Abstract
We report a case of Peutz-Jeghers syndrome (PJS) in a 33-year-old female patient with synchronous uterine cervical minimal deviation adenocarcinoma (MDA) and gastric type adenocarcinoma (GTA). The patient was diagnosed with PJS at the age of 10. At the time of consultation, she complained of watery discharge. Magnetic resonance imaging of the pelvis showed a poorly circumscribed mass in the uterine cervix. Histologically, both MDA and GTA components, as well as their transitional area, were observed. Both components were diffusely positive for MUC6, CK7 and, robustly, for p16. Moreover, the components were negative for ER, PgR and CEA, while HIK1083 and CK20 positive cells were found focally. Ki-67 labeling index in the MDA component was 5% while that in the GTA component was 50%. This case of GTA accompanied by MDA in a patient with PJS is distinct from the single previously-reported comparable case of which we are aware, with respect to the overexpression of p16 protein, an event considered rare in these tumors, and the continuity between the MDA and GTA components. This continuity favors the hypothesis that GTA arises from the dedifferentiation of MDA.
Keywords: P16, immunohistochemistry, minimal deviation adenocarcinoma, gastric type adenocarcinoma, cervical adenocarcinoma, Peutz-Jeghers syndrome
Introduction
Peutz-Jeghers syndrome (PJS) is an autosomal dominant genetic syndrome characterized by mucocutaneous pigmentation and polyposis of the gastrointestinal tract. In the female genital tract, minimal deviation adenocarcinoma (MDA), sex-cord tumors with annular tubules and bilateral ovarian mucinous tumors are known to occur in association with PJS patients [1-3].
Gastric type adenocarcinoma (GTA) is a newly established entity of cervical mucinous adenocarcinoma, distinct morphological features of which are clear and abundant cytoplasm and distinct cell borders, and a gastric immunophenotype characterized by expression of HIK1083 or MUC6 [4]. GTA is classified as an independent histologic subtype of mucinous adenocarcinoma of uterine cervix in the new WHO Classification of Tumors of Female Reproductive Organs (Fourth edition, 2014), which also describes MDA as an extremely well differentiated form of GTA.
To our knowledge, there has been only a single previously-reported case of GTA accompanied by MDA in a patient with PJS [5]. Unlike that case, in which the two components were spatially separate and did not express p16, the current case exhibits continuity of the MDA and GTA components, and robust expression of p16 protein in each.
Case report
A 32-year-old woman, G0P0, who had previously been diagnosed with PJS based on mucocutaneous pigmentation and hamartomatous polyps in her gastrointestinal tract at the age of 10 (Figure 1A and 1B), underwent an annual gynecological examination. She had previously undergone small intestine partial resections due to intestinal obstructions at the ages of 10 and 20, and small intestinal polypectomies at the ages of 24, 26 and 30. Most of these resected polyps demonstrated typical hamartomatous polyps. No other family members showed any manifestations of PJS, indicating the sporadic nature of this case.
Figure 1.
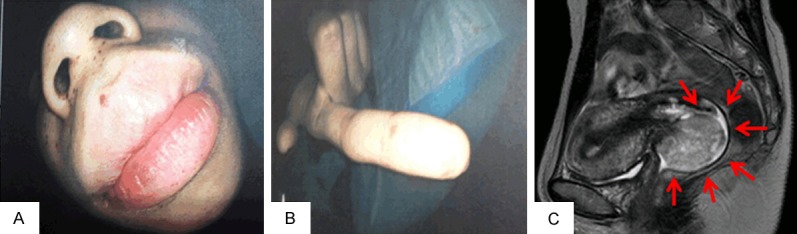
Physical and Imaging findings. The pigmented macules on the lips and fingers (A and B). Magnetic resonance imaging of the pelvis shows a poorly circumscribed 40 mm mass in the uterine cervix (C).
At the time of consultation, the patient had no apparent gynecologic symptoms with the exception of a watery vaginal discharge. Magnetic resonance imaging (MRI) of the pelvis showed a poorly circumscribed 40 mm mass in the uterine cervix (Figure 1C). Brushing cytological study was performed. Papanicolaou staining of the tumor cells identified both isolated and clustered cells in a mucinous background. The cells were columnar-shaped, and their nuclei were enlarged with prominent nucleoli in them. Some tumor cells appeared as disordered and tightly crowded sheets, Abundant yellowish intracytoplasmic mucin was detected. Some infiltrating neutrophils and lymphocytes among the cells in the lesion were also observed (Figure 2).
Figure 2.
