10.4314/ajtcam.v11i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manna P, Sinha M, Sil PC. Aqueous extract of Termilalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complement Altern Med. 2006;6:33–43. doi: 10.1186/1472-6882-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shyamal S, Latha PG, Shine VJ, Sula SR, Rajasekaran S, Devi TG. Hepatoprotective effects of Pittosporumneelgherrense Wight and Arn. , a popular Indian ethanomedicine. J Ethnopharmacol. 2006;107:151–155. doi: 10.1016/j.jep.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a poly herbal formulation. J Ethanoparamcol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Bera TK, Chatterjee K, Jana K, Ali KM, De D, Maity S, Ghosh D. Antihepatotoxic effect of “Livshis”, a polyherbal formulation against carbon tetrachloride-induced hepatotoxicity in male albino rat. J Nat Pharm. 2012;3:17–24. [Google Scholar]
- 28.Mistry S, Dutt KR, Jena J. Protective effect of Sidacordata leaf extract against CCl4 induced acute liver toxicity in rats. Asian Pac J Trop Med. 2013;6:280–284. doi: 10.1016/S1995-7645(13)60057-7. [DOI] [PubMed] [Google Scholar]
- 29.Shenoy KA, Somayaji SN, Bairy KL. Hepatotoxic effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Ind J Pharamcol. 2001;33:260–266. [Google Scholar]
- 30.Fahmy SR, Hamdi SA, Abdel-Salam HA. Curative effect of dietary freshwater and marine crustacean extract on carbon tetrachloride induced nephrotoxicity. Aust J Basic Appl Sci. 2009;3:2118–2129. [Google Scholar]
- 31.Jayakumar T, Ramesh E, Geraldine P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl(4)-induced liver injury in rats. Food Chem Toxicol. 2006;44:1989–1996. doi: 10.1016/j.fct.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Lu H, Huang H, Hwang G, Tzen JT. Sesame Lignans Significantly Alleviate Liver Damage of Rats Caused by Carbon Tetrachloride in Combination with Kava. J Food Drug Anal. 2010;18:249–255. [Google Scholar]


