Abstract
Objective: to investigate the role of EphB4 and IGF-1R in the proliferation and migration of breast cancer. Methods: The relative mRNA levels of EphB4 were measured by RT-PCR. The proliferation of the cells was determined by MTT assay, and cells migration and invasive ability was analyzed using the scratch migration assay. Results: The expression of EphB4 in control group was significantly decreased when compared with IGF-I group (P < 0.001). The expression of EphB4 in IGF-I+LY and LY group were lower than that of the control group (P < 0.001).The cell proliferation and migration ability of the cells in IGF-I group increased significantly compared to the cells in the control group (P < 0.001), while the cells in IGF-1+LY group and LY group showed a decreased proliferation and migration ability compared to the control group (P < 0.001). Conclusion: IGF-IR might be a upstream gene of EphB4. Besides, higher expression of EphB4 shows increased tumor proliferation and migration in breast cells. The study of EphB4 upstream gene and signaling pathway can provide more targeted anti-tumor point selection for targeted therapy.
Keywords: EphB4, IGF-IR, breast cancer cell
Introduction
Breast cancer is one of the most malignant tumors in female, its occurrence and development is a complex and multistage process. The exact molecular mechanism of malignant epithelial breast cancer is unclear; as molecular targeted therapy was applied in clinical, more attention had been paid to genetic and molecular mechanism study of breast cancer.
The erythropoietin-producing hepatoma (Eph) receptors represent the largest class of receptor tyrosine kinases (RTKs). Recent studies have shown that Eph signaling plays an important role in the growth, differentiation, aggregation, migration, and apoptosis of cancer cells [1-5]. The expression of Eph receptors is increased in many kinds of cancers, including prostate, colon, breast, and ovarian cancers [6-9]. EphB4, a member of the Eph receptor family, has been reported to be frequently amplified in some cancers [10-12], and has been shown to overexpress in a wide range of tumor cells and tissues, including cancers found in the ovary [6,13], bladder [14], prostate [14,15], breast [16], and colon [12,17]. Insulin-like growth factor I receptor (IGF-IR) also has high expression levels in a variety of human tumor cells. In pathological state, when combined with insulin-like growth factor (IGF), IGF-IR can induce cells to the malignant phenotype transformation, promote proliferation of tumor cells, and inhibit tumor cell apoptosis [18].
In this study, we evaluated the effect of EphB4 on breast cancer cells, the relation between IGF-IR mediated signaling pathway and EphB4.
Materials and methods
Patients and tissue specimens
In the study, we selected 208 patients who had histologically been confirmed as breast cancer and had undergone radical surgeries at the first Hospital of China Medical University between December 1997 and October 2003. All patients underwent standardized comprehensive treatment. The median follow-up time was 69 months. The inclusion criteria were (a) curative surgeries, (b) pathological examination of resected specimens, (c) pathological examination of more than 15 lymph nodes after surgery, and (d) availability of complete medical records. All the participants provided their written informed consent to participate in this study, and the ethics committee of China Medical University approved the consent procedure and this study’s protocol.
Tissue microarray construction
We fixed thin slices of breast cancer tissue and normal breast epithelial tissue of all patients in a 4% formaldehyde solution (pH 7.0) for periods not exceeding 24 h. The tissues were processed routinely for paraffin embedding, and 4-μm thick sections were cut and placed on glass slides. Tissue samples were stained with hematoxylin and eosin to define diagnostic areas, and a representative 1-mm core was obtained from each case and inserted in a grid pattern into a recipient paraffin block. According to this procedure, four tissue microarray blocks were constructed.
Cell lines and cell culture
MCF-7 cells were obtained from the American Type Culture Collection (Rockville, MD, USA), LY294002 was from Abcam, IGF-I was from Abcam. The cells were cultured in RPMI 1640 Medium (GIBCO, Carlsbad, USA) supplemented with 10% fetal bovine serum (GIBCO, Carlsbad, USA), and they were incubated at 37°C with 5% CO2. After the cells reach 80-90% confluence, they are passaged with the use of 0.25% trypsin. Starvation medium contains all of the above ingredients except 0.1% bovine serum albumin instead of fetal bovine serum.
