Abstract
Adenomatoid tumor (AT) is an extremely rare benign tumor in the testis of infants. A case of 14-month-old boy with testicular adenomatoid tumor was reported in this study. On physical examination, a smooth solid nodule sized 8 mm could be palpated with little tenderness on the head of the right testis. It could be clearly revealed by B ultrasonic scanning and computerized tomography. The patient underwent right radical orchiectomy. In postoperative histopathological study, the tumor was characterized by diffuse sheets of epithelioid cell and desmo-stroma structures. There was positive immunohistochemical staining of mesothelioma-associated antigens. The tumor should be differentiated from the tumor of the male genital tract including benign and malignant tumors of both epithelial and stromal origin. And we followed the case and no nodule was found in his scrotum by physical examination and scrotal ultrasonography after 3, 6, 12, 18, 24, 30, 36, 42, 48, 54, 60 months. These findings have important implications that the histogenesis of adenomatoid tumor of the testis is unclear yet. The diagnosis depends on pathologic studies, and should be differentiated from paratesticular malignant mesothelioma and sclerosed lipogranuloma. Radical surgery is the common choice, and as a result of getting a good prognosis.
Keywords: Adenomatoid tumor, testicle, infant, case report, review
Introduction
Adenomatoid tumor (AT) is a benign neoplasm that usually occurs in the genital tracts of both genders. In women, it characteristically arises in the uterus, fallopian tube, and rarely in the ovary, and in men it has been most frequently found in the epididymis, testicular tunica, and spermatic cord. Extragenital ATs have been reported to occur in the omentum [1], umbilical skin [2], mesentery of the small intestine [3], pancreas [4], mediastinal lymph node [5], pleura [6], and heart [7]. Primary adenomatoid tumor of the testis is substantial clinical very rare begin tumor, and testicular adenmatiod tumor of the infant is extremely rare.
Case report
A 14-month-old Chinese boy was represented with a 3 months history of the right scrotal mass with no pain. No urinary or constitutional symptoms were complained. Physical examination revealed a firm hard lump, 8×7 mm in size, adherent to the inferior pole of the testis, with no ulceration and no inguinal lymphadenopathy. Serum tumor markers including β-human chorionic gonadotropin (<0.5 IU/L) and αfetoprotein (5.23 μg/L) were normal.
Scrotal ultrasonography examination revealed a hypo echoic lesion in the right testis, color Doppler flow image shows color Doppler signals surrounding in the periphery of testicular parenchyma, and RI was 0.72. Pelvic CT examination was performed, revealing a right testicular mass, with no ulceration and no inguinal lymphadenopathy (Figure 1).
Figure 1.
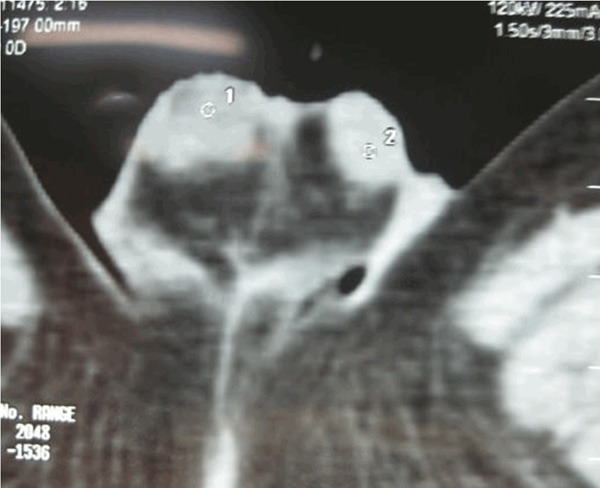
CT image of scrotum. The 8 mm-sized slightly lower density solid nodule on the head of right testicular parenchyma is detected and the border is unclear.
The patient underwent exploration via a right inguinal approach. The tumor could not be seen but could be touched after isolating the spermatic cord. Suspicion biopsy was performed for intraoperative frozen section analysis. The results were suggestive of a mesothelium neoplasm and malignancy could not be excluded. A right radical orchiectomy with high ligation of the cord was therefore performed. The gross appearance of adenomatoid tumor indicated that a relatively well circumscribed solid pink nodule occupied predominantly the substance (Figure 2). Final pathological analysis showed mesothelium cell invasion from the tunica albuginea into the testicular parenchyma. Light microscope showed that the tumor was consisted of two main components: the elements from the epithelial cells and fibrous stroma (Figure 3).
Figure 2.
