Abstract
Bile duct adenoma (BDA) is a comparatively rare disease and there are relatively few reported cases in the English-language literature. Herein, we present a 63-year-old woman, who was incidentally found to have a liver-occupying lesion during a routine medical examination. Ultrasonography suggested “quick wash-in and wash-out” sign with an obvious nodular enhancement in the peripheral of the right intrahepatic nodular. Computed tomography revealed a 33 mm×25 mm×28 mm mass in the right hepatic segment. The patient underwent a liver tumor resection. Histological examination showed that the tumor was consisted of small heterogeneous tubular ducts with fibrous tissues and several inflammatory cells, without cell atypia and mitotic activity. Immunohistochemically, the tumor cells were positive for CK19, CK7, CD56 and CD10. The final histopathological diagnosis was intrahepatic BDA.
Keywords: Liver, bile duct adenoma, peribiliary gland hamartoma
Introduction
Intrahepatic bile duct adenoma (BDA) is a rare benign liver tumor arising from the epithelium of the intrahepatic bile ducts. Bhathal et al [1] suggests that BDA should be called a peribiliary gland hamartoma because of a similarity between bile duct adenoma and peribiliary glands in their secretory gland cell phenotype. And accounting for 1.3% of primary liver tumors [2]. Craig et al. found only five BDA in 50000 autopsies [3]. BDA has been reported to show benign behavior and have limited growth potential, but one report pointed out that it can malignantly transformate into cholangiocarcinoma [4]. BDA are still confused with metastatic well-differentiated adenocarcinoma by surgeons and pathologists. Herein, we report a case of BDA and present a brief review of the literature. To the best of our knowledge, less than twenty cases reported in the literature to date from 1991.
Case report
A 63-year-old Chinese female was incidentally found to have a liver-occupying lesion during a routine medical examination. Ultrasonography revealed a 30 mm×20 mm mass in the right lobe with “quick wash-in and wash-out” sign. Computed tomography revealed a 33 mm×25 mm×28 mm mass in the right hepatic segment. MRI revealed that the mass showed heterogeneous high signal intensity on T2-weighted images and low signal intensity on T1-weighted images (Figure 1A, 1B); dynamic enhanced scanning the mass with an enhancement effect persisting into the portal phase, arterial early phase mass began to strengthen, the arterial late phase enhancement obviously (Figure 1C, 1D). Routine laboratory investigations were normal, including complete blood count and serum urea and electrolyte levels. The liver function tests were within normal limits. Tumor markers, including carcinoembryonic antigen, α-fetoprotein, and carbohydrate antigen 19-9, were all within normal limits. Serum hepatitis viral markers were negative. The patient underwent a liver tumor resection. Surgery revealed the mass is subcapsular and well-cirumscribed but non-encapsulated. The patient has been free from tumor recurrence in the 8 months since surgery.
Figure 1.
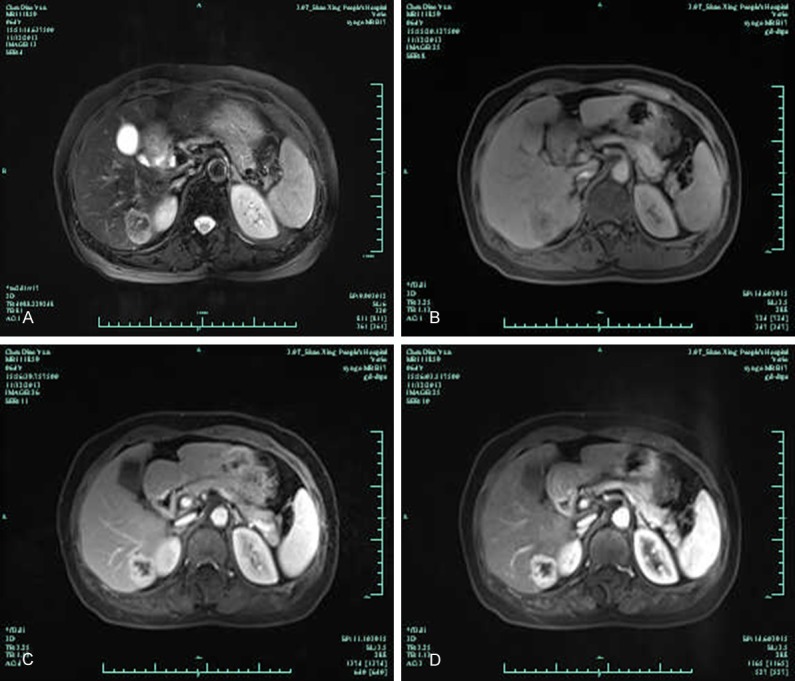
MRI revealed that the mass showed heterogeneous high signal intensity on T2-weighted images and low signal intensity on T1-weighted images (A and B); dynamic enhanced scanning the mass with an enhancement effect persisting into the portal phase, arterial early phase mass began to strengthen, the arterial late phase enhancement obviously (C and D).
