Abstract
Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) was first described by Chan et al in 1991. It is characterized by nest or strands of epidermoid tumor cells with squamous differentiation, rare mucous cells, prominent sclerotic stroma, eosinophilic and lymphoplasmacytic infiltration, and a background of chronic lymphocytic thyroiditis in the non-neoplastic thyroid gland. It is important to recognize SMECE of thyroid and differentiate it from squamous cell carcinoma or other neoplasms with squamous differentiation/metaplasia. In published cases, the SMECE of thyroid gland predominantly occurs in women. We report a case of SMECE of thyroid in a 45-year-old male patient. All cases in male patients were Caucasian described in English literature, and our case is the first one in Asian.
Keywords: Sclerosing mucoepidermoid carcinoma, eosinophilia, male, thyroid gland, Hashimoto thyroiditis
Introduction
Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) was first described by Chan et al in 1991 [1]. It is characterized by nests or strands of epidermoid tumor cells with squamous differentiation, rare mucous cells, prominent sclerotic stroma, eosinophilic and lymphoplasmacytic infiltration, and a background of chronic lymphocytic (Hashimoto) thyroiditis in the non-neoplastic thyroid gland. It is a distinctive entity that can be separated from conventional mucoepidermoid carcinoma (MEC) of thyroid gland morphologically and immunochemically [2]. It is important to differentiate it from primary or secondary squamous cell carcinoma (SCC) in thyroid gland, and from other cases of thyroid tumors with squamous differentiation. Recently, tumors showing similar morphology of SMECE have been also reported in major or minor salivary glands and esophagus [3-6]. In the published cases, SMECE of thyroid gland has a predominant occurrence in female. We report a case in a male patient. It is the 4th such example in English literature and the first reported case in an Asian male [7-9].
Case report
A 45-year-old man without remarkable medical history was found to have a painless mass in right neck during a regular health examination in July 2008. Physical examination showed a palpable and hard tumor in right thyroid. Ultrasound scan revealed an ill-defined, heterogeneous, and hypoechoic nodule in right thyroid, about 2.5 cm in diameter, with some microcalcification spots. The remaining part of thyroid showed homogenous isoechoic pattern. Fine needle aspiration cytology revealed moderate cellularity with anisocytosis, prominent nucleoli and colloid, and a malignant tumor was suspected. The laboratory data including free-T4, thyroid stimulating hormone and blood cell count were within normal limits. The patient received right lobectomy and isthmusectomy in March 2009.
The resected thyroid tissue was measured 4.3 × 3.5 × 2.5 cm and weighed 13.3 grams, with a circumscribed, yellow and firm tumor measuring 1.5 × 1.5 × 1.5 cm. Microscopically, it was a partially circumscribed mass with an ill-defined border. The tumor was composed of small irregular nests or anastomosing strands of epidermoid cells in a sclerotic stroma. Squamous differentiation and keratin peals were easily found (Figure 1A). The tumor cells were round to polygonal, with eosinophilic or clear cytoplasm, open nuclear chromatin, and small but prominent nucleoli. Rare mucous cells arranged in single cells or small nests were seen (Figure 1B). Infiltration of abundant eosinophils, lymphocytes, and plasma cells was observed (Figure 1C). Occational lymphoid follicles were also noted. Areas of prominent sclerosis were also seen (Figure 1D). Perinural invasion was found. The non-tumor thyroid tissue showed lymphocytic thyroiditis. The dissected 4 peritrachial lymph nodes were negative for malignancy.
Figure 1.