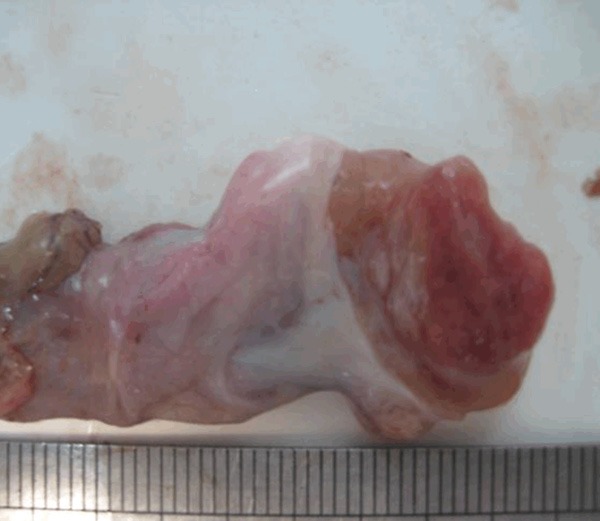
Gross appearance of adenomatoid tumor. The figure shows the cut surface of the right testis in lateral view. A relatively well circumscribed solid pink nodule occupied predominantly the substance.
Figure 3.
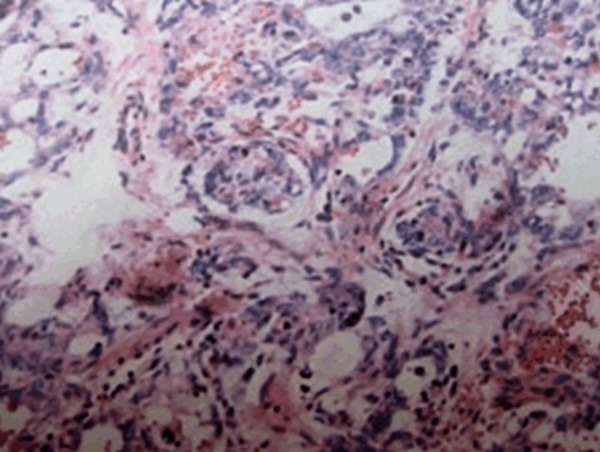
Micrograph of adenomatoid tumor of the testis. The tumor is characterized by diffuse sheets of epithelioid cell and desmo-stroma structures (hematoxylin-eosin, original magnification ×100).
The tumor cells showed negative staining by the PAS method, but they were positive for cytokeratin, epithelial membrane antigen (EMA), α-trypsin, calcium retinal protein (calretinin), vimentin (Vim). They were weak in carcinoembryonic antigen (CEA), negative for P53, factor VIII, AFP and placental alkaline phosphatase (PLAP). See Table 1.
Table 1.
Immunohistochemicalstaining of adenomatoid tumours
| Antibody | Staining intensity |
|---|---|
| Vim | (++) |
| Cytokeratin | (+++) |
| EmA | (+++) |
| Calretinin | (+++) |
| α-Trypsin | (+) |
| CEA | (+) |
| AFP | (+/-) |
| PLAP | (-) |
| factor VIII | (-) |
| P53 | (-) |
On physical examination after 3, 6, 12, 18, 24, 30, 36, 42, 48, 54, 60 months, no nodule was found in the scrotum. No neoplasm was found by scrotal ultrasonography and pelvic CT. Β-human chorionic gonadotropin and α-fetoprotein levels were within normal limits.
Discussion
Adenomatoid tumor (AT) is a rare benign tumor, and adenomatoid tumors of the male (paratesticular) and female (uterus and fallopian tubes) reproductive tracts have been recognized since the report of Golden and Ash in 1945. The origin of these tumors was debated for years, but more recent studies utilizing electron microscopy and immunohistochemical staining point toward a likely mesothelial origin. Their mesothelial origin is controversial, but like normal mesothelial cells, the tumor may produce hyaluronic acid which, in association with cellular architecture, supports this embryological theory [8,9]. There are four theories on the origin: mesoyhelial source, endothelial source, mensonephros and Müller tube [10]. AT typically present as a hard painless mass discovered incidentally on physical examination in men between the third and seventh decades, and more common in 30-40 years old. Adenomatoid tumor of the testis is the second most common benign tumor of the paratestis. The great majority of tumors in men are found in the epididymis (about 77%), the testis sheath or tunica albuginea (about 22%), with only rare case reports in the spermatic cord (about 1%) from the seminal vesicles. AT in the testicular parenchyma is quite rare and it is extremely rare in the testis of infants. In this case report, we revealed a 14-month-old boy with AT in testicular parenchyma [9,11-15]. AT of the testis typically presents as small, firm and asymptomatic mass, most often localizes to the lower pole of the testis. Adenomatoid tumors have generally been described as having similar or greater echogenicity than testicular parenchyma on ultrasonography. Kassis A [16] reported that iso-, hypo- and hyperechogenicity were present in five of their patients, and a case similar to the present patient. Some of the present clinical characteristics were helpful in planning surgical treatment in these patients. Superficial and lower pole localization may be considered as special features, but most important were the pain and mobility of the lesion over the underlying normal parenchyma on palpation. The combination of these clinical characteristics, with the results of ultrasonography, seems to be more rewarding than relying on ultrasonography alone in deciding the management of these rare and challenging tumors. This present tumor appeared hyperechogenicity on ultrasonography, and pelvic CT revealed a slightly low density, ill-defined tumor in the right testis [17-19].