Cell stimulation and inhibition
Cells were cultured in RPMI 1640 medium with 10% fetal bovine serum until 80-90% confluent and then washed twice with phosphate-buffered saline (Biofluids, Rockville, MD). For stimulation experiments, medium was changed to serum-free medium (SFM) for 24 h. Cells were treated with IGF-I and LY294002 as described below, in growth assay. Medium was replaced with SFM plus indicated growth factors for 10 min at the following concentrations, unless noted otherwise: 10 ng/ml IGF-I, 50 µM LY294002.
Cell proliferation assay
Cells were plated in 96-well plates with 1000 cells/well in serum-containing medium. Cells were switched to serum-free media (SFM) for 24 h and then treated separately with 10 ng/ml IGF-I for 10 min, 50 µM LY294002 for 10 min, 50 µM LY294002 for 10 min before treated with IGF-I for 10 min. All treatments were done in triplicates. Growth was measured 3 days after treatment. Growth was assayed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. After incubation for 4 h at 37°C, wells were aspirated and formazan crystals were lysed with 500 µl of solubilization solution (95% DMSO +5% IMEM). Then, absorbance at 450 nm was measured using a microplate reader, wherein the absorbance value indicated the proliferative capacity.
Scratch-wound assay
Cells were plated onto 6-well plates at a concentration of 2 × 105 cells/well. Cell monolayers were carefully wounded by scratching with a sterile plastic pipette tip. The cells were washed twice with PBS and incubated for a further 48 h. Photographs for each wound was captured in the same fields at different times up to 48 h. The distance between the wound edges was analyzed using Image J version 1.42 (National Institutes of Health, Bethesda, MD). The percentage of wound occupied was calculated by dividing the non-recovered area at 24 h by the initial wound area at 0 h and subtracting this value as a percentage from 100%.
RNA extraction and reverse-transcription PCR
Gene amplification was analyzed by Reverse-Transcription polymerase chain reaction (PCR). RNA was extracted from MCF-7 cells using RNAiso Plus (Takara), and reverse transcribed to cDNA using the cDNA Archive Kit (Applied Biosystems) according to the manufacturer’s instructions. Reverse-Transcription PCR was performed on MyCycle PCR system (BIO-RAD, US) using Takara RNA PCR Kit (AMV) Ver. 3.0 according to the manufacturer’s instructions with thermal profile of 95°C for 10 minutes followed by 40 cycles of 95°C for 30 seconds, 60°C for 1 minute, and then 72°C for 1 minute. The primers were EphB4-forward, 5’-AATGTCACCACTGACCGAGAG-3’; EphB4-reverse, 5’-CCGATGAGATACTGTCCGTGT-3; β-actin-forward, 5’-TGACCCAGATCATGTTTGAGA-3’; β-actin-reverse, 5’-ACTCCATGCCCAGGAAGGA-3’. PCR reaction products were electrophoresed on 1.0% agarose gels and stained with ethidium bromide. Densitometric quantification of band intensity was analyzed by Bio Spectrum Imaging System Chemi 410 (UVP, US).
Statistical analysis
All experiments were repeated at least three times. Statistical analysis was performed using the SPSS statistics 19.0 software package. The Student’s t-test was used to evaluate the differences between groups. P values less than 0.05 were considered significant.
Results
EphB4 expression in breast cancer and its relationship with clinicopathological characteristics
To score EphB4 as immuno-positive staining, the positive cells appeared as a brown color in the cytoplasm and membrane. Immunohistochemical examination showed that EphB4 was located in the cytoplasm and membrane of the breast cancers (Figure 1). In total, 66.9% (115/172) of the breast cancer cases showed high EphB4 expression.