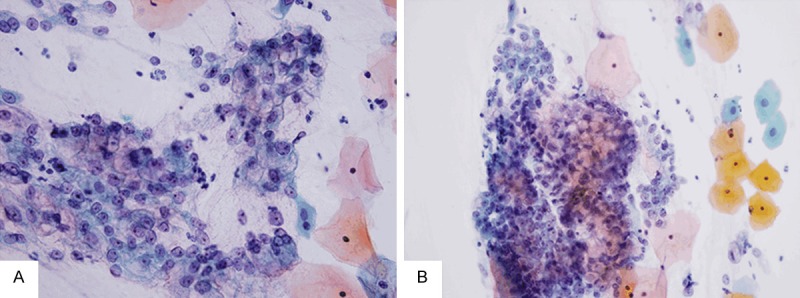
Cytological findings. Papanicolaou staining reveals isolated cells as well as clustered cells in the mucinous background. The tumor cells exhibit architectural disarray, and their nuclei are enlarged with prominent nucleoli in them (A). Tumor cells containing yellowish intracytoplasmic mucin are identified. Some infiltrating neutrophils and lymphocytes among the cells are also observed (B).
Based upon the result of the biopsy, which indicated mucinous adenocarcinoma, total hysterectomy was performed. Hematoxylin and eosion (HE) staining identified two histologically distinct components. The majority of the tumor was composed of dilated glands lined by single-layer columnar cells without prominent atypia. The nuclei were basally located and the cytoplasm was abundant and clear. Some glands in the deep area of uterine cervix had a distorted, irregular shape, suggesting MDA (Figure 3A).
Figure 3.
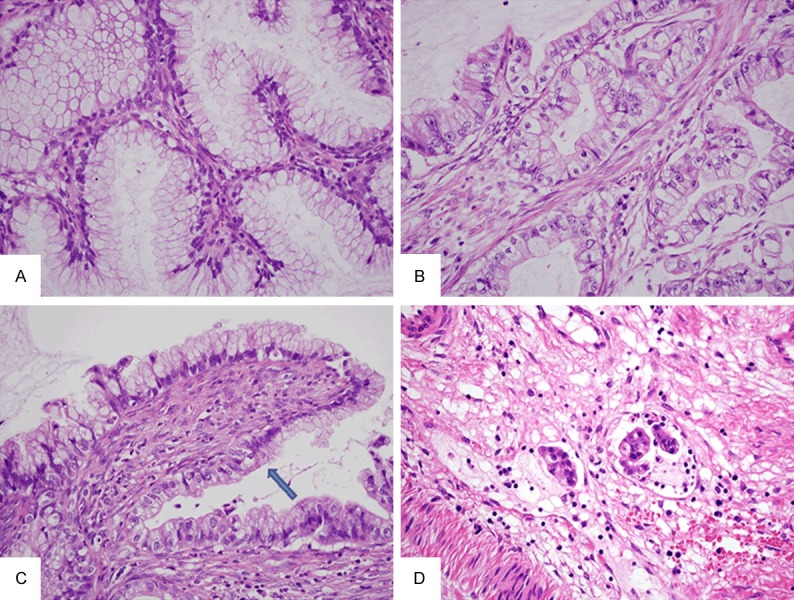
Histological findings. Irregularly shaped glands are detected. The glands are lined by single-layer columnar cells without prominent atypia. The nuclei are basally located and the cytoplasm is abundant and clear, suggesting MDA (A). Other glands contain cells with markedly enlarged atypical nuclei and prominent nucleoli. The tumor cells have abundant clear cytoplasm with prominent cell borders, reflecting the morphological features of GTA (B). Transitional area (arrow) between the MDA and GTA components is detected (C). Lymphatic involvements were observed (D).
Other glands contained cells with markedly enlarged atypical nuclei and prominent nucleoli. These glands were irregular in shape and exhibited stromal desmoplasia in some areas. The tumor cells had abundant clear cytoplasm with prominent cell borders, reflecting the morphological features of GTA (Figure 3B). Transitional area between the MDA and GTA components was detected (Figure 3C) as were lymphatic involvements (Figure 3D).
