Abstract
Carcinoid tumor of the ovary is uncommon. We herein report a very rare case of primary ovarian carcinoid tumor with aggressive histology and an unusual immunophenotype. A 21-year-old woman presented with a palpable abdominal mass. Computed tomographic scan revealed a large, extensively necrotic solid mass in the left ovary. The patient underwent a left salpingo-oophorectomy. Grossly, the left adnexa showed a large, vaguely lobulated ovarian tumor measuring 22×15×13 cm. Histologically, the tumor had a readily identifiable neuroendocrine growth pattern, but some areas showed solid growth pattern associated with mild nuclear pleomorphism and multiple foci of punctate necrosis. Furthermore, mitotic figures were recognized in 8 per 10 high-power fields, and a few foci of large coagulative tumor necrosis were also noted. In addition, the tumor tissue exhibited uniform, strong nuclear β-catenin immunoreactivity, indicating the nuclear accumulation of β-catenin in the individual tumor cells. In summary, we described the first case of primary ovarian carcinoid tumor with loss of neuroendocrine growth pattern, increased mitotic activity and large areas of coagulative tumor necrosis. According to the WHO classification of pulmonary carcinoid tumor, this case may be classified as “atypical” carcinoid. However, currently, no primary ovarian atypical carcinoid exists in the classification system. Due to its rarity, there are no established diagnostic criteria and clinical data on patient outcomes for ovarian carcinoid tumors with aggressive histology. Additional reports are clearly necessary. We also showed for the first time the nuclear accumulation of β-catenin in carcinoid tumor cells, suggestive of a role for β-catenin in the tumorigenesis of ovarian atypical carcinoid tumor or its aggressive histology.
Keywords: Neuroendocrine tumor, carcinoid, atypical carcinoid, ovary
Introduction
Primary carcinoid tumors of the ovary are rare neoplasms. Carcinoid tumors mostly occur in the gastrointestinal tract and the lungs [1]. Ovarian carcinoid tumors constitute only 0.5% of all carcinoid tumors and 0.1% of all ovarian neoplasms [2-4]. We recently encountered a case of primary ovarian carcinoid tumor with aggressive histology including loss of “neuroendocrine” growth pattern, frequent mitotic figures and foci of coagulative tumor necrosis. Ovarian monodermal teratomas of carcinoid type, including insular, trabecular, strumal and mucinous carcinoids, have been the subject of several studies during the last 3 decades, resulting in a well-established classification system [5-9]. However, there is no available data on aggressive histological features of ovarian carcinoid tumors. We herein report for the first time a case of primary ovarian carcinoid tumor with aggressive histological features and an unusual immunophenotype.
Case presentation
A 21-year-old Korean woman was referred to the Department of Obstetrics and Gynecology, Samsung Medical Center (Seoul, Republic of Korea), presenting with a palpable abdominal mass. Physical and pelvic examination revealed a large pelvic mass, which was the size of an adult head. Her past medical history was unremarkable. Hematological profile revealed hemoglobin of 12.0 g/dL with normal leukocyte and platelet counts. Serum levels of cancer antigen (CA) 125 (44.8 U/mL) and lactate dehydrogenease (2,396.0 IU/L) were increased, whereas those of β-human chorionic gonadotropin, carcinoembryonic antigen and CA 19-9 were within normal limits. Computed tomographic (CT) scan of the abdomen revealed a large, necrotic solid mass in the left ovary (Figure 1A). Tumor involvement was not observed in the uterus, right ovary and lymph nodes; and peritoneal seeding and hematogenous metastasis were absent. Based on imaging and laboratory findings, both germ cell tumor and malignant epithelial tumor were suspected. A left salpingo-oophorectomy was performed. The postoperative course was uneventful, and the patient left the hospital 5 days later. Four months after surgery, abdominal CT scan showed no evidence of recurrence. Whole body positron emission tomographic scan showed no abnormal hypermetabolic lesion.