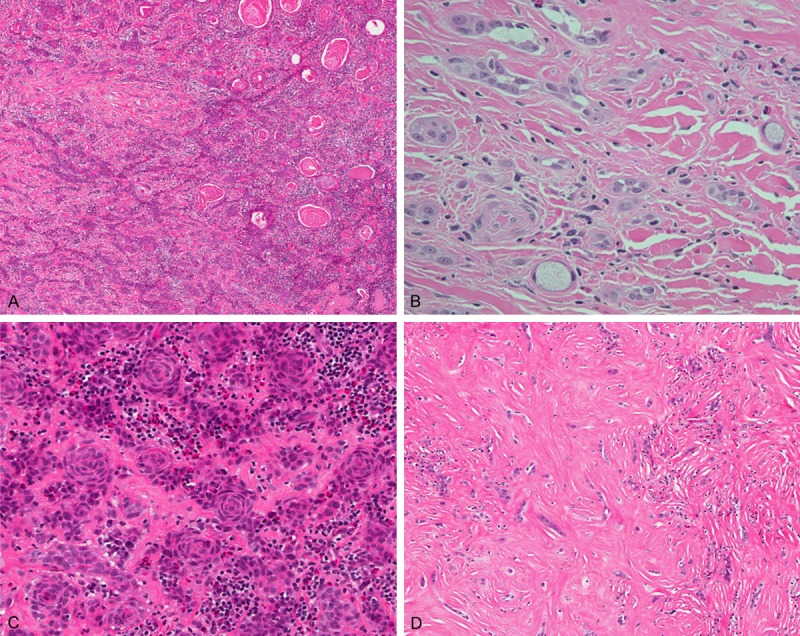
A. Anastomosing epidermoid tumor cells in a fibrohyaline background with abundant inflammatory cell infiltrates. The squamous differentiation and keratin peals were easily seen. (hematoxylin and eosin 100 ×). B. Rare mucous cells in single cells or small aggregates adjacent to epidermoid component. (hematoxylin and eosin 400 ×). C. Anastomosing epidermoid tumor cells with squamous differentiation. Note the infiltration of eosinophil-rich inflammatory cells in the stroma and tumor nests. (hematoxylin and eosin 200 ×). D. In sclerosing area, the tumor cells were arranged in files, small clusters, or single cells. (hematoxylin and eosin 200 ×).
Immunohistochemically, the tumor cells showed strong positivity for CD10, p63, and beta-catenin. The tumor cells were negative for thyroglobulin, calcitonin, thyroid transcription factor-1 (TTF-1), carcinoembryonic antigen (CEA), vimentin, smooth muscle actin (SMA), and S-100 protein. Rare mucous cells were highlighted by mucicarmine stain.
After operation, the patient was under thyroxine sodium supplement and was regularly followed up at our outpatient department. There was no evidence of tumor recurrence until the last follow up of the patient in December 2014.
Discussion
Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) was first described by Chan et al in 1991, as a low-grade malignancy in thyroid gland occurring in a background of Hashimoto thyroiditis [1]. Sim et al compared SMECE with conventional MEC of thyroid gland and supported SMECE as a distinctive entity [2]. Hunt et al revealed the expression of p63 in SMECE of thyroid gland suggesting a tumor origin of ultimobranchial body/solid cell nest rather than follicular epithelial cells [9].
The SMECE of thyroid gland most commonly presents as painless neck mass or cold nodule on thyroid scan [10]. Non-specific symptoms such as hoarseness occur depending on tumor extension [11]. In published cases of SMECE in thyroid gland, there is a prominent female preponderance. Only 4 male patients have been reported in English literature over 37 published cases (including our case). The patient age ranges from 32 to 89 years (mean 55.2 years) [9,10,12-16].
Morphologically, it is often a yellow to white, firm and ill-defined mass on gross examination. Under the microscopy, the majority of epidermoid tumor cells arranged in thin strands and small nests with mild to moderate nuclear pleomorphism, distinct nucleoli, and eosinophilic or pale cytoplasm infiltrated in abundant dense fibrohyaline stroma. Foci of squamous differentiation including intercellular bridges, keratinocyte, keratin peals, and keratinolysis forming pesudovascular appearance could be seen. Rare single or small aggregates of mucous cells, and small mucin pools can be identified by morphology or special stain in most cases. Lymphoplasmacytic infiltration with abundant eosinophils is seen in the sclerosing stroma and epidermoid tumor nests. Perineural invasion and obliteration of blood vessels, especially median-sized vessels, are common. The non-tumor thyroid always presents Hashimoto thyroiditis or lymphocytic thyroiditis [1,2,10,11,14-16].
