Abstract
Warthin-Like tumor of the thyroid is a recently described rare variant of papillary thyroid cancer. The distinct histological feature of this variant is papillary architecture lining oncocytic epithelial cells with nuclear characteristics of papillary carcinoma, accompanied by prominent lymphocytic infiltration in the papillary stalks. Here, we present a case of occult Warthin-like papillary thyroid carcinoma, 0.5-cm in maximum dimension, underwent left thyroid lobectomy in a 65 years old Chinese woman. In this case, there was no extrathyroid extension, vascular invasion and lymphatic metastasis, as well as no complication of lymphocytic thyroiditis. Immunohistochemistry staining revealed that the tumor cells were positive for Leu-M1, HBME-1, 34βE12, and MIB-1 labeling index was low. RET/PTC expression was absent in tumor cells. Furthermore, activated point mutations of BRAF V600E and V600K were concurrently detected by DNA sequencing. Further studies are needed to elucidate the prevalence and role of BRAFV600K mutation in papillary thyroid carcinoma, and long-term follow-up for the patient is needed to clarify the biological behavior of this variant with dual BRAF mutations.
Keywords: Warthin-like, papillary thyroid carcinoma, BRAF mutation
Introduction
Papillary carcinoma (PC) is the most common histotype of thyroid carcinoma, and often has a favorable prognosis than other carcinoma types. Warthin-like papillary thyroid carcinoma (WLPTC) is an uncommon variant which has papillary architecture with prominent lymphocytic stroma in their stalks, histologically resembling Warthin tumor of the salivary gland. The neoplastic cells lining the papillae have nuclear features of usual PC with oncocytic cytoplasm [1-12]. Recent studies demonstrated that papillary carcinoma have frequently genetic alterations involving in BRAF mutation or RET arrangement which leads to activation of the MAPK signaling pathway. So far, the BRAF mutation detected in PTC has been the V599E or V600E in exon 15, only rarely has other point mutation of BRAF gene been found in PTC [13-16]. The presence of BRAF mutation in PTC is an early event and has been associated with older age of patient, advanced tumor stages, higher recurrence and mortality [17,18]. Furthermore, most studies demonstrated that BRAF mutation and RET/PTC rearrangement rarely overlap in the same tumor [13-16].
Here, we reported a case of Warthin-like papillary thyroid microcarcinoma diagnosed at our hospital in August 2014. The clinicopathological features were analyzed and DNA sequencing of PCR-amplified exon 15 of BRAF gene was performed in this case.
Clinical history
A 65-year-old Chinese female was admitted to Shanghai Tongji Hospital in August 2014 because of Type I diabetes. She had no history of chronic thyroiditis or Hashimoto’s disease. Routine ultrasound examination of thyroid revealed a lesion of approximately 1.4×0.8 cm in size in the upper pole of the left lobe, which showed an uneven density of low echo with a hyperechoic spot, irregular margins and rich intra- and perilesional vascularization. Routine laboratory studies, including thyroid function test, proved no abnormalities. Fine needle aspiration cytology (FNAC) was performed on the left thyroid nodule and diagnosed as being suspicious for malignancy. Subsequently, the patient underwent a left thyroid lobectomy with left central neck lymphadenectomy.
Materials and methods
H&E and immunohistochemistry staining
The surgical specimen was fixed in 10% buffered formaldehyde and embedded in paraffin. The 4-μm-thick sections were stained with haematoxylin and eosin (H&E) for histological examination.
Immunostains for HBME-1, 34βE12, Galectin-3, Cyclin D1, Ki67 (MIB-1), TPO, CD15 (Leu-M1), Cytokeratin 19, CD20, CD79a, CD3, UCHL-1 (DAKO, Denmark) and anti-RET rabbit serum (Santa Cruz Biotechnology, Santa Cruz, CA) were carried out using EnVision two-step method. The tissues were incubated with primary antibodies diluted at 1:100 for all except for RET, 1:300 at 4°C overnight, after a prior high pressure antigen retrieval (10 minutes in citrate buffer, pH 6.0). Then slides were washed and incubated with horseradish peroxidase-linked secondary antibodies for 1 h at room temperature, followed by incubation with diaminobenzidine (DAB) for indicated time. Slides were counterstained with Mayer’s hemotoxylin for 2 min, dehydrated and mounted.