Figure 1.
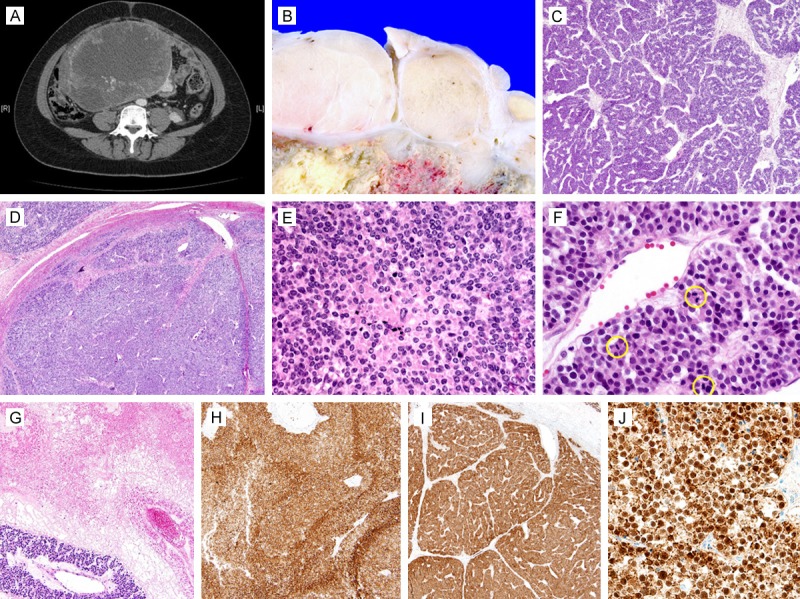
Clinicopathological findings of primary “atypical” ovarian carcinoid. A. CT scan revealed a huge, solid adnexal mass. B. Grossly, conglomeration of relatively well-circumscribed, whitish yellow, solid nodules surrounded central areas of tumor necrosis. C. Histologically, organoid and trabecular growth patterns typical of neuroendocrine tumor were evident. D. However, some areas displayed a loss of “neuroendocrine” growth pattern. E. The solid growth pattern was associated with mild nuclear pleomorphism with chromatin condensation, apoptotic bodies and punctate necrosis. F. Mitotic figures (yellow circles) were frequently identified. Three mitotic figures were detected in a single high-power field. G. A few foci of extensive coagulative tumor necrosis were also noted. Immunohistochemically, the tumor tissue was positive for H. CD56 and I. β-catenin. J. In particular, the tumor cells exhibited strong, uniform nuclear β-catenin immunoreactivity.
Pathologic findings
Grossly, the left adnexa showed a large, vaguely lobulated ovarian tumor measuring 22×15×13 cm. There were no gross areas of capsular breach. On serial sectioning, the left ovary was completely replaced by the tumor, which was whitish yellow, predominantly solid with necrotic areas of varying size. The solid component was a conglomeration of multiple, relatively well-circumscribed nodules (Figure 1B). No normal ovarian tissue was identified in the left adnexal mass. The left salpinx was grossly unremarkable. Histologically, the tumor displayed a readily identifiable neuroendocrine growth pattern that consists of various architectural types. Tumor cells were arranged in a mainly trabecular (Figure 1C) and nested pattern, and had a polygonal contour, with ample amounts of granular cytoplasm. Their nuclei were centrally located, monotonous and round, without marked nuclear pleomorphism or prominent nucleoli. However, in some areas, the tumor exhibited a solid growth pattern (Figure 1D), associated with mild nuclear pleomorphism, chromatin condensation and multiple foci of punctate necrosis (Figure 1E). Furthermore, mitotic figures were recognized in 8 per 10 high-power fields (Figure 1F). A few large foci coagulative tumor necrosis were obvious (Figure 1G). The ovarian surface or left salpinx was not involved. Immunohistochemically, the tumor cells showed strong membranous reactivity for CD56 (Figure 1H) and synaptophysin, but they were negative for OCT4, CD117, D2-40, inhibin-α and calretinin. The tumor cells were also positive for β-catenin (Figure 1I), with uniform and strong nuclear staining of individual tumor cells (Figure 1J).
