Summary
Heat shock proteins (HSPs) are a super family of highly conserved molecular chaperone proteins, which are induced in response to stress. HSP70 has been demonstrated to inhibit apoptosis induced by a number of chemotherapeutic agents. Previous investigations have suggested the development of drug resistance in multiple myeloma (MM) cells after adhesion to stroma. This study used MM cell lines and primary plasma cells to determine if HSP70 had a role in development of chemo resistance. Adhesion of MM cells to either bone marrow stromal cells or fibronectin (FN) enhanced HSP70 expression. Inhibition of the HSP70 expression decreased 8226 cell adhesion to stroma or FN and induced more apoptosis in FN-adhered 8226 cells than in suspension cultures at 24 h. Further, HSP70 inhibitors enhanced melphalan-induced apoptosis and reversed melphalan-induced cell adhesion-mediated drug resistance (CAM-DR) phenotype. In addition, compared to parental cells, KNK-437, a heat shock factor inhibitor caused more apoptosis in melphalan-resistant 8226/LR5 cells and sensitized them to melphalan. Primary CD138 positive cells showed high expression of HSPA4 mRNA, and KNK-437 caused apoptosis in these cells. In conclusion, our data suggest inhibition of HSP70, reduced adhesion and caused apoptosis of both acquired and de novo drug resistant MM cells.
Keywords: HSP70, multiple myeloma, drug resistance, adhesion, cell adhesion mediated resistance
In spite of high dose chemotherapy and novel targeted therapies, multiple myeloma (MM) remains incurable with a median survival of 3–6 years, mainly because of relapse after chemotherapy (Preisler, et al 1989a, Preisler, et al 1989b). Improved survival requires new strategies to prevent relapse.
An increasing body of evidence suggests that the bone marrow microenvironment is the primary site for minimal residual disease (MRD) leading to relapse after chemotherapy (Matsunaga, et al 2003, Preisler, et al 1989a). Adhesion of MM cells to extra-cellular matrix (ECM) components, such as fibronectin (FN) via β1 integrin, has been demonstrated to confer resistance to a host of chemotherapeutic agents (Damiano, et al 1999, Shain, et al 2000). This anti-apoptotic phenomenon, termed “cell adhesion-mediated drug resistance” (CAM-DR), is a form of de novo drug resistance (Damiano, et al 1999, Shain, et al 2000). Therefore, identification of mediators of cell adhesion may elucidate novel targets for MM therapy and inhibition of this target could potentially overcome CAM-DR.
In addition to CAM-DR, chemo-resistance in MM is characterized by a concomitant insensitivity to the drugs used in therapy, as well as to other unrelated cytotoxic agents - a phenomenon known as acquired multidrug resistance (MDR) (Bellamy, et al 1991, Bhalla, et al 1994, Dalton, et al 1986, Ross 2000). This acquired drug resistance has been shown to develop following chemotherapy.
Recently, our group has reported genotypic and phenotypic profiles of acquired and de novo melphalan resistance in an isogenic human myeloma cell line (Hazlehurst, et al 2003). Gene expression changes associated with de novo resistance were significantly less complex compared with acquired resistance (Hazlehurst, et al 2003). This indicates that myeloma cell adhesion promotes a form of de novo drug resistance by protecting cells from melphalan-induced cytotoxic damage and that this transient protection allows cells to acquire a more permanent and complex drug resistance phenotype associated with a reduction in drug induced DNA damage.
Heat shock proteins (HSPs) are highly conserved proteins, which are induced in plant, yeast, bacterial and mammalian cells in response to an array of physiological and environmental stress cues (Welch 1992). HSP70 has been shown to be preferentially expressed in high-grade malignant tumors compared to low-grade tumors and surrounding tissues (Ralhan and Kaur 1995, Santarosa, et al 1997). HSP70 was also associated with chemotherapeutic resistance in many forms of leukemias (Chant, et al 1995, Santarosa, et al 1997, Sliutz, et al 1996). Though heat shock proteins were initially discovered to function as molecular chaperones, there is increasing evidence to suggest that they play a key role in survival of cancer cells (Beere, et al 2000, Jaattela, et al 1998). HSP70 has been reported to prevent cell death initiated by various apoptotic stresses, such as heat shock, ceramide, ionizing radiation, tumour necrosis factor-alpha (TNF-α) and ischemia (Geginat, et al 1993) (Jaattela, et al 1998). Further, HSP70 has been shown to inhibit mitochondria-induced apoptosis by physically interacting with and inhibiting Apaf-1 and apoptosis-inducing factor (AIF) resulting in suppression of caspase-dependent and -independent apoptosis, respectively (Beere, et al 2000, Ravagnan, et al 2001). In spite of the extensive studies of HSP70 in apoptosis and drug resistance there is lack of information on its role in tumor microenvironment and MRD in cancer therapy.
The present study demonstrated that HSP70 expression was enhanced when 8226 myeloma cells were attached to stromal cells. HSP70 inhibition reduced adhesion of myeloma cells to FN or stromal cells, caused apoptosis of acquired and de novo drug resistant myeloma cells, and sensitized them to chemotherapeutic agents. These results suggest that HSP70 is a key modulator of bone marrow microenvironment in MM that results in CAM-DR.