Figure 1.
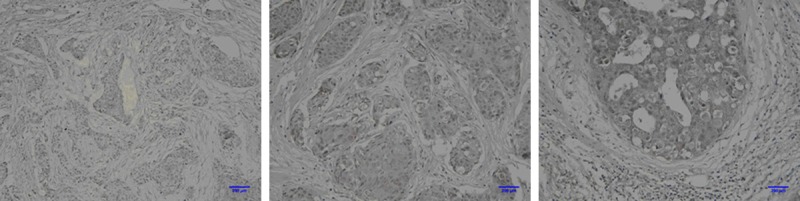
Immunohistochemical examination showed that EphB4 was located in the cytoplasm and membrane of the breast cancers.
EphB4 expression status was not balanced among TNM stage, lymph node metastasis, ER status, and PR status (P = 0.001, 0.004, 0.007, and 0.041 respectively) (Table 1). Spearman correlation regression analysis showed that EphB4 expression has a linear correlation to TNM stage, lymph node metastasis, ER status, and PR status (P < 0.01, respectively).
Table 1.
Correlations between EphB4 expression and clinicopathological features (n = 172)
| Variables | N | FSIP1+ | FSIP1– | P value |
|---|---|---|---|---|
| Age | 0.868 | |||
| ≤ 50 Y | 105 | 71 | 34 | |
| > 50 Y | 67 | 44 | 23 | |
| Tumor size | 0.070 | |||
| T1 | 70 | 41 | 29 | |
| T2, T3 | 102 | 74 | 28 | |
| Family history of cancer | 0.652 | |||
| Yes | 26 | 16 | 10 | |
| No | 146 | 99 | 47 | |
| Metastatic nodes | 0.001 | |||
| Positive | 86 | 68 | 18 | |
| Negative | 86 | 47 | 39 | |
| ER status | 0.007 | |||
| Positive | 65 | 49 | 16 | |
| Negative | 107 | 66 | 41 | |
| PR status | 0.041 | |||
| Positive | 70 | 52 | 18 | |
| Negative | 102 | 63 | 39 | |
| Her-2 status | 0.361 | |||
| Negative-+ | 111 | 70 | 41 | |
| ++ | 38 | 28 | 10 | |
| +++, ++++ | 23 | 17 | 6 | |
| TNM stage | 0.004 | |||
| II | 50 | 25 | 25 | |
| III, IV | 122 | 90 | 32 |
Regulation of EphB4 expression in breast cancer cells by IGF-I and LY294002
We first examined EphB4 expression in MCF-7 cells by IGF-I and LY294002 (LY). Cells were cultured in RPMI 1640 medium with 10% fetal bovine serum until 80-90% confluent, The control group was changed to serum-free medium (SFM) for 24 h. IGF-I and LY group cells were treated with 10 ng/ml IGF-I, 50 µM LY294002 for 10 min after SFM. The expression of EphB4 was shown in Figure 1. The expression of EphB4 in control group was significantly decreased when compared with IGF-I group (P < 0.001). The expression of EphB4 in IGF-I+LY and LY group were lower than that of the control group (P < 0.001).
Effects of cell proliferation in response to IGF-I and LY294002
After give IGF-I or LY, growth was measured 24 h, 48 h and 72 h respectively. All treatments were done in triplicates. Growth was assayed by the (MTT) assay. The MTT assay showed that, compared to the cells in the control group, cells in IGF-I group increased significantly, (P < 0.001), while cells in IGF-I+LY group and LY group show decreased proliferation when compared to the cells in the control group, especially in LY group, P < 0.001 (Figure 2).
Figure 2.