Macrodissection of tumor tissue
Macrodissection from paraffin-embedded specimens to obtain ‘pure’ tumor tissues was performed as follows: areas exhibiting exclusively tumor tissue were marked on histological slides and the tumor areas were obtained from at least ten slices of tissue (4 μm/one slice) with a scraper blade for subsequent DNA extraction.
Identification of BRAF mutation
For analysis of BRAF gene mutation, genomic DNA was isolated from the macrodissected tissues. Briefly, the sample was depafaffinized by xylene/ethanol treatment. DNA was extracted using the QIAamp DNA FFPE Tissue kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). Exon 15 of BRAF was amplified by polymerase chain reaction (PCR) with the primers, as followed: forward, 5’-TCT CAC CTC ATC CTA ACA CAT-3’; reverse, 5’-GTT TGA AAT ACA CTG AAA CTG GT-3’. The PCR conditions were initially denaturation at 95°C for 2 min, followed by 40 cycles (denaturation at 95°C for 40 s; annealing at 56°C for 30 s; synthesis at 72°C for 45 s) and a final extension at 72°C for 2 min. The amplicon size for exon 15 was 504 bp, then PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and sequenced using the forward and reverse primers described earlier and the Big Dye terminator kit (Applied Biosystems, Foster City, CA) by the automated ABI PRISM DNA sequencer (Applied Biosystems). Two M13 recombinant plasmids (wild type and mutation type) were used as negative and positive controls. Paratumor tissues of the case were used as an internal control.
Results
Cytopathological findings
Cytological examination showed papillary pattern and clusters with a background of mature lymphocytes. The clusters were composed of atypical cells which had eosinophilic cytoplasm and enlarged oval nucleoli with clear chromatin and nuclear grooves (Figure 1A).
Figure 1.
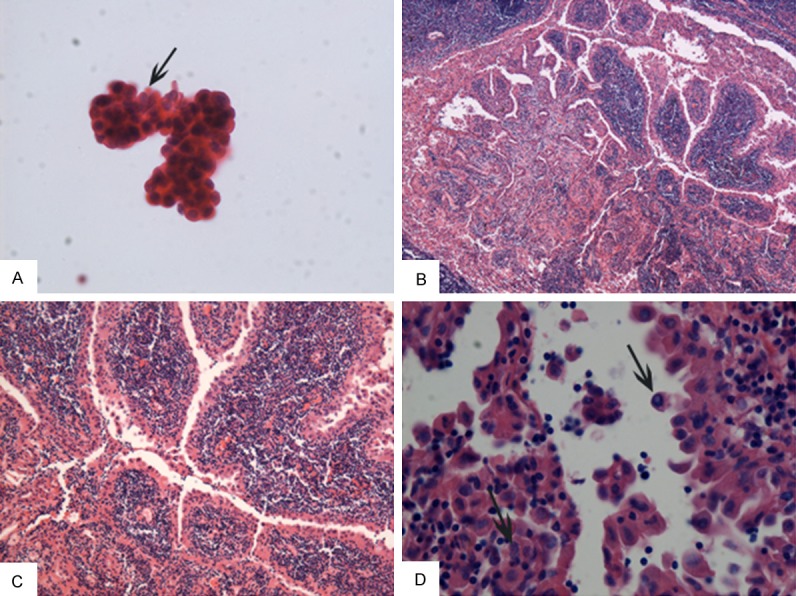
Morphological examination for the case of Wathin-like papillary thyroid carcinoma (HE stain). A: Fine needle aspiration cytology. Oncocytic papillary thyroid epithelium cells with elongated nuclei, clear chromatin and nuclear grooves (arrow indicated) and scattered lymphocytes in the background (×400). B-D: Histology revealed papillae with striking lymphocytic infiltration in papillary stalks. The lining neoplastic cells exhibited eosinophilic crytoplasm and irregular, clear, overlapping nuclei with nuclear grooves (arrow) and intranuclear pseudoinclusions (arrow) (×40, ×100, ×400 respectively).