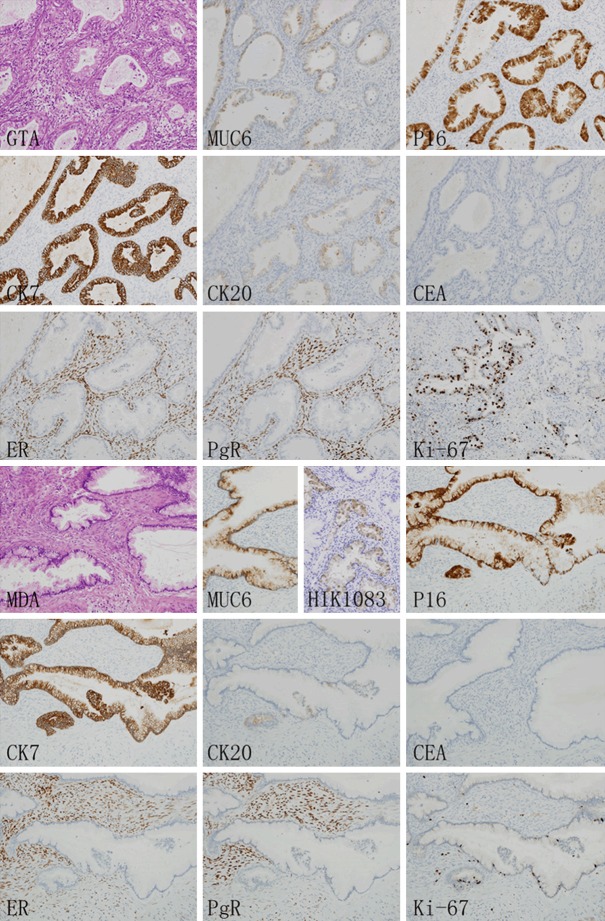
An immunohischemical study (Figure 4) of the GTA and MDA components using the primary antibodies listed in Table 1 [6]. Immunohistologically, both MDA and GTA showed cytoplasmic positivity for MUC 6. Moreover, certain MDA glands exhibited focally positive expression of HIK1083. MDA and GTA cells were diffusely positive for p16 and CK7, focally positive for CK20, and negative for CEA, ER and PgR. In addition, The Ki-67 labeling indices of the MDA and GTA components were 5% and 50% respectively, indicating the striking difference in proliferation between the two components.
Figure 4.
Immunohischemical study. Both GTA (upper half) and MDA (lower half) showed cytoplasmic positivity for MUC 6. Although no positive cells were found in GTA component, some MDA glands exhibited focally positive expression of HIK1083. Both GTA and MDA components were diffusely positive for p16 and CK7, focally positive for CK20, and negative for CEA, ER and PgR. The Ki-67 labeling indices of the GTA and MDA components were 50% and 5% respectively.
Table 1.
Primary antibodies used in this study
| Antibody | Clone | Type | Dilution |
|---|---|---|---|
| CEA | COL-1 | mm | Ready to use |
| CK7 | OV-TL 12/30 | mm | 1:50 |
| CK20 | Ks20.8 | mm | 1:50 |
| ER | SP1 | mr | Ready to use |
| MUC6 | CLH5 | mm | 1:100 |
| P16 | E6H4 | mm | Ready to use |
| PgR | iE2 | mr | Ready to use |
mm: mouse monoclonal, rp: rabbit polyclonal.
Discussion
Compared with the general population, patients with PJS have an increased incidence of malignant tumors. Although uterine cervical MDA is well characterized in PJS patients, GTA is a relatively unfamiliar entity in cervical mucinous adenocarcinoma. Both MDA and GTA are characterized by negative expression of p16, aggressive clinical courses and worse prognosis [4]. Although MDA shares many common features with GTA, and is classified as an extremely well differentiated form of GTA in the new WHO Classification of Tumors of Female Reproductive Organs, it has to date not been clear whether GTA arises from the dedifferentiation of MDA. Using aCGH and CISH analyses, McCluggage et al. identified common genetic aberrations between MDA and GTA indicating a possible link between these tumors [5]. In contrast to that study however, the MDA and GTA components in the current case were continuous in nature, and had similar immunophenotypes, favoring the hypothesis that GTA may arise from dedifferentiation of MDA.
Unlike the majority of usual type cervical adenocarcinomas, both MDA and GTA are generally not related to HPV infection [7-9], which complicates an early diagnosis of these tumors, even when a HPV DNA test is used. In the case of MDA in particular, obscure cell atypia makes it difficult to differentiate from normal cells. Familiarity with the cytological and histological features of these tumors is therefore important for prompt and accurate diagnosis. Cytologically, MDA is characterized by columnar-shaped cells with abundant yellowish mucinous cytoplasm in the Papanicolaou stain. However, since a yellowish appearance reflects a neutral, gastric/pyloric-type phenotype, it may also indicate GTA and similar staining has also been observed in endocervical glandular hyperplasia with pyloric gland metaplasia [10,11]. In addition, the cytological characteristics of MDA include a feathery appearance of cell clusters, nuclear enlargement, mitoses, irregularity of nuclear contour and loss of polarity. Compared with MDA, GTA has obvious malignant cytological features such as enlarged nuclei, prominent nucleoli, irregular arrangement of tumor cells, and prominent borders between cells, which distinguish this tumor from other usual type cervical adenocarcinomas.