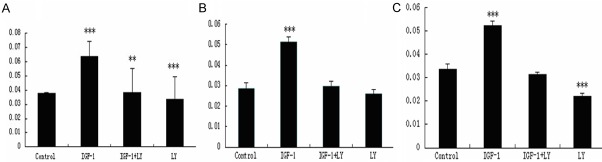
Serum-starved cells were given IGF-I (10 ng/ml) with or without preincubation with 50 mM LY294002 (LY) for 10 min. The proliferation capacity was measured by absorbance value using a microplate reader. A. Cells were harvested 24 h after stimulation or inhibition. B. Cells were harvested 48 h after stimulation or inhibition. C. Cells were harvested 72 h after stimulation or inhibition. Results are expressed as the means ± SD; ***P < 0.001, **P < 0.01. The representative results from three independent experiments are shown.
Effect of cell migration and invasion in response to IGF-I and LY294002
The scratch-wound assay showed that after 24 h, compared to the cells in the control group, cells in IGF-I group increased significantly, indicating that IGF-I stimulate the migration of breast cancer cells. While IGF-I+LY group and LY group show decreased migration when compared to the cells in the control group, indicating that LY inhibited the migration of breast cancer cells (Figure 3).
Figure 3.
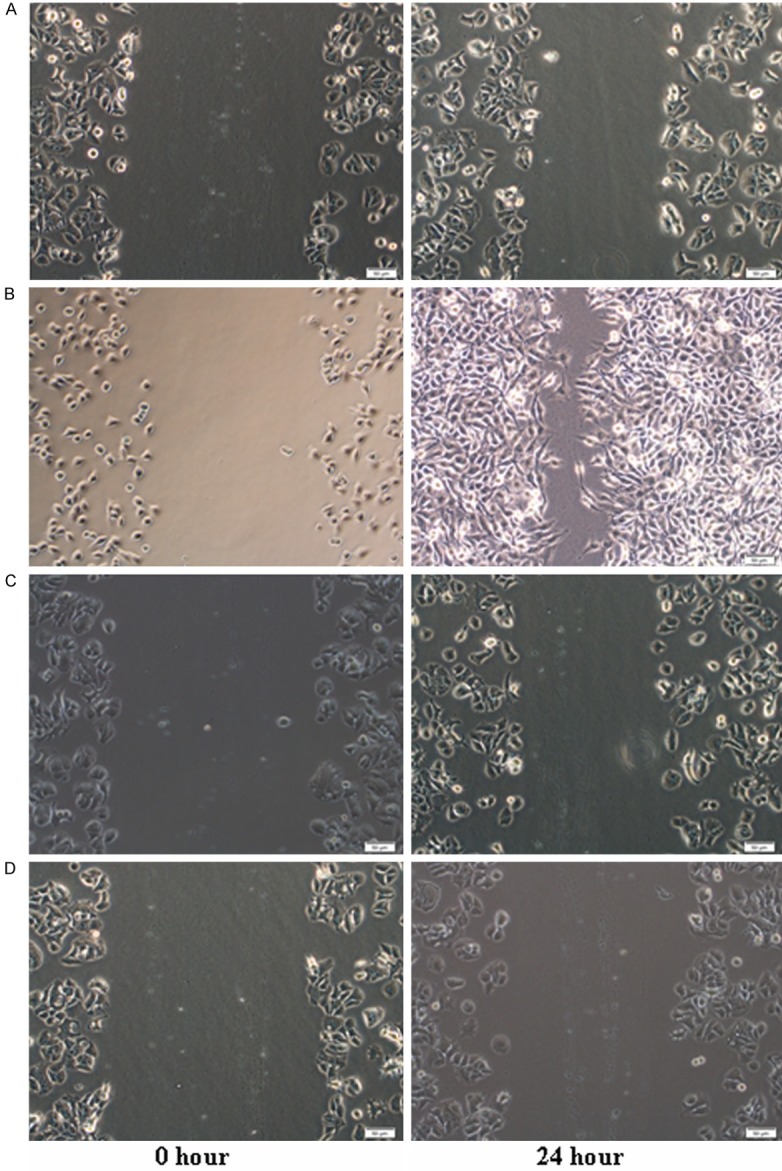
Migration and Invasion of MCF-7 cells in response to IGF-I and LY294002 A. control group. B. IGF-1 group. C. IGF-1+LY group. D. LY group. Serum-starved cells were given IGF-I (10 ng/ml) with or without pre-incubation with 50 mM LY294002 (LY) for 10 min. The migration and invasion assay was measured by scratch-wound assay. Subconfluent cultures of MCF-7 cells were wounded with a sterile pipette tip and repopulation of the wounded area by migrating cells observed 24 hour later. The extent of repopulation in three repeat experiments was quantitated using Image J software.