Immunohistochemically, Shehadeh et al reviewed 23 published cases showing tumor cells were positive for cytokeratin (19/19 cases, 100%) and CEA (13/16 cases, 81.2%), and negative for thyroglobulin (19/21 cases, 90.5%) and calcitonin (19/19 cases, 100%) [10]. Hunt et al examined 3 cases which were positive for p63 and negative for TTF-1 [9]. A study of 6 cases by Quiroga-Garza et al revealed similar result (positivity of CK19 and p63 in 6/6 cases, positivity of thyroglobulin in 2/6 cases, and positivity of TTF-1 in 2/6 cases) [16].
Extrathyroidal extension is seen in about a half of cases, and regional lymph node metastasis is also common [10]. SMECE of thyroid was initially thought to be a low-grade malignancy with indolent clinical behavior [1]. In early literature, some patients survived with or without disease up to 12 years after initial diagnosis, even with extrathyroidal extension and regional recurrence [2,10]. However, few cases of distant metastasis were also reported [2,8,10,16]. The most common site of metastasis was lung (5 of 7 cases), and other reported locations included bone, liver, mediastinum, and non-continuous lymph nodes. In a recent study by Quiroga-Garza et al, five of 6 patients had died within 1.5 to 8 years after initial diagnosis, 3 of them had distant metastasis, although definite cause of death was not documented [16]. The behavior of SMECE of thyroid gland appears to be more aggressive than we thought before, and the definite prognostic factors need to be established by evaluating more cases with longer period of follow up.
Conventional MEC of thyroid gland shares similar features with SMECE of thyroid gland. Sim et al had compared SMECE with conventional MEC of thyroid, which displays larger sheets and nests of epidermoid cells, more obvious glandular or cystic spaces, and lack of eosinophilic infiltration and vascular invasion and obliteration. According to his study, only half of MEC of thyroid related with a lymphocytic thyroiditis (12/23 cases), and is more commonly associated with papillary carcinoma (7/23 cases). Immunohistochemical study revealed thyroglobulin positivity in 14 of 21 cases (67%) of MEC of thyroid [2], while thyroglobulin positivity was shown in 2 of 21 cases (9.5%) of SMECE of thyroid gland [10].
The SMECE of thyroid gland should be differentiated from primary or secondary SCC, especially when mucous component is inconspicuous. Most SCC in thyroid gland is secondary; primary SCC of thyroid gland is rare but very aggressive. Therefore, the recognition of SMECE and distinguish it from SCC is important to avoid unnecessary search for primary site or overtreatment of the patient. SCC usually has more prominent pleomorphism and atypia, frequent mitosis, and rarely shows stromal sclerosis with prominent eosinophilic infiltration [10].
In addition, SMECE of thyroid gland should be distinguished from thyroid tumors with squamous differentiation such as papillary carcinoma, medullary carcinoma, carcinoma with thymus-like differentiation, and anaplastic carcinoma. Careful examination for histologic features is mandatory.
On the other hand, when SMECE develops nodal metastasis, the sclerosis and eosinophil infiltration could mimic nodular sclerosing Hodgkin’s disease. The cohesive morphology, lack of Reed-Sternberg cells, and a penal of immunohistochemical stain including negative for CD15, CD30 and CD45 are helpful for differential diagnosis [17].
In summary, SMECE of thyroid gland is a rare entity with a distinctive morphology characterized by squamoid tumor cell nests or stands, rare mucous component, in a fibrohyaline stroma with eosinophil-rich infiltration. It always has a background of chronic lymphocytic (Hashimoto’s) thyroiditis, and predominantly occurs in women. It has been though as an indolent disease earlier, but more and more cases of distant metastasis and death have been reported. It should be carefully differentiated from secondary or primary SCC, conventional MEC of thyroid, and other thyroid tumors with squamous differentiation.
Acknowledgements
This study was supported by grants from the Ministry of Education, Taiwan, ROC (EMRPD1A0391, to C. H.).
Disclosure of conflict of interest
None.
