Abstract
We present a case of metastatic malignant melanoma in a patient initially diagnosed with glioblastoma multiforme, giant cell variant. A forty year old female presented to our institution for a re-resection of a recurrent right parietal lobe mass, presumed to be recurrent glioblastoma multiforme. PET scan during preoperative evaluation revealed a 3 cm left lower lobe lung mass. Metastatic glioblastoma to lung was considered in the differential diagnosis. Resection of the brain mass revealed a highly pleomorphic giant and spindle cell lesion with an immunophenotype strongly supportive of melanoma. Immunostains for melanocytic markers were subsequently performed on the lung biopsy specimen, and demonstrated diffuse staining of the atypical cells, supporting the diagnosis of malignant melanoma in the lung. This case demonstrates the importance of considering melanoma in the differential in any tumor with high grade features.
Keywords: Melanoma, giant cell variant glioblastoma multiforme, pleormorphic lung carcinoma
Introduction
There are a plethora of histologic variants of malignant melanoma that have been reported in the literature. These variants may pose a considerable diagnostic challenge, especially on needle core biopsy specimens where there is limited diagnostic material. We present a case of malignant melanoma with morphologic features that mimic glioblastoma multiforme, giant cell variant, WHO grade IV. This case demonstrates the importance of considering melanoma in the differential in any tumor with high grade features.
Case report
The patient is a forty year old female who presented with generalized seizures at an outside institution, and an 18-month history of headaches prior to her presentation. She was found to have a right parietal lobe mass which was resected and initially diagnosed as glioblastoma multiforme (GBM), giant cell variant, WHO grade IV. She was subsequently treated with Temozolomide and radiation therapy. Follow up MRI of the brain a few months later demonstrated a 4.6 cm mass at the previous resection site with associated mass effect, cerebral edema, and midline shift (Figure 1A). The patient presented to our institution for resection of the mass for further workup and treatment. PET scan during preoperative evaluation revealed a peripheral 3 cm mass in the left lower lobe of the lung (Figure 1B). The patient did not have a history of ventriculoperitoneal shunt placement.
Figure 1.
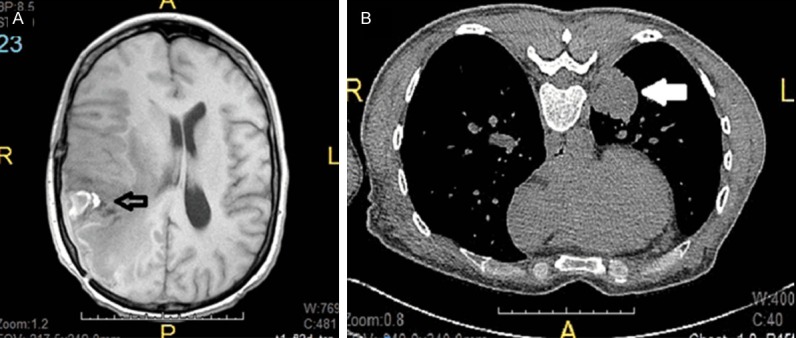
Brain and lung imaging studies. A. Magnetic resonance imaging showing a 4.6 cm necrotic and heterogeneous mass at the temporoparietal junction (empty arrow). B. Computed tomography of chest showing a 3.0 cm demarcated and solid mass abutting the posterior medial pleura (white arrow).
The patient underwent resection of the recurrent brain tumor. The surgical specimen consisted of multiple fragments of friable tan-white and slightly hyperemic brain with focal necrosis and no grossly identifiable pigment, which measured 10.5 × 8.6 × 2.3 cm in aggregate. Microscopic examination of the resection specimen revealed a poorly differentiated high grade neoplasm consisting of pleomorphic giant and spindle cells (Figure 2A) with atypical mitotic figures and focal areas of pseudopalisading necrosis that were morphologically similar to the lung biopsy specimen (Figure 3A). The brain and leptomeninges were involved. Immunostains for S100, MART1 (Figure 2B), HMB45, tyrosinase, and MITF were strongly and diffusely positive. Scattered atypical multinucleated giant cells showed weak expression of neurofilament. GFAP showed focal positivity in reactive glial cells. p53 was positive and mildly increased. Ki67 was increased with overall activity estimated at 30%, with focal activity estimated at 70%. Immunostains for CD34, desmin, SMA, keratin 20, CDX2, CEA, synaptophysin, chromogranin, keratin 18, p63, keratin CAM 5.2, EMA, and HCG, were all negative. The immunophenotype was supportive of melanoma. No V600E mutation was detected in the BRAF gene via cobas® 4800 BRAF mutation test.