On gross inspection, they tend to be white or pink, superficial and very well circumscribed. They are firm and are usually <3 cm in diameter. The histological features are of a gland-like structure with cuboidal cells; the stroma is fibrous and dense. On microscopic examination, AT may show a variety of histologic patterns. Adenomatoid tumor can be divided into four types: adenoid tissue, vascular tumor-like, solid and cystic cord strips. More commonly, the tumor is composed of irregular acinar spaces lined by flattened epithelial-like cells within a loose fibrous tissue stroma rich in reticulin. The present tumor was adenoid tissue in this case, and on light microscopic examination, there are two major components: epithelial-like cells and fibrous stroma (Figure 3). On transmission electron microscopy, adenomatoid tumor typically shows features in common with mesothelial cells, with prominent desmosomes, and long microvilli on the luminal surface of tumor acini and on the surface of intercellular canaliculi. These features have been confirmed in several studies and the ultrastructural similarity between adenomatoid tumor, and mesothelial cells and epithelial mesothelioma has been observed. The similarity of ultrastructural features of adenomatoid tumor and epididymal duct cells has also been noted and it has been suggested that adenomatoid tumor is derived from coelomic cells as they differentiate towards genital epithelium [20].
The tumor cells show variable staining for glycogen by the PAS method but usually show positive staining for acid mucopolysaccharide with Alcian blue, colloidal iron, or toluidine blue, which is abolished by hyaluronidase digestion. More rarely, the tumor has a predominantly syncytial or plexiform appearance composed of individual cells, cell nests, cords, and sheets. The tumor cells exhibit a stellate pattern focally, and cytoplasmic processes may be seen extending into adjacent stroma. In syncytial AT, the cytoplasm may be vacuolated, and transitional forms showing the development of cytoplasmic acini are usually seen. In most tumors, either the syncytial or acinar pattern predominates; however, in rare instances only a small focus of the minor pattern may be identified [17]. In common with mesothelial cells, AT universally express vimentin and cytokeratin AE1/AE3, while cytokeratin 34βE12 expression is seen in 25% of cases [21]. Epithelial membrane antigen is present in both the cytoplasm and cell membrane of tumor cells [21]. Thrombomodulin is all expressed on the surface of microvilli of the acinar component of the tumor, while staining is seen in occasional cells in the cords of the solid component. Both solid and acinar areas are strongly positive for calretinin [22,23]. Unlike the situation in malignant mesotheliomas, the stromal component of AT does not express calretinin and is thus reactive in nature [22]. Immuno-histochemical markers expressed in a variety of carcinomas (Leu M1, AUA-1, CA19.9, Ber-EP4, CEA, HEA-125, B72.3, MOC-31, and LEA.135) are negative, as are markers of endothelial cells (CD34 and factor VIII). Negative expression of p53 is consistent with the benign nature of these tumors [20,21]. The present tumor cells showed positive staining for cytokeratin, α-trypsin, calretinin, Vim and EMA. Expression of p53 protein and factor VIII were not observed. Moreover, there was negative immunohistochemical staining of either germ cell or sex cord stromal origin-associated antigens, for example AFP and PLAP [24]. The immunohistochemical staining characteristics of the tumors in the study are summarized in Table 1.