Histopathological findings
Grossly, the surgical resected specimen revealed a 1.1×1×0.5 cm crimson lump, which has a white rigid region of about 0.4×0.3 cm. Microscopically, the lesion range was approximately 0.5×0.3 cm in size. The tumor showed a papillary architecture with prominent lymphatic stroma at the papillary stalk, which was circularly covered with one to several layers of atypical epithelial cells. These epithelial cells had clear, irregular, overlapping nuclei with grooves and pseudoinclusions and oncocytic cytoplasm (Figure 1B-D). Lymphocytes of the stroma showed no atypia. Furthermore, there was no metastasis found in three lymph nodes.
The neoplastic cells lining the papillae stained strongly for HBME-1, 34βE12, Galectin-3, Cyclin D1, CD15 (leu-M1) and Cytokeratin 19 (CK19) but absent for TPO and RET/PTC expression. The MIB-1 positive index showed a low rate of approximately 3%. The lymphocytic infiltrate in the papillae and surrounding thyroid gland showed a mixed population composed of B-cells, CD20 and CD79a positive, and T-cells, CD3 and UCHL1 positive (Figure 2).
Figure 2.
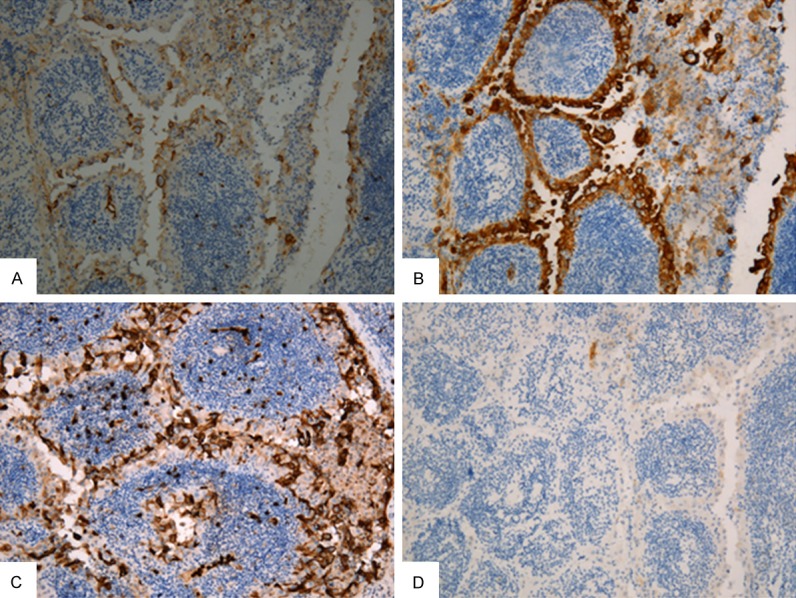
Immunohistochemistry staining. Neoplastic epithelial cells show positivity at membrane or cytoplasm for HBME-1 (A), 34βE12 (B), Leu-M1 (C), respectively (×100). (D) RET/PTC expression using anti-RET rabbit serum. No signal present in the cytoplasm and membrane of neoplastic epithelial cells (×100).
Mutation analysis of BRAF in exon 15
Genomic DNA was amplified by PCR and subjected to pyrosequencing for the BRAF status (Figure 3). Point mutations of BRAF V600E and V600K were synchronously detected (black line labeled). While paratumor tissues showed none of BRAF mutations. Two M13 recombinant plasmids (wt and mt) were used as negative and positive controls.