Figure 2.
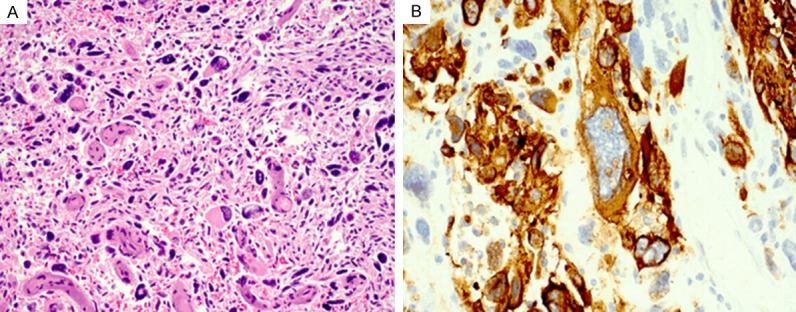
Metastatic melanoma in the brain. (A) Histologic section of the resected brain tumor demonstrates a high grade malignant neoplasm with a pleomorphic giant and spindle cell proliferation. (B) Immunhistochemical staining shows that the tumor cells are strongly and diffusely positive for MART1. Hemotoxylin-eosin stain, original magnification 200× (A); immunohistochemical stain, original magnification, 1000× (B).
Figure 3.
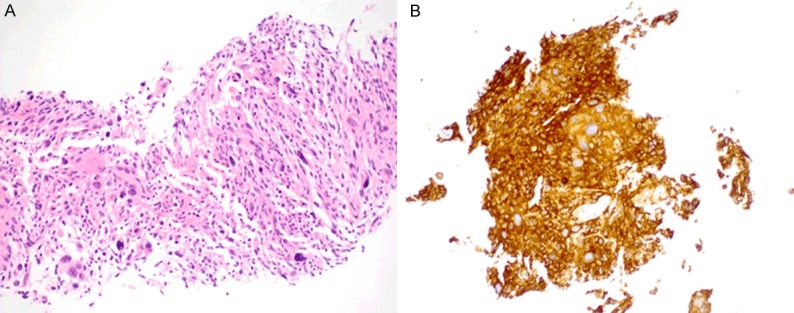
Metastatic melanoma in the lung. (A) Histologic section of the lung biopsy specimen demonstrates a high grade malignant neoplasm with a pleomorphic giant and spindle cell proliferation. (B) Immunhistochemical staining shows that the tumor cells are strongly and diffusely positive for MART1. Hemotoxylin-eosin and immunohistochemical stains, original magnification 200× (A).
Transthoracic compute tomography (CT)-guided biopsy of the lung mass demonstrated a high grade malignant neoplasm that consisted of non-pigmented atypical giant and spindle cells (Figure 3A) which was morphologically compatible with the patient’s history of giant cell glioblastoma. Pleomorphic lung carcinoma and other metastatic malignant tumors were considered in the differential diagnosis. The touch preparation of the biopsy specimen also showed atypical epithelioid to spindled cells arranged in tightly to loosely cohesive variably-sized clusters. The ovoid to elongated nuclei were enlarged, with prominent nucleoli, irregular nuclear contours, and fine to clumped chromatin distribution. Scattered giant cells with bizarre forms were also noted. However, immunohistochemical stains for S100, HMB45, MART1 (Figure 3B), MITF, Tyrosinase, and vimentin showed strong and diffuse positivity, and was negative for GFAP. Immunostains for keratin AE1/3 and TTF-1 demonstrated focal positivity in reactive type 2 pneumocytes, and was negative for p40, keratin 7, keratin 20, keratin CAM 5.2, EMA, and keratin 5/6. The immunophenotype again was supportive of melanoma.