References
- 1.Chan JK, Albores-Saavedra J, Battifora H, Carcangiu ML, Rosai J. Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto’s thyroiditis. Am J Surg Pahol. 1991;15:438–448. [PubMed] [Google Scholar]
- 2.Sim SJ, Ro JY, Ordonez NG, Cleary KR, Ayala AG. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: report of two patients, one with distant metastasis, and review of the literature. Hum Pathol. 1997;28:1091–1096. doi: 10.1016/s0046-8177(97)90064-2. [DOI] [PubMed] [Google Scholar]
- 3.Urano M, Abe M, Horibe Y, Kuroda M, Mizoguchi Y, Sakurai K, Naito K. Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary glands. Pathol Res Pract. 2002;198:305–310. doi: 10.1078/0344-0338-00259. [DOI] [PubMed] [Google Scholar]
- 4.Tasaki T, Matsuyama A, Tabata T, Suzuki H, Yamada S, Sasaguri Y, Hisaoka M. Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary gland: Case report and review of the literature. Pathol Int. 2013;63:125–131. doi: 10.1111/pin.12035. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Satoh K, Aizawa T, Urano M, Kuroda M, Mizutani H. Local recurrence of sclerosing mucoepidermoid carcinoma with eosinophilia in the upper lip: a case report. J Med Case Rep. 2015;9:41. doi: 10.1186/s13256-015-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mewa Kinoo S, Maharaj K, Singh B, Govender M, Ramdial PK. Primary esophageal sclerosing mucoepidermoid carcinoma with “tissue eosinophilia”. World J Gastroenterol. 2014;20:7055–7060. doi: 10.3748/wjg.v20.i22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenig BM, Adair CF, Heffess CS. Primary mucoepidermoid carcinoma of the thyroid gland: a report of six cases and a review of the literature of a follicular epithelial-derived tumor. Hum Pathol. 1995;26:1099–1108. doi: 10.1016/0046-8177(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 8.Geisinger KR, Steffee CH, McGee RS, Woodruff RD, Buss DH. The cytomorphologic features of sclerosing mucoepidermoid carcinoma of the thyroid gland with eosinophilia. Am J Clin Pathol. 1998;109:294–301. doi: 10.1093/ajcp/109.3.294. [DOI] [PubMed] [Google Scholar]
- 9.Hunt JL, LiVolsi VA, Barnes EL. p63 expression in sclerosing mucoepidermoid carcinomas with eosinophilia arising in the thyroid. Mod Pathol. 2004;17:526–529. doi: 10.1038/modpathol.3800021. [DOI] [PubMed] [Google Scholar]
- 10.Shehadeh NJ, Vernick J, Lonardo F, Madan SK, Jacobs JR, Yoo GH, Kim HE, Ensley JF. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: a case report and review of the literature. Am J Otolaryngol. 2004;25:48–53. doi: 10.1016/s0196-0709(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 11.Chung J, Lee SK, Gong G, Kang DY, Park JH, Kim SB, Ro JY. Sclerosing mucoepidermoid carcinoma wth eosinophilia of the thyroid glands: a case report with clinical manifestation of recurrent neck mass. J Korean Med Sci. 1999;14:338–341. doi: 10.3346/jkms.1999.14.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondeson L, Bondeson AG. Cytologic features in fine-needle aspirates from a sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. Diagn Cytopathol. 1996;15:301–305. doi: 10.1002/(SICI)1097-0339(199611)15:4<301::AID-DC10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Sharma K, Nigam S, Khurana N, Chaturvedi KU. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid--a case report. Indian J Pathol Microbiol. 2003;46:660–661. [PubMed] [Google Scholar]
- 14.Das S, Kalyani R. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid. Indian J Pathol Microbiol. 2008;51:34–36. doi: 10.4103/0377-4929.40389. [DOI] [PubMed] [Google Scholar]
- 15.Frazier WD, Patel NP, Sullivan CA. Pathology quiz case 1. Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) Arch Otolaryngol Head Neck Surg. 2008;134:333, 335. doi: 10.1001/archotol.134.3.333. [DOI] [PubMed] [Google Scholar]
- 16.Quiroga-Garza G, Lee JH, El-Naggar A, Black JO, Amrikachi M, Zhai QJ, Ayala AG, Ro JY. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: more aggressive than previously reported. Hum Pathol. 2015;46:725–731. doi: 10.1016/j.humpath.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Solomon AC, Baloch ZW, Salhany KE, Mandel S, Weber RS, LiVolsi VA. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia. mimic of Hodgkin disease in nodal metastases. Arch Pathol Lab Med. 2000;124:446–449. doi: 10.5858/2000-124-0446-TSMCWE. [DOI] [PubMed] [Google Scholar]