Pathological examination
On gross examination, the segmental liver resection specimen measured 40 mm×30 mm×25 mm, cross-sections revealed a yellowish white, well circumscribed, subcapsular firm tumor and measured 25 mm×20 mm×10 mm. Microscopically, the tumor had no capsule, but was well circumscribed from the surrounding liver parenchyma (Figure 2A); the liver parenchyma showed no fatty change; the tumor was mainly composed of a proliferation of small, uniform small-sized ducts with cuboidal cells that had regular nuclei; near the periphery of the tumor was consisted of densely packed proliferation of simple tubular ducts combined with some chronic inflammatory cell infiltration, cuboidal epithelium resembled that of interlobular bile ducts without cell atypia or mitotic activity (Figure 2B); in the central fibrosis was denser, the density of small bile duct was decreased and was separated by fibrous septa, squeezed into a slit like or irregular in shape (Figure 2C, 2D); and focal areas showed clusters of calcification. Immunohistochemically, tumor cells were strongly reactive for CK19 (Figure 3A), CK7 (Figure 3B), CD56 (Figure 3C) and CD10 (Figure 3D), Ki-67 were 5% positive, but were negative for AFP, P53, CDX2, CK20, TTF-1 and Syn. The histologic diagnosis was intrahepatic BDA.
Figure 2.
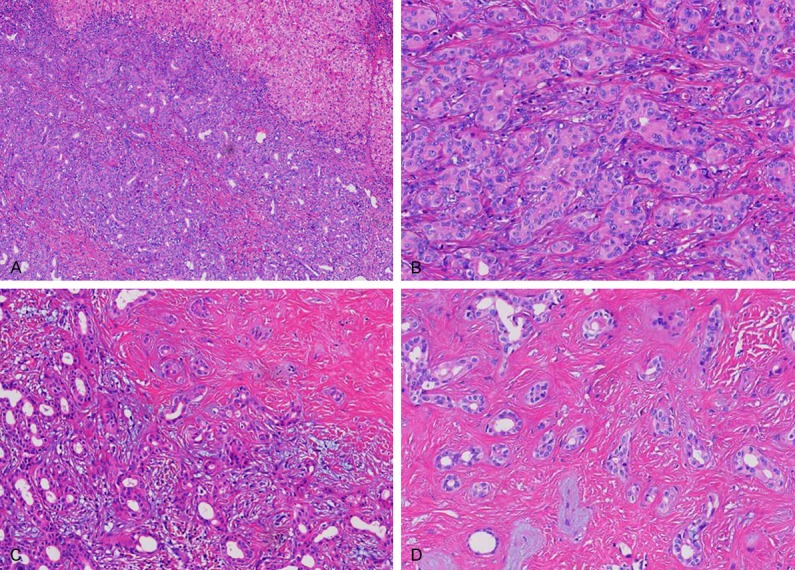
Microscopically, the tumor had no capsule, but was well circumscribed from the surrounding liver parenchyma (A); the tumor was mainly composed of a proliferation of small, uniform small-sized ducts with cuboidal cells that had regular nuclei; near the periphery of the tumor was consisted of densely packed proliferation of simple tubular ducts combined with some chronic inflammatory cell infiltration (B and C); in the central fibrosis was denser, small bile duct was decreased and was separated by fibrous septa, squeezed into a slit like or irregular in shape (D).
Figure 3.
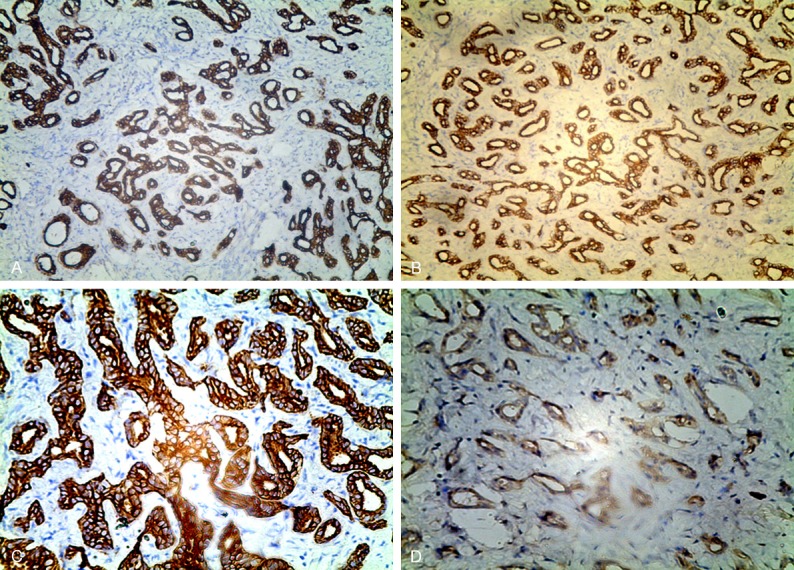
Immunohistochemically, tumor cells were strongly reactive for CK19 (A), CK7 (B), CD56 (C) and CD10 (D).