Figure 3.
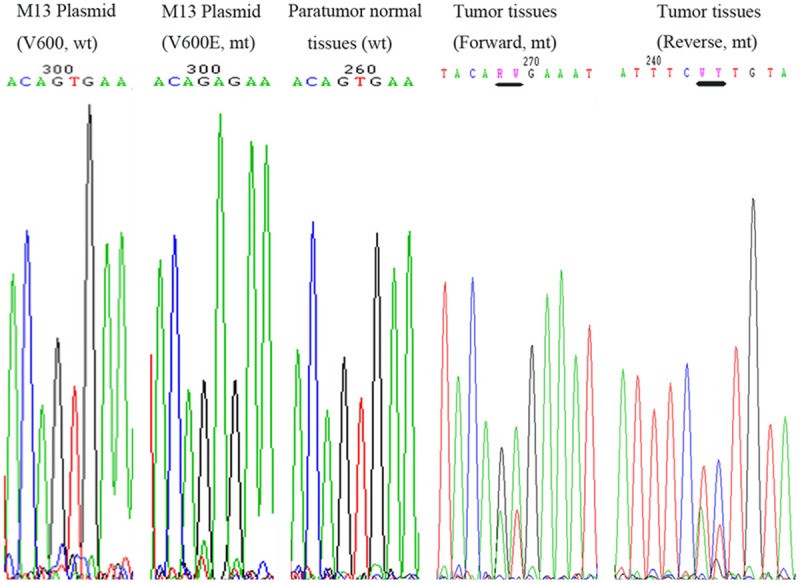
BRAF mutation by DNA sequencing. Sequence chromatogram of PCR (exon 15) product from two M13 recombinant plasmids, one for V600 wild type and the other for V600E mutation, used as negative and positive controls respectively. DNA sequence for paratumor tissues of the case of WLPTC showing no mutation, was used as an internal control. The corresponding tumor tissues were found harboring dual mutations of V600E and V600K (Lines indicated).
Discussion
WLPTC was first described by Apel et al in 1995 as an uncommon variant of papillary thyroid carcinoma in which neoplastic cells with oncocytic changes and clear nuclei, lining the papillary fronds that have lymphocytic stroma in their stalks. They named this tumor as “Warthin-like” because of its resemblance to Warthin tumor of the salivary gland. The onset age of patients with WLPTC ranged from 19 to 85 years, and the tumor size was 0.3-5 cm. Female patients account for a great proportion [1-12].
Regarding the rate of complication of thyroid cancer with chronic thyroiditis, the cases reports in the literature point out that lymphocytic thyroiditis or Hashimoto thyroiditis is an associating condition in most of the Warthin-like tumors [1-12]. In our case, there is only a small quantity of lymphocytes infiltration in the non-neoplastic thyroid tissue, but they are not enough to diagnose Hashimoto thyroiditis accompanying the tumor.
BRAF activated point mutation, which is the most common genetic hallmark in PTC, has been identified in 75% of WLPTCs. BRAF belongs to the RAF family of serine/threonine kinases that play an important role in regulating activity of Ras-Raf-MEK-ERK cascade, which induced cell growth, differentiation and apoptosis. Activating missense mutations of BRAF gene often occurs in the kinase domain of exons 15. The most common mutations of BRAF gene are attributable to a single-base thymidine substitution at the nucleotide position 1796 or 1799 with adenosine, which leads to the conversion of valine at codon 599 (V599E) or 600 (V600E) to glutamic acid [13-16]. BRAF V600K mutation, which occurs replacements of two nucleotides at nucleotide position 1798 and 1799 (GTG to AAG), substituting lysine for valine, was more frequent in melanoma [19]. It has been not reported in PTC so far. Currently, dual mutations of BRAF V600E and V600K were simultaneously detected in tumor tissues instead of paratumor tissues, suggesting that these two activated point mutations can cooperate with each other and participate in carcinogenesis of WLPTC. Additionally, there is no harboring V599E mutation in this case. Although RET/PTC rearrangement is frequently genetic alteration of PTC and rarely overlapping with BRAF mutation in previous more studies, a recent study by Guerra et al found 19.4% of PTC with concomitant BRAFV600E mutation and RET/PTC rearrangement [20]. To test whether BRAF mutation concurs with RET/PTC rearrangement in this case, we conducted immunohistochemistry staining to determine the status of RET/PTC rearrangement. The result revealed that RET/PTC expression was absent in the cytoplasm of the tumor cells, which suggests RET/PTC rearrangement is not principle factor in initiating its tumorigenesis.