After the diagnosis of malignant melanoma, the patient revealed that she underwent a biopsy of a red, scaly, and persistent skin lesion on the right upper arm in 2006. This lesion was considered premalignant and a subsequent excision at another institution revealed a malignant melanoma. Unfortunately, the slides were not available for review since this was done in another country. Follow-up imaging a month later demonstrated 10 new enhancing lesions throughout the left cerebral hemisphere concerning for metastatic disease. The patient was enrolled in a clinical study to evaluate the safety and efficacy of MK-3475 compared to Ipilimumab in patients with advanced melanoma. She was also treated with stereotactic radiosurgery for the multiple brain metastases.
Discussion
Melanoma is the third most common metastatic tumor to the brain [1]. Although the incidence of malignant melanoma is increasing, the early identification of these lesions usually leads to curative surgical excision. On the other hand, metastatic melanoma is much more difficult to manage since the majority of these patients have widespread systemic micrometastatic disease [2]. The most frequent cause of death from metastatic melanoma is either respiratory failure or intracranial metastases [3]. Brain metastases in patients with malignant melanoma in general have a poor prognosis. The median survival of such patients was four months, with one year survival rates between 9-19 percent [5]. The median survival of patients with leptomeningeal involvement by metastatic melanoma is less than two months [8]. Quality of life is usually the principle goal with respect to the treatment of these patients. Resection should be reserved for relief and/or prevention of morbidity due to local tumor growth, even though survival may not be enhanced [4]. Systemic chemotherapy such as temozolamide has shown some activity against brain metastases, but does not have an established role in the management of patients with metastatic melanoma to the brain. In general, systemic chemotherapy for patients with metastatic melanoma only has limited activity, and has been largely replaced by immunotherapy and targeted therapy for the initial treatment of metastatic disease [6,7].
Giant cell glioblastoma multiforme is a rare variant of GBM that encompasses 2-5% of GBM diagnoses [10], and may be associated with improved survival relative to the reported prognosis in GBM [11]. The median survival time in patients with GBM is approximately 1 year [14]. In a retrospective study looking at 113 patients with GBM, 5% (5 patients) had a survival more than 5 years after diagnosis, three of which were diagnosed with giant cell GBM [14]. This lesion is characterized by the presence of prominent multinucleated giant cells and lymphocytic infiltration in addition to the classical features of GBM, namely, necrosis and atypia. They are known to stain variably for S100, vimentin, GFAP, p53, EGFR, and class III β-tubulin [12]. It has also been suggested to be involved in some patients with Turcot’s syndrome, a rare GBM-forming genetic disorder with biallelic mutation of the DNA mismatch repair genes MSH6, MLH1, PMS2, and MSH2, along with favorable prognosis despite a high proliferative index and degree of anaplasia [13].
The different therapeutic strategies and prognosis of these two entities demonstrates the importance for their distinction and accurate histopathological diagnosis. The biopsy of the lung mass that was found incidentally during the preoperative workup demonstrated large atypical multinucleated giant cells that were morphologically consistent with the patient’s presumed history of giant cell glioblastoma multiforme. The differential for the left lower lobe lung mass included pleomorphic carcinoma of the lung, poorly differentiated squamous cell carcinoma and/or adenocarcinoma, and sarcoma. The negative staining for Keratin AE1/3, Keratin 7, Keratin CAM 5.2, EMA, and Keratin 5/6 did not support the diagnosis of pleomorphic carcinoma. The possibility of metastatic GBM to the lung, which has been reported in a few cases in literature, was also considered [9]. The resection specimen showed similar high grade morphology that was seen in the lung biopsy. S100, HMB45, MART1, Tyrosinase, and MITF all showed strong and diffuse expression in the atypical giant cells, strongly supporting the diagnosis of melanoma. In addition, the atypical cells showed aberrant expression of Neurofilament. Aberrant Neurofilament protein expression is very rare in melanoma, and has been reported in sinonasal and primary cutaneous small cell melanomas [15,16].