Since it is a newly established entity with a generally worse prognosis than usual type cervical adenocarcinomas, recognition of GTA is important. The histological features of MDA are well known [12,13], while those of GTA include columnar-shaped cells with abundant clear or eosinophilic cytoplasm, clear cell borders, and inflammatory cells infiltration. Immunohistochemically, GTA tumor cells are typically positive for HIK1083 or MUC6, diffusely positive for CK7, focally positive for CK20 and usually negative for ER and PgR. In addition, diffuse p53 and CEA positivity are commonly seen, whereas p16 is typically negative [2]. Consistent with previous reports, the current case was diffusely positive for MUC6 and CK7, focally positive for CK20, and negative for ER and PgR. HIK1083 was focally positive in MDA. Unexpectedly however, p16 was diffusely positive in both the MDA and GTA components. In unusual types of cervical adenocarcinoma, including MDA and GTA, p16 expression is not necessarily associated with the presence of oncogenic HPV, and is considered an indirect phenomenon of an aberrant Rb functional pathway. A similar trend has also been observed in some benign cervical lesions, such as mesonephric hyperplasia and lobular endocervical glandular hyperplasia [7,14].
To our knowledge, this is the first report of continuous MDA and GTA lesions in a case of PJS. Moreover, our case exhibits overexpression of p16 protein, which has been considered very rare in both MDA and GTA. Additional studies are needed to determine the association between p16 overexpression and GTA in patients with PJS.
Acknowledgements
This work was supported in part by Grants-in-Aid for Clinical Rebiopsy Bank Project for Comprehensive Cancer Therapy Development to Zenya Naito from Ministry of Education, Culture, Sport, Science, and Technology, Japan (S1311022).
Disclosure of conflict of interest
None.
References
- 1.Li Y, Zeng Q, Liao Z, Zhang G, Xiao R, Wen H. Peutz-Jeghers syndrome and family survey: a case report. Int J Clin Exp Pathol. 2013;6:982–984. [PMC free article] [PubMed] [Google Scholar]
- 2.McCluggage WG. New developments in endocervical glandular lesions. Histopathology. 2013;62:138–160. doi: 10.1111/his.12012. [DOI] [PubMed] [Google Scholar]
- 3.Folkins AK, Longacre TA. Hereditary gynaecological malignancies: advances in screening and treatment. Histopathology. 2013;62:2–30. doi: 10.1111/his.12028. [DOI] [PubMed] [Google Scholar]
- 4.Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, Nishimura R. Gastric Morphology and Immunophenotype Predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–672. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- 5.McCluggage WG, Harley I, Houghton JP, Geyer FC, MacKay A, Reis-Filho JS. Composite cervical adenocarcinoma composed of adenoma malignum and gastric type adenocarcinoma (dedifferentiated adenoma malignum) in a patient with Peutz Jeghers syndrome. J Clin Pathol. 2010;63:935–941. doi: 10.1136/jcp.2010.080150. [DOI] [PubMed] [Google Scholar]
- 6.Peng WX, Kudo M, Fujii T, Teduka K, Naito Z. Altered expression of fibroblast growth factor receptor 2 isoform IIIc: relevance to endometrioid adenocarcinoma carcinogenesis and histological differentiation. Int J Clin Exp Pathol. 2014;7:1069–1076. [PMC free article] [PubMed] [Google Scholar]
- 7.Houghton O, Jamison J, Wilson R, Carson J, McCluggage WG. p16 Immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010;57:342–350. doi: 10.1111/j.1365-2559.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 8.Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, Juretzka MM, Pirog EC. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 9.Kusanagi Y, Kojima A, Mikami Y, Kiyokawa T, Sudo T, Yamaguchi S, Nishimura R. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. doi: 10.2353/ajpath.2010.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raab SS. Can glandular lesions be diagnosed in pap smear cytology? Diagn Cytopathol. 2000;23:127–133. doi: 10.1002/1097-0339(200008)23:2<127::aid-dc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Hata S, Mikami Y, Manabe T. Diagnostic significance of endocervical glandular cells with “golden-yellow” mucin on pap smear. Diagn Cytopathol. 2002;27:80–84. doi: 10.1002/dc.10140. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Jiang W, Gui S, Xu C. Minimal deviation adenocarcinoma of the uterine cervix. Int J Gynaecol Obstet. 2010;110:89–92. doi: 10.1016/j.ijgo.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Young RH, Clement PB. Endocervical adenocarcinoma and its variants: their morphology and differential diagnosis. Histopathology. 2002;41:185–207. doi: 10.1046/j.1365-2559.2002.01462.x. [DOI] [PubMed] [Google Scholar]
- 14.Hashi A, Xu JY, Kondo T, Hashi K, Yuminamochi T, Nara M, Murata S, Katoh R, Hoshi K. p16INK4a overexpression independent of human papillomavirus infection in lobular endocervical glandular hyperplasia. Int J Gynecol Pathol. 2006;25:187–94. doi: 10.1097/01.pgp.0000179612.63085.70. [DOI] [PubMed] [Google Scholar]