Although long-term follow-up data are needed, WLPTC is generally reported to have an indolent clinical behavior. Some authors consider existence of lymphocytic stroma in thyroid cancer as an indicator of favorable prognosis [4]. However, Mai et al reported that Hürthle cell PTC associated with Warthin-like features or tall cell variant usually has worse prognosis than conventional papillary carcinoma [21]. Lam et al reported a WLPTC case with dedifferentiation and aggressive biological behavior. The patient developed distant metastases and died of the disease 18 months after operation [9]. Besides, more studies supported BRAF mutation was associated with nodal metastasis and recurrence, and RET/PTC rearrangements were less frequent in the more aggressive PTCs [17,18,22]. Although the clinicopathological features (small size <1 cm, absence of extrathyroidal extension, vascular invasion and lymph nodes metastasis) suggest a more favorable behavior in this case, its harboring BRAF gene dual mutations needs to be valued and long-term follow-up for the patient is necessary to evaluate prognosis.
In conclusion, we first reported the presence of BRAFV600K mutation in a case of WLPTC. Further studies were needed to investigate and confirm the frequency and role of BRAFV600K mutation in papillary thyroid carcinoma.
Acknowledgements
This work has been supported by a grant from the National Natural Science Foundation of China (No. 81201838) for Dr Fei Han.
Disclosure of conflict of interest
None.
References
- 1.Apel RL, Asa SL, LiVolsi VA. Papillary Hürthle cell carcinoma with lymphocytic stroma. “Warthin-like tumor” of the thyroid. Am J Surg Pathol. 1995;19:810–814. [PubMed] [Google Scholar]
- 2.Vera-Sempere FJ, Prieto M, Camanas A. Warthin-like tumor of the thyroid: a papillary carcinoma with mitochondrion-rich cells and abundant lymphoid stroma. A case report. Pathol Res Pract. 1998;194:341–347. doi: 10.1016/S0344-0338(98)80058-7. [DOI] [PubMed] [Google Scholar]
- 3.Fadda G, Mule A, Zannoni GF, Vincenzoni C, Ardito G, Capelli A. Fine needle aspiration of a warthin-like thyroid tumor. Report of a case with differential diagnostic criteria vs. other lymphocyterich thyroid lesions. Acta Cytol. 1998;42:998–1002. doi: 10.1159/000331984. [DOI] [PubMed] [Google Scholar]
- 4.Baloch ZW, LiVolsi VA. Warthin-like papillary carcinoma of the thyroid. Arch Pathol Lab Med. 2000;124:1192–1195. doi: 10.5858/2000-124-1192-WLPCOT. [DOI] [PubMed] [Google Scholar]
- 5.D’Antonio A, De Chiara A, Santoro M, Chiappetta G, Losito NS. Warthin-like tumour of the thyroid gland: RET/PTC expression indicates it is a variant of papillary carcinoma. Histopathology. 2000;36:493–498. doi: 10.1046/j.1365-2559.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 6.Ludvikova M, Ryska A, Korabecna M, Rydlova M, Michal M. Oncocytic papillary carcinoma with lymphoid stroma (Warthinlike tumour) of the thyroid: A distinct entity with favourable prognosis. Histopathology. 2001;39:17–24. doi: 10.1046/j.1365-2559.2001.01154.x. [DOI] [PubMed] [Google Scholar]