Discussion
EphB4 is normally expressed on endothelial cells of venous and arterial lineage, and the interaction between these cells is critical for new vessel formation, fusion between vessel compartments, and blood flow [19-21]. Besides EphB4 shows oncogenic activities in several tumors, including prostate, breast, endometrial, head and neck, bladder, and ovarian cancers, as well as mesothelioma [22-25]. It has become clear that IGF-1R has high expression levels in a variety of human tumor cells, when combined with IGF-I, IGF-IR can stimulate both normal and malignant breast cell proliferation [18]. In this study, we evaluate the role of EphB4 in breast cancer cells and whether IGF-IR is the upstream gene of EphB4.
Xia and colleagues [14] demonstrated that EGF/EGFR and IGF-I/IGF-IR can induce high expression of EphB4 in prostate cancer cells. Many studies have found that when combined with IGF-I, IGF-IR can induce product downstream insulin receptor substrate I (IRS-1) tyrosine phosphorylation, then respectively activate two signaling ways: the Ras-MAPK pathways and PI3K-Akt/PKB pathway [8], while other study showed [26], PI3K-Akt/PKB pathway play a main role when induced by IGF-I in the breast cancer cells. We showed IGF-I/IGF-IR can induce high expression of EphB4 in MCF-7 cells, RT-PCR showed IGF-1 group had higher expression of EphB4 than other three groups, with statistical significance. LY294002 (LY) is PI3K inhibitor, in IGF-I+LY group and LY group ,the expression of EphB4 were significantly less than the control group and IGF-I group, with statistical significance, indicating that IGF-I/IGF-IR can induce high expression of EphB4 through PI3K-Akt/PKB signaling pathway. Besides, higher expression of EphB4 shows increased tumor proliferation and migration in MTT assay and scratch-wound assay, and IGF-1 group shows significantly increased tumor proliferation and migration.
These findings are also supported by studies that have shown EphB4 as a survival factor in tumor cells by interfering with apoptotic pathways and by promoting tumor cell migration and invasion [14,16]. For example, Xia and colleagues [14] demonstrated a decrease in tumor cell proliferation and increase in apoptosis following EphB4 knockdown in a murine bladder cancer model. Downregulation of EphB4 expression in breast cancer cell lines led to decreased cell viability and activation of caspase-8-mediated apoptosis [23]. Furthermore, the EphB4 receptor is correlated to the initiation, progression, and angiogenesis of the tumor [27,28].
The results of our study imply that IGF-IR might be the upstream gene of EphB4, when combined with IGF-I can induce higher expression of EphB4 through PI3K-Akt/ PKB signaling pathway. In recent year, genetic and molecular mechanism study of breast cancer has mostly focused on EphB4 and its downstream signaling pathway, targeting EphB4 is a potentially effective therapy for cancer, while few reports about upstream genes or signaling pathway. Studying of EphB4 upstream target genes and signaling pathways means more targeted anti-tumor point selection for targeted therapy, therefore, intervention of EphB4 upstream target genes and signaling pathways can restrain the progress of breast cancer, which is expected to become the new target goals. Our understanding of the complex roles of IGF-IR, EphB4 and signaling pathway in cancer is still evolving, and more information is needed to resolve the many confusing and controversial issues. Future research will determine whether EphB4-based therapeutic strategies can be effective for the treatment of cancers that overexpress EphB4 and in which types of cancer, different therapeutic approaches may be most appropriate.