Discussion
According to the WHO classification of pulmonary tumor [10], carcinoid tumors are characterized by growth patterns that suggest neuroendocrine differentiation, including organoid, insular, trabecular, palisading, ribbon and rosette-like arrangements. Individual tumor cells have uniform cytologic features with moderate eosinophilic, finely granular cytoplasm and nuclei with a finely granular chromatic pattern. Pulmonary carcinoid tumors are classified as typical or atypical, depending on the mitotic activity and the presence of necrosis. Typical carcinoid is defined as a carcinoid tumor with < 2 mitoses per 10 high-power fields (2 mm2) and lacking necrosis. In contrast, atypical carcinoid is defined as a carcinoid tumor with 2-10 mitosis per 10 high-power fields and/or foci of necrosis. There are no established histological criteria for discriminating between typical and atypical ovarian carcinoid tumors. Since the incidence of ovarian carcinoid is very low with no reported cases of ovarian carcinoid with aggressive histological features, there was no previous necessity for establishing criteria for atypical carcinoid tumor of the ovary. According to the WHO classification of pulmonary carcinoid tumor, it is reasonable to classify the current case as “atypical” carcinoid. The presence of large areas of coagulative tumor necrosis favors a neuroendocrine carcinoma rather than atypical carcinoid, but necrosis per se is not diagnostic of neuroendocrine carcinoma [11]. Necrosis may also occur extensively in atypical carcinoid tumors [10,12,13]. Furthermore, tumor cells did not exhibit obvious nuclear pleomorphism or prominent nucleoli. Despite thorough searches, mitotic figures were < 10 per 10 high-power fields. The distinction of atypical carcinoid from other neuroendocrine tumors should be based on morphological parameters that include the amount and pattern of necrosis, cell size, amount of cytoplasm, nuclear chromatin and degree of pleomorphism, nucleoli, and most importantly, the mitotic rate [14]. Due to the absence of diagnostic criteria, primary ovarian atypical carcinoid tumors might be underdiagnosed and often unrecognized. Additional reports are necessary to better understand the nature of ovarian atypical carcinoid.
Interestingly, the tumor cells displayed uniform, strong nuclear immunoreactivity for β-catenin. Nuclear accumulation of β-catenin is reported in pulmonary neuroendocrine and gastrointestinal carcinoid tumors [15,16]. Pelosi and colleagues [15] reported that the nuclear accumulation of β-catenin is present exclusively in pulmonary small- and large-cell neuroendocrine carcinoma and atypical carcinoid tumors, whereas normal and hyperplastic neuroendocrine cells have uniform membranous β-catenin. They found that an aberrant β-catenin staining pattern is an independent predictor of lymph node metastasis in patients with atypical carcinoid tumor, and suggest a possible role for the impaired intracellular β-catenin signaling apparatus in the development and progression of pulmonary neuroendocrine tumors. In contrast, Su and colleagues [16] reported that even though the nuclear accumulation of β-catenin occurs in gastrointestinal carcinoid tumors, β-catenin and APC gene mutation is absent in all cases with nuclear β-catenin expression, suggesting that β-catenin mutation is less likely to contribute to the pathogenesis of gastrointestinal carcinoid tumors. To the best of our knowledge, we reported for the first time the nuclear accumulation of β-catenin in primary ovarian carcinoid tumor. Tumor involvement of lymph nodes could not be ruled out since we did not conduct pelvic lymphadenectomy; and close follow-up is crucial based on the previous observation of Pelosi and colleagues [15]. More studies are required to clarify the role of β-catenin and its genetic alteration in the tumorigenesis and progression of ovarian carcinoid tumors.