- 7.Urano M, Abe M, Kuroda M, Mizoguchi Y, Horibe Y, Kasahara M, Tanaka K, Sudo K, Hirasawa Y. Warthin-like tumor variant of papillary thyroid carcinoma: case report and literature review. Pathol Int. 2001;51:707–712. doi: 10.1046/j.1440-1827.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 8.Anwar F. The phenotype of Hürthle and Warthin-like papillary thyroid carcinomas is distinct from classic papillary carcinoma as to the expression of retinoblastoma protein and E2F-1 transcription factor. Appl Immunohistochem Mol Morphol. 2003;11:20–27. doi: 10.1097/00129039-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lam KY, Lo CY, Wei WI. Warthin tumor-like variant of papillary thyroid carcinoma: a case with dedifferentiation (anaplastic changes) and aggressive biological behavior. Endocr Pathol. 2005;16:83–89. doi: 10.1385/ep:16:1:083. [DOI] [PubMed] [Google Scholar]
- 10.Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hürthle (oncocytic) cell and its associated pathologic conditions. A surgical pathology and cytopathology review. Arch Pathol Lab Med. 2008;132:1241–1250. doi: 10.5858/2008-132-1241-TTHOCA. [DOI] [PubMed] [Google Scholar]
- 11.Kim HH, Myssiorek D, Heller KS, Zahurullah F, Bhuiya T. Warthin-like tumor of the thyroid gland: an uncommon variant of papillary thyroid cancer. Ear Nose Throat J. 2006;85:56–59. [PubMed] [Google Scholar]
- 12.Ersen A, Durak MG, Canda T, Sevinc AI, Saydam S, Kocdor MA. Warthin-like papillary carcinoma of the thyroid: a case series and review of the literature. Turk Patoloji Derg. 2013;29:150–155. doi: 10.5146/tjpath.2013.01168. [DOI] [PubMed] [Google Scholar]
- 13.Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, Refetoff S, Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 14.Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Maximo V, Botelho T, Seruca R, Sobrinho-Simoes M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 15.Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 16.Trovisco V, Vieira de Castro I, Soares P, Maximo V, Silva P, Magalhaes J, Abrosimov A, Guiu XM, Sobrinho-Simoes M. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol. 2004;202:247–251. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforov YE, Fagin JA. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 18.Guerra A, Fugazzola L, Marotta V, Cirillo M, Rossi S, Cirello V, Forno I, Moccia T, Budillon A, Vitale M. A high percentage of BRAFV600E alleles in papillary thyroid carcinoma predicts a poorer outcome. J Clin Endocrinol Metab. 2012;97:2333–2340. doi: 10.1210/jc.2011-3106. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein JC, Sznol M, Pavlick AC, Ariyan S, Cheng E, Bacchiocchi A, Kluger HM, Narayan D, Halaban R. Incidence of the V600K mutation among melanoma patients with BRAF mutations and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67–69. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra A, Zeppa P, Bifulco M, Vitale M. Concomitant BRAFV600E mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. 2014;24:254–259. doi: 10.1089/thy.2013.0235. [DOI] [PubMed] [Google Scholar]
- 21.Mai KT, Thomas J, Yazdi HM, Commons AS, Lamba M, Stinson AW. Pathologic study and clinical significance of Hürthle cell papillary thyroid carcinoma. Appl Immunohistochem Mol Morphol. 2004;12:329–337. doi: 10.1097/00129039-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Guerra A, Sapio MR, Marotta V, Campanile E, Moretti MI, Deandrea M, Motta M, Limone PP, Fenzi G, Rossi G, Vitale M. Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr J. 2011;58:31–38. doi: 10.1507/endocrj.k10e-260. [DOI] [PubMed] [Google Scholar]