Acknowledgements
The study was supported by National Science Foundation. This study was done in The Key Lab for Basic Research of Difficult and Critical Diseases with Integrated Traditional
Disclosure of conflict of interest
None.
References
- 1.Wang B. Cancer cells exploit the Eph-ephrin system to promote invasion and metastasis: tales of unwitting partners. Sci Signal. 2011;4:pe28. doi: 10.1126/scisignal.2002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu SL, Li D, Park R, Liu R, Xia ZX, Guo JC, Krasnoperov V, Gill PS, Li ZB, Shan H. PET imaging of colorectal and breast cancer by targeting EphB4 receptor with Cu-64-labeled hAb47 and hAb131 antibodies. J Nucl Med. 2013;54:1094–1100. doi: 10.2967/jnumed.112.116822. [DOI] [PubMed] [Google Scholar]
- 3.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Scehnet JS, Ley EJ, Krasnoperov V, Liu R, Manchanda PK, Sjoberg E, Kostecke AP, Gupta S, Kumar SR, Gill PS. The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma and implications of EphrinB2 blockade. Blood. 2009;113:254–263. doi: 10.1182/blood-2008-02-140020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spannuth WA, Mangala LS, Stone RL, Carroll AR, Nishimura M, Shahzad MM, Lee SJ, Moreno-Smith M, Nick AM, Liu R, Jennings NB, Lin YG, Merritt WM, Coleman RL, Vivas-Mejia PE, Zhou Y, Krasnoperov V, Lopez-Berestein G, Gill PS, Sood AK. Converging evidence for efficacy from parallel EphB4-targeted approaches in ovarian carcinoma. Mol Cancer Ther. 2010;9:2377–2388. doi: 10.1158/1535-7163.MCT-10-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol. 2010;12:1194–U1175. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- 8.Chen T, Liu X, Yi S, Zhang J, Ge J, Liu Z. EphB4 is overexpressed in gliomas and promotes the growth of glioma cells. Tumor Biol. 2013;34:379–385. doi: 10.1007/s13277-012-0560-7. [DOI] [PubMed] [Google Scholar]
- 9.Yang NY, Lopez-Bergami P, Goydos JS, Yip D, Walker AM, Pasquale EB, Ethell IM. The EphB4 receptor promotes the growth of melanoma cells expressing the ephrin-B2 ligand. Pigment Cell Melanoma Res. 2010;23:684–7. doi: 10.1111/j.1755-148X.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spannuth WA, Mangala LS, Stone RL, Carroll AR, Nishimura M, Shahzad MM, Lee SJ, Moreno-Smith M, Nick AM, Liu R, Jennings NB, Lin YG, Merritt WM, Coleman RL, Vivas-Mejia PE, Zhou Y, Krasnoperov V, Lopez-Berestein G, Gill PS, Sood AK. Converging Evidence for Efficacy from Parallel EphB4-Targeted Approaches in Ovarian Carcinoma. Mol Cancer Ther. 2010;9:2377–2388. doi: 10.1158/1535-7163.MCT-10-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafleur K, Huang D, Zhou T, Caflisch A, Nevado C. Structure-based optimization of potent and selective inhibitors of the tyrosine kinase erythropoietin producing human hepatocellular carcinoma receptor B4 (EphB4) J Med Chem. 2009;52:6433–6446. doi: 10.1021/jm9009444. [DOI] [PubMed] [Google Scholar]
- 12.Dopeso H, Mateo-Lozano S, Mazzolini R, Rodrigues P, Lagares-Tena L, Ceron J, Romero J, Esteves M, Landolfi S, Hernández-Losa J, Castaño J, Wilson AJ, Ramon y Cajal S, Mariadason JM, Schwartz S Jr, Arango D. The receptor tyrosine kinase EPHB4 has tumor suppressor activities in intestinal tumorigenesis. Cancer Res. 2009;69:7430–7438. doi: 10.1158/0008-5472.CAN-09-0706. [DOI] [PubMed] [Google Scholar]