As a consequence of the variety of morphologic patterns observed for AT, the differential diagnosis of these tumors is quite extensive and includes benign and malignant tumors of both epithelial and stromal origin [15,25]. The differential diagnosis for adenomatoid tumor of the male genital tract includes carcinoma of the rete testis, embryonal carcinoma, yolk sac tumor, metastatic carcinoma, histiocytoid (epithelioid) haemangioma and malignant mesothelioma [9,26]. Adenocarcinoma of the rete testis is less likely to cause diagnostic difficulty due to the presence of an infiltrative growth pattern and marked nuclear pleomorphism. In problematic cases, the diagnosis may be confirmed by immunostaining for carcinoembryonic antigen which is usually positive in these tumors, as may be the differential factor for embryonal carcinoma. Yolk sac tumors exhibiting either a microcystic or polyvesicular vitelline pattern may be differentiated from AT by AFP immunopositivity, although this is not universally positive for yolk sac tumors. Morphologically, yolk sac tumors do not possess the typical stroma of AT and there is usually a much greater degree of nuclear pleomorphism. The absence of staining of epithelial markers is of utility in excluding carcinomas and germ cell tumors from the differential diagnosis, while negativity of vascular markers (CD34, factor VIII) excludes a diagnosis of histiocytoid (epithelioid) haemangioma. The diagnosis of mesothelioma relies on a panel of immunohistochemical stains, although more recently a variety of markers have been claimed to be of value in the identification of tumors of mesothelial cell origin [10]. In addition, sclerosing lipogranuloma is included in the differential diagnosis of AT largely on clinical grounds. Sclerosing lipogranuloma of the testis contains a diffuse inflammatory infiltrate consisting of histiocytes, foreign body type giant cells, and eosinophils within a dense sclerotic stroma.
In summary, adenomatoid tumors confined to the testicle are rarely seen. Adenomatoid tumors are invariably benign but their importance lies in the preoperative differential diagnosis with malignant tumors of the testis. The prognosis of almost all benign adenomatoid tumors is good. The clinical pertinence of the present case report is its influence upon the urological surgeon’s intraoperative decision to either excise the local lesion (and risk leaving a malignancy) or to perform radical orchiectomy. Typical location (in the epididymis) and scrotal ultrasound are helpful in this differentiation, but one must be certain of the benign nature of the tumor. Obviously, it is necessary that a suspicion biopsy was sent for intraoperative frozen section analysis. Even so, due to difficulty in differentiating a benign from a malignant neoplasm pre- or intraoperatively, radical orchiectomy remains the most common treatment, as in this case. The present case report emphasizes that benign tumors must be suspected, even in unusual locations. The urologist should discuss the possibility of a benign tumor with an experienced pathologist intraoperatively as the infiltrative pattern of growth of the adenomatoid could lead to needless orchiectomy.
Acknowledgements
We are grateful to Drs. Zhang Guanjun, Ren Xiaoping and Tang Kaifa for technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Hanrahan JB. A Combined papillary mesothelioma and adenomatoid tumor of the omentum; report of a case. Cancer. 1963;16:1497–1500. doi: 10.1002/1097-0142(196311)16:11<1497::aid-cncr2820161108>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Adem C, Schneider M, Hoang C. Pathologic quiz case: an unusual umbilical mass. Arch Pathol Lab Med. 2003;127:e303–304. doi: 10.5858/2003-127-e303-PQCAUU. [DOI] [PubMed] [Google Scholar]
- 3.Craig JR, Hart WR. Extragenital adenomatoid tumor: Evidence for the mesothelial theory of origin. Cancer. 1979;43:1678–1681. doi: 10.1002/1097-0142(197905)43:5<1678::aid-cncr2820430518>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Overstreet K, Wixom C, Shabaik A, Bouvet M, Herndier B. Adenomatoid tumor of the pancreas: a case report with comparison of histology and aspiration cytology. Mod Pathol. 2003;16:613–617. doi: 10.1097/01.MP.0000072803.37527.C8. [DOI] [PubMed] [Google Scholar]
- 5.Isotalo PA, Nascimento AG, Trastek VF, Wold LE, Cheville JC. Extragenital adenomatoid tumor of a mediastinal lymph node. Mayo Clin Proc. 2003;78:350–354. doi: 10.4065/78.3.350. [DOI] [PubMed] [Google Scholar]