In conclusion, this case highlights the importance of considering melanoma in the differential for any high grade tumor, since numerous histolopathological variants of malignant melanoma exist. A detailed clinical history and thorough physical examination are essential prior to giving a definitive diagnosis. Melanocytic immunohistochemical markers may help arrive at the correct diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337. [PubMed] [Google Scholar]
- 2.Cole BF, Gelber RD, Kirkwood JM, Goldhirsch A, Barylak E, Borden E. Quality-of-life-adjusted survival analysis of interferon alfa-2b adjuvant treatment of high-risk resected cutaneous melanoma: an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1996;14:2666. doi: 10.1200/JCO.1996.14.10.2666. [DOI] [PubMed] [Google Scholar]
- 3.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 4.Leo F, Cagini L, Rocmans P, Cappello M, Geel AN, Maggi G, Goldstraw P, Pastorino U. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer. 2000;83:569. doi: 10.1054/bjoc.2000.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. Neurosurg. 1998;88:11. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 6.Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J. Clin. Oncol. 2004;22:2101. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Schadendorf D, Hauschild A, Ugurel S, Thoelke A, Egberts F, Kreissig M, Linse R, Trefzer U, Vogt T, Tilgen W, Mohr P, Garbe C. Dose-intensified bi-weekly temozolomide in patients with asymptomatic brain metastases from malignant melanoma: a phase II DeCOG/ADO study. Ann Oncol. 2006;17:1592. doi: 10.1093/annonc/mdl148. [DOI] [PubMed] [Google Scholar]
- 8.Champagne MA, Silver HK. Intrathecal dacarbazine treatment of leptomeningeal malignant melanoma. J Natl Cancer Inst. 1992;84:1203–4. doi: 10.1093/jnci/84.15.1203. [DOI] [PubMed] [Google Scholar]
- 9.Blume C, von Lehe M, van Landeghem F, Greschus S, Boström J. Extracranial glioblastoma with synchronous metastases in the lung, pulmonary lymph nodes, vertebrae, cervical muscles and epidural space in a young patient - case report and review of literature. BMC Res Notes. 2013;6:290. doi: 10.1186/1756-0500-6-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karsy M, Gelbman M, Shah P, Balumbu O, Moy F, Arslan E. Established and emerging variants of glioblastoma multiforme: review of morphological and molecular features. Folia Neuropathol. 2012;50:301–21. doi: 10.5114/fn.2012.32361. [DOI] [PubMed] [Google Scholar]
- 11.Palma L, Celli P, Maleci A, Di Lorenzo N, Cantore G. Malignant monstrocellular brain tumours. A study of 42 surgically treated cases. Acta Neurochir (Wien) 1989;97:17–25. doi: 10.1007/BF01577735. [DOI] [PubMed] [Google Scholar]
- 12.Louis DN, Ohgaki HH, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. Albany: WHO Press; 2007. Astrocytic tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lusis EA, Travers S, Jost SC, Perry A. Glioblastomas with giant cell and sarcomatous features in patients with Turcot syndrome type 1: a clinicopathological study of 3 cases. Neurosurgery. 2010;67:811–817. doi: 10.1227/01.NEU.0000375513.12925.5C. discussion 817. [DOI] [PubMed] [Google Scholar]
- 14.Shinojima N, Kochi M, Hamada JI, Nakamura H, Yano S, Makino K, Tsuiki H, Tada K, Kuratsu J, Ishimaru Y, Ushio Y. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg. 2004;101:219–226. doi: 10.3171/jns.2004.101.2.0219. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387–402. doi: 10.1046/j.1365-2559.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 16.Coli A, Giacomini PG, Bigotti G, Ferraro S, Alessandrini M, Del Vecchio M, Massi G. Aberrant neurofilament protein and synaptophysin expression in malignant melanoma of the nasal cavity. Histopathology. 2004;44:193–5. doi: 10.1111/j.1365-2559.2004.01784.x. [DOI] [PubMed] [Google Scholar]