- 13.Wu Q, Suo Z, Kristensen GB, Baekelandt M, Nesland JM. The prognostic impact of EphB2/B4 expression on patients with advanced ovarian carcinoma. Gynecol Oncol. 2006;102:15–21. doi: 10.1016/j.ygyno.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, Quinn DI, Weaver FA, Gill PS. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–780. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 15.Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, Quinn DI, Henshall SM, Sutherland RL, Pinski JK, Daneshmand S, Buscarini M, Stein JP, Zhong C, Broek D, Roy-Burman P, Gill PS. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 16.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. AmJ Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA, Beart RW, Singh G, Nguyen C, Kahn M, Gill PS. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–3745. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- 18.Gualberto A, Pollak M. Clinical development of inhibitors of the insulin like growth factor receptor in oncology. Curr Drug Targets. 2009;10:923–936. doi: 10.2174/138945009789577945. [DOI] [PubMed] [Google Scholar]
- 19.Krasnoperov V, Kumar SR, Ley E, Li X, Scehnet J, Liu R, Zozulya S, Gill PS. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. Am J Pathol. 2010;176:2029–2038. doi: 10.2353/ajpath.2010.090755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsuta H, Fukushima Y, Maruyama K, Hirashima M, Nishida K, Nishikawa S, Uemura A. EphrinB2-EphB4 signals regulate formation and maintenance of funnel-shaped valves in corneal lymphatic capillaries. Invest Ophthalmol Vis Sci. 2013;54:4102–8. doi: 10.1167/iovs.12-11436. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AC, Mendel TA, Mason KE, Degen KE, Yates PA, Peirce SM. Attenuation of ephrinB2 reverse signaling decreases vascularized area and preretinal vascular tuft formation in the murine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53:5462–70. doi: 10.1167/iovs.11-8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, Gill PS. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer. 2007;96:1083–91. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia G, Kumar SR, Masood R, Koss M, Templeman C, Quinn D, Zhu S, Reddy R, Krasnoperov V, Gill PS. Up-regulation of EphB4 in mesothelioma and its biological significance. Clin Cancer Res. 2005;11:4305–15. doi: 10.1158/1078-0432.CCR-04-2109. [DOI] [PubMed] [Google Scholar]
- 24.Sinha UK, Kundra A, Scalia P, Smith DL, Parsa B, Masood R, Gill PS. Expression of EphB4 in head and neck squamous cell carcinoma. Ear Nose Throat J. 2003;82:866, 869–70, 887. [PubMed] [Google Scholar]
- 25.Berclaz G, Karamitopoulou E, Mazzucchelli L, Rohrbach V, Dreher E, Ziemiecki A, Andres AC. Activation of the receptor protein tyrosine kinase EphB4 in endometrial hyperplasia and endometrial carcinoma. Ann Oncol. 2003;14:220–6. doi: 10.1093/annonc/mdg072. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Lin M, van Golen KL, Yoshioka K, Itoh K, Yee D. Multiple signaling pathways are activated during insulin-like growth factor-I (IGF-I) stimulated breast cancer cell migration. Breast Cancer Res Treat. 2005;93:159–168. doi: 10.1007/s10549-005-4626-8. [DOI] [PubMed] [Google Scholar]
- 27.Allington TM, Schiemann WP. The Cain and Abl of epithelial-mesenchymal transition and transforming growth factor-β in mammary epthelial cells. Cells Tissues Organs. 2011;193:98–113. doi: 10.1159/000320163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rappa G, Anzanello F, Lorico A. Imatinib mesylate enhances the malignant behavior of human breast cancinoma cells. Cancer Chemother Pharmacol. 2011;67:919–926. doi: 10.1007/s00280-010-1394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