- 6.Minato H, Nojima T, Kurose N, Kinoshita E. Adenomatoid tumor of the pleura. Pathol Int. 2009;59:567–571. doi: 10.1111/j.1440-1827.2009.02407.x. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan S, Luthringer DJ, Fishbein MC. Adenomatoid tumor of the heart: report of a case. Am J Surg Pathol. 1997;21:1378–1380. doi: 10.1097/00000478-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Delahunt B, Eble JN, King D, Bethwaite PB, Nacey JN, Thornton A. Immunohistochemical evidence for mesothelial origin of paratesticular adenomatoid tumour. Histopathology. 2000;36:109–115. doi: 10.1046/j.1365-2559.2000.00825.x. [DOI] [PubMed] [Google Scholar]
- 9.Barry P, Chan KG, Hsu J, Quek ML. Adenomatoid tumor of the tunica albuginea. Int J Urol. 2005;12:516–518. doi: 10.1111/j.1442-2042.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao JH, Jing XD, Luan ZQ. Epididymis testicular adenomatoid tumors: report of 11 cases. Chinese: J Post Med. 2003;26:35, 39. [Google Scholar]
- 11.Zhou XH, Cao J. Adenomatoid tumor: a clinicopathologic study of 12 cases. Chinese Journal of Misdiagnostics. 2007;28:6910–6911. [Google Scholar]
- 12.Horstman WG, Sands JP, Hooper DG. Adenomatoid tumor of testicle. Urology. 1992;40:359–361. doi: 10.1016/0090-4295(92)90390-i. [DOI] [PubMed] [Google Scholar]
- 13.Tammela TL, Karttunen TJ, Makarainen HP, Hellstrom PA, Mattila SI, Kontturi MJ. Intrascrotal adenomatoid tumors. J Urol. 1991;146:61–65. doi: 10.1016/s0022-5347(17)37715-7. [DOI] [PubMed] [Google Scholar]
- 14.Piccin A, Conneally E, Lynch M, McMenamin ME, Langabeer S, McCann S. Adenomatoid tumor of the testis in a patient on imatinib therapy for chronic myeloid leukemia. Leuk Lymphoma. 2006;47:1394–1396. doi: 10.1080/10428190600556098. [DOI] [PubMed] [Google Scholar]
- 15.Fields JM, Russell SA, Andrew SM. Case report: ultrasound appearances of a malignant mesothelioma of the tunica vaginalis testis. Clin Radiol. 1992;46:128–130. doi: 10.1016/s0009-9260(05)80318-6. [DOI] [PubMed] [Google Scholar]
- 16.Kassis A. Testicular adenomatoid tumours: clinical and ultrasonographic characteristics. BJU Int. 2000;85:302–304. doi: 10.1046/j.1464-410x.2000.00445.x. [DOI] [PubMed] [Google Scholar]
- 17.Feuer A, Dewire DM, Foley WD. Ultrasonographic characteristics of testicular adenomatoid tumors. J Urol. 1996;155:174–175. [PubMed] [Google Scholar]
- 18.Somers WJ. The sonographic appearance of an intratesticular adenomatoid tumor. J Clin Ultrasound. 1992;20:479–480. doi: 10.1002/jcu.1870200712. [DOI] [PubMed] [Google Scholar]
- 19.Gerscovich EO. High-resolution ultrasonography in the diagnosis of scrotal pathology: II. Tumors. J Clin Ultrasound. 1993;21:375–386. doi: 10.1002/jcu.1870210603. [DOI] [PubMed] [Google Scholar]
- 20.Xie YC, Liu WL, Li KS. Adenomatoid tumor: a study on its clinicopathological and immunohistochemical characteristics. Medicine and Pharmacy of Yunnan. 2004;25:392–394. [Google Scholar]
- 21.Delahunt B, Eble JN, Nacey JN, Thornton A. Immunohistochemical evidence for mesothelial origin of paratesticular adenomatoid tumour. Histopathology. 2001;38:479. doi: 10.1046/j.1365-2559.2001.1163a.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagata S, Aishima S, Fukuzawa K, Takagi H, Yonemasu H, Iwashita Y, Kinoshita T, Wakasugi K. Adenomatoid tumour of the liver. J Clin Pathol. 2008;61:777–780. doi: 10.1136/jcp.2007.054684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz EJ, Longacre TA. Adenomatoid tumors of the female and male genital tracts express WT1. Int J Gynecol Pathol. 2004;23:123–128. doi: 10.1097/00004347-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Favilla V, Cimino S, Madonia M, Morgia G. New advances in clinical biomarkers in testis cancer. Front Biosci (Elite Ed) 2010;2:456–477. doi: 10.2741/e105. [DOI] [PubMed] [Google Scholar]
- 25.Huang WQ, Ji P, Wei HJ. Clinical pathological analysis of 20 cases adenomatoid tumors in testis and epididymides with special dyeing and immunohistochemical methods. Chn Hebei Med. 2001;7:388–390. [Google Scholar]
- 26.Sangoi AR, McKenney JK, Schwartz EJ, Rouse RV, Longacre TA. Adenomatoid tumors of the female and male genital tracts: a clinicopathological and immunohistochemical study of 44 cases. Mod Pathol. 2009;22:1228–1235. doi: 10.1038/modpathol.2009.90. [DOI] [PubMed] [Google Scholar]