Materials and methods
Cell lines, cytokines, and other chemicals
Myeloma cell lines RPMI-8226 and H929 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and HS.5 bone marrow-derived transformed stromal cells were a kind gift from Beverly Totok-Storb at the Fred-Hutchinson Cancer Center (Seattle,WA). Melphalan-resistant 8226/LR5 cell line was developed in our laboratory and previously described. (Bellamy, et al 1991) Sensitive and resistant 8226 cells and HS.5 were maintained in RPMI medium (Cell Gro MediaTech, Herndon, VA) supplemented with 5% or 10% fetal bovine serum (Omega Scientific, Tarzana, CA), 1% penicillin/streptomycin and 100 mmol/l L-glutamine (Gemini Bio-Products, Calabasas, CA). The H929 cell medium was also supplemented with 0·05 mol/l 2-mer-captoethanol (Sigma, St. Louis, MO). Recombinant human interleukin 6 (rhIL-6) was obtained from R&D System (Minneapolis, MN). Human plasma FN was obtained from Invitrogen Life Technologies (Carlsbad, CA). KNK-437, an inhibitor of heatshock factor-1, was purchased from Calbiochem (San Diego CA). Based on its molecular weight, a 10 mmol/l stock solution was prepared in 50% dimethyl sulphoxide (DMSO). Final concentration of DMSO was 0·2% and this was used to treat control cells.
Adhesion assays
Myeloma cells (RPMI-8226 and H929) were adhered to FN as previously described (Hazlehurst and Dalton 2001). Cells were preincubated with different concentrations of KNK-437, (5, 10 and 20 ng/ml) for 2 h or RNAi/HSP70 for 8 h. Cells were then washed twice with serum free medium and adhered to FN-or bovine serum albumin (BSA)-coated 96-well plate at a concentration of 1 × 105 cells/well. After 2 h adhesion at 37°C, wells were washed twice in serum-free media to remove unattached cells and adherent cells were fixed in 70% methanol for 5 min. Following media aspiration, wells were allowed to dry and then stained with 0·02% crystal violet/0·2% ethanol for an additional 10 min. After solubilization with 100 µl Sorenson buffer, absorbance was read at 540 nm with an automated microtitre plate reader. In specific experiments, cells were preincubated for 2 h with anti b1 or VLA4 or VLA5 or isotype control before application to the wells.
In experiments utilizing stromal cells instead of FN, HS.5 cells were plated at a density of 1·0 × 105 cells per well in 96 well plates for 16 h. Adhesion was measured using a previously described cell-labeling technique (Nefedova, et al 2003). Briefly, myeloma cells were labeled with 5 µmol/l cell tracker Green CFMDA (5-chloromethylfluorescein diacetate), (Molecular Probes, Eugene, OR) for 30 min, then washed with phosphate-buffered saline (PBS) and resuspended in serum-free medium and then incubated either with anti b1 or very late antigen (VLA)4 or VLA5 isotype-specific antibody or for 2 h before application to the wells containing HS.5 cells. After 2 h of adhesion at 37°C, wells were washed twice in serum-free media to remove unattached cells and adherent cells were washed once with cold PBS and permeabilized with 50 µl of 0·1% Nonidet-P-40 (NP-40). Fluorescence was read at 485–535 nm using a Victor Wallac fluorescence micro plate reader.
Cell death assessment
After drug treatments, cells were resuspended in 100 µl staining solution containing annexin-V fluorescein isothiocyanate (FITC) and 7-Amino-actinomycin D (7-AAD) in a HEPES buffer according to manufacturers instructions (Boehringer-Mannheim, Indianapolis, IN). Briefly, cells were washed with PBS and resuspended in 200 µl of 1×annexin binding buffer obtained from BD Biosciences, at a concentration of 1 × 106 cells/ml. 5 µl of annexin V-FITC was added and the cells were incubated in the dark for 15 min at room temperature. The labelled cells were then added to 10 µl 7-AAD and analyzed immediately with a FACSCALIBUR cytometer (Becton–Dickinson, UK). Data from at least 10 000 events per sample were recorded and processed using the CellQuest software (Becton–Dickinson, UK). The percentage of specific cell death was determined by subtracting background death in untreated samples; for these calculations we used annexin V and propidium iodide (PI) + samples.
Measurement of HSP70 in the culture medium
Human HSP70 expression from myeloma cell lines were quantitated using a sandwich enzyme-linked immunosorbent assay (ELISA) obtained from Stressgen Biotechnologies (San Diego, CA) Cells were either placed in suspension culture or adhered to FN or to HS.5 cells for 2 h, then non-adhered cells were aspirated and cells were cultured in serum-free medium. Cell culture supernatant was collected at indicated time points and 100 µl of the medium was used for ELISA according to the manufacturer’s instructions. In some experiments myeloma cells (8226, H929), as well as HS.5 cells, were pre incubated with either KNK-437 for two h, or RNAi/HSP70 for 8 h then cells were co-cultured for 8 h, then culture supernatant was collected for assaying HSP70.
Cellular levels of HSP-70 in myeloma cells
Myeloma cells untreated or treated with either indicated doses of KNK-437 or RNAi/HSP70 retrovirus for 24 h were collected, washed twice with ice-cold PBS, and lysed in RIPA buffer. Equal protein was separated by either 10 or 12% sodium dodecyl sulphate polyacrylamide gel electroporesis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membrane and blocked with 5% milk in Tris-buffered saline-Tween (TBS/T). The membrane was then probed with antiserum to the indicated protein, washed with TBS/T, and incubated with HRP-conjugated anti-rabbit or mouse secondary antibody for 1 h. Anti HSP70, HSP90, and HSP27 antibodies