In summary, we demonstrated the first case of primary ovarian carcinoid tumor with aggressive histological features, including loss of neuroendocrine growth pattern, increased mitotic activity and large areas of coagulative tumor necrosis. Our case may be classified as “atypical” carcinoid according to the WHO classification of pulmonary carcinoid tumor. The rarity poses a challenge to the diagnosis, histological subclassification and assessment of clinical outcome. Additional reports are clearly necessary to better understand the nature of ovarian “atypical” carcinoid. We also showed for the first time the nuclear accumulation of β-catenin in ovarian carcinoid tumor cells, suggestive of its role in the tumorigenesis or the development of its aggressive histological phenotype.
Disclosure of conflict of interest
None.
References
- 1.Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician. 2006;74:429–434. [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Talerman A. Germ cell tumors of the ovary. Curr Opin Obstet Gynecol. 1997;9:44–47. [PubMed] [Google Scholar]
- 5.Robboy SJ, Norris HJ, Scully RE. Insular carcinoid primary in the ovary. A clinicopathologic analysis of 48 cases. Cancer. 1975;36:404–418. doi: 10.1002/1097-0142(197508)36:2<404::aid-cncr2820360216>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Robboy SJ, Scully RE. Strumal carcinoid of the ovary: an analysis of 50 cases of a distinctive tumor composed of thyroid tissue and carcinoid. Cancer. 1980;46:2019–2034. doi: 10.1002/1097-0142(19801101)46:9<2019::aid-cncr2820460921>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Talerman A. Carcinoid tumors of the ovary. J Cancer Res Clin Oncol. 1984;107:125–135. doi: 10.1007/BF00399383. [DOI] [PubMed] [Google Scholar]
- 8.Baker PM, Oliva E, Young RH, Talerman A, Scully RE. Ovarian mucinous carcinoids including some with a carcinomatous component: a report of 17 cases. Am J Surg Pathol. 2001;25:557–568. doi: 10.1097/00000478-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Choi YH, Chae SW, Park HR, Lee MC, Park YE. A case report of strumal carcinoid of the ovary. Korean J Pathol. 1994;28:307–312. [Google Scholar]
- 10.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. [Google Scholar]
- 11.Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29:179–187. doi: 10.1097/01.pas.0000149690.75462.29. [DOI] [PubMed] [Google Scholar]
- 12.Corrin B, Nicholson AG. Pathology of the Lungs. London: Churchill Livingstone; 2011. [Google Scholar]
- 13.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. World Health Organization International Histological Classification of Tumours: Histological Typing of Lung and Pleural Tumours. Berlin: Springer; 1999. Berlin: Springer; 1999. [Google Scholar]
- 14.Moghe G, Jambhekar NA, Deshpande RK, Hejmadi R, Vyas H. Atypical carcinoid tumors of lung: clinicopathologic study of six cases. Asian Cardiovasc Thorac Ann. 2000;8:41–45. [Google Scholar]
- 15.Pelosi G, Scarpa A, Puppa G, Veronesi G, Spaggiari L, Pasini F, Maisonneuve P, Iannucci A, Arrigoni G, Viale G. Alteration of the E-cadherin/beta-catenin cell adhesion system is common in pulmonary neuroendocrine tumors and is an independent predictor of lymph node metastasis in atypical carcinoids. Cancer. 2005;103:1154–1164. doi: 10.1002/cncr.20901. [DOI] [PubMed] [Google Scholar]
- 16.Su MC, Wang CC, Chen CC, Hu RH, Wang TH, Kao HL, Jeng YM, Yuan RH. Nuclear translocation of beta-catenin protein but absence of beta-catenin and APC mutation in gastrointestinal carcinoid tumor. Ann Surg Oncol. 2006;13:1604–1609. doi: 10.1245/s10434-006-9072-2. [DOI] [PubMed] [Google Scholar]