Discussion
Intrahepatic BDA, also known as peribiliary gland hamartoma, is uncommon tumor which is usually found incidentally during surgery or at autopsy, accounting for 1.3% of primary liver tumors, however the true incidence is unknown [1-6]. In the largest series of BDA, Allaire et al. [2] found 152 cases between 1943 and 1986. Of these 152 BDA, 103 patients did not present with clinical symptoms and were discovered incidentally during abdominal surgery, 49 patients were found at autopsy. Our patient was also found to have liver occupying lesions incidentally when undergoing an abdominal B ultrasound examination during a routine medical examination.
There has been considerable controversy in the etiology, pathogenesis and origin of BDA. Early study has described BDA with alcoholic cirrhosis, biliary obstruction, hemangioma, and focal nodular hyperplasia [1,2,5,7], but a few cases with chronic hepatitis related with hepatitis C virus have been reported [8]. Our patient doesn’t have liver cirrhosis and hepatitis virus infection. The pathogenesis of BDA has been considered to be a reactive process to a focal bile ductular injury caused by trauma or inflammation. Bhathal et al [1] demonstrated a similarity in the secretory gland cell phenotype between BDA and peribiliary glands, suggesting that BDA represents disorganized peribiliary glands.
There are no tumor markers or imaging characteristics that allow a preoperative diagnosis, the diagnosis is based entirely on histopathological and immunohistochemical findings. BDA is usually single, usually <2 cm in size, and is subcapsular and well-circumscribed but non-encapsulated [9-12], also there have been several very large BDA reports and its diameter can be up to 92 mm [13]. Pathologically BDA is composed of a proliferation of small, uniform small-sized ducts with cuboidal cells that have regular nuclei; these ducts have no or little lumen and can elaborate mucin; their associated fibrous stroma shows varying degrees of chronic inflammation, collagenization and calcification [14]. Also BDA can appear oncocytic [15-17], signet-ring [18] and clear cell features [19].
Immunohistochemical stains for CD10, CK19, CK7 and CD56 are positive, whereas for AFP and p53 stains are negative. BDA and peribiliary glands share common antigens, suggesting a common line of differentiation and leading some to consider it a peribiliary gland hamartoma. Some reports have shown that CD10 and CD56 was negative in malignant extrahepatic bile duct lesions but it was positive in benign lesions, CD10 and CD56 expression in our case suggests that this tumor is a benign adenoma rather than an invasive carcinoma. CD10 and CD56 staining may be useful in distinguishing intrahepatic BDA from intrahepatic cholangiocarcinoma [6].
Our patient was an elderly woman, without any clinical symptoms, who was incidentally found to have a liver-occupying lesion during a routine medical examination, and experimental examination also had no abnormal findings. Combined with postoperative routine HE morphology and immunohistochemical features, the tumor was conformed to the diagnosis of intrahepatic BDA. Even when conducting a frozen section examination, it is still difficult to identify BDA from cholangiocarcinoma or liver metastasis. If the pathologist was lack of experience, it was easily misdiagnosed as metastatic adenocarcinoma or high differentiation cholangiocarcinoma.
The differential diagnosis included cholangiocarcinoma and metastatic adenocarcinoma, biliary adenofibroma, Von Meyenberg syndrome and primary sclerosing cholangitis. Cholangiocarcinoma and metastatic adenocarcinoma cells often have moderate to severe atypia, and the latter usually has a history of adenocarcinoma of the other body parts. Biliary adenofibroma is characterized by a complex tubulocystic biliary epithelium without mucin production, together with abundant fibroblastic stromal components, and it might be related Von Meyenburg complex on the basis of similar morphological architecture and epithelial expression of CD10. Von Meyenburg complex is small, up to several millimetres in diameter, these lesions are usually multiple and are adjacent to a portal tract and, if widespread, may be an indication of fibropolycystic disease of the liver.
BDA is regarded as a benign tumor, the prognosis is favorable, so far there is no report about tumor recurrence in the literature, but it still has the possibility of carcinogenesis. Hasebe et al. [4] reported a case in which BDA developed into cholangiocarcinoma. Therefore, surgical resection is still needed as a way of treatment and local excision is also feasible.
In summary, BDA is extremely rare benign tumor of the liver, which is difficult to distinguish it from intrahepatic cholangiocarcinoma. Its main pathological characteristic is a large quantity of proliferated bile ducts mixing varying amounts of collagen and inflammatory cells infiltration, immunohistochemical stains for CD10, CK19, and CD56 are positive, whereas for AFP and p53 stains are negative. Surgical resection is the main treatment method for BDA.
Disclosure of conflict of interest
None.
References
- 1.Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858–64. doi: 10.1097/00000478-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708–15. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 3.An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol. 2013;14:769–75. doi: 10.3348/kjr.2013.14.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, Fukamachi S, Hirohashi S. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426:209–13. doi: 10.1007/BF00192644. [DOI] [PubMed] [Google Scholar]
- 5.Aishima S, Tanaka Y, Kubo Y, Shirabe K, Maehara Y, Oda Y. Bile duct adenoma and von Meyenburg complex-like duct arising in hepatitis and cirrhosis: pathogenesis and histological characteristics. Pathol Int. 2014;64:551–9. doi: 10.1111/pin.12209. [DOI] [PubMed] [Google Scholar]
- 6.Wu WW, Gu M, Lu D. Cytopathologic, histopathologic, and immunohistochemical features of intrahepatic clear cell bile duct adenoma: a case report and review of the literature. Case Rep Pathol. 2014;2014:874826. doi: 10.1155/2014/874826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes NR, Goodman ZD, Bhathal PS. An immunohistochemical profile of the so-called bile duct adenoma: clues to pathogenesis. Am J Surg Pathol. 2010;34:1312–8. doi: 10.1097/PAS.0b013e3181ead722. [DOI] [PubMed] [Google Scholar]
- 8.Tajima T, Honda H, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Taguchi K, Shimada M, Masuda K. Radiologic features of intrahepatic bile duct adenoma: a look at the surface of the liver. J Comput Assist Tomogr. 1999;23:690–5. doi: 10.1097/00004728-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Xu MY, Chen F. Bile duct adenoma: a case report and literature review. World J Surg Oncol. 2014;12:125. doi: 10.1186/1477-7819-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández Fernández JM, Muriel Cueto P, Sánchez Sánchez AM, Dulanto Vargas M, Bados Nieto P. Bile duct adenoma (hamartoma of peribiliary glands): Importance of an adequate diagnosis. Cir Esp. 2013 doi: 10.1016/j.ciresp.2013.06.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Takumi K, Fukukura Y, Nagasato K, Nakajo M, Natsugoe S, Higashi M. Intrahepatic bile duct adenoma mimicking hepatic metastasis: case report and review of the literature. Magn Reson Med Sci. 2013;12:141–5. doi: 10.2463/mrms.2012-0078. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Rha SE, Oh SN, Jung SE, Shin YR, Choi BG, Byun JY, Jung ES, Kim DG. Imaging findings of intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and literature review. Korean J Radiol. 2010;11:560–5. doi: 10.3348/kjr.2010.11.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga F, Tanaka H, Takamatsu S, Baba S, Takihara H, Hasegawa A, Yanagihara E, Inoue T, Nakano T, Ueda C, Ono W. A case of very large intrahepatic bile duct adenoma followed for 7 years. World J Clin Oncol. 2012;3:63–6. doi: 10.5306/wjco.v3.i4.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda E, Uozumi K, Kato N, Akahane M, Inoh S, Inoue Y, Beck Y, Goto A, Makuuchi M, Ohtomo K. Magnetic resonance findings of bile duct adenoma with calcification. Radiat Med. 2006;24:459–62. doi: 10.1007/s11604-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 15.Hastir D, Verset L, Demetter P. Intrahepatic bile duct adenoma with oncocytic features. Liver Int. 2013;33:273. doi: 10.1111/j.1478-3231.2012.02866.x. [DOI] [PubMed] [Google Scholar]
- 16.Johannesen EJ, Wu Z, Holly JS. Bile duct adenoma with oncocytic features. Case Rep Pathol. 2014;2014:282010. doi: 10.1155/2014/282010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arena V, Arena E, Stigliano E, Capelli A. Bile duct adenoma with oncocytic features. Histopathology. 2006;49:318–20. doi: 10.1111/j.1365-2559.2006.02441.x. [DOI] [PubMed] [Google Scholar]
- 18.Gambarotti M, Medicina D, Baronchelli C, Bercich L, Bonetti F, Facchetti F. Alpha-1-antitrypsin-positive “signet-ring” bile duct adenoma in a patient with M (MALTON) mutation. Int J Surg Pathol. 2008;16:218–21. doi: 10.1177/1066896907306968. [DOI] [PubMed] [Google Scholar]
- 19.Albores-Saavedra J, Hoang MP, Murakata LA, Sinkre P, Yaziji H. Atypical bile duct adenoma, clear cell type: a previously undescribed tumor of the liver. Am J Surg Pathol. 2001;25:956–60. doi: 10.1097/00000478-200107000-00016. [DOI] [PubMed] [Google Scholar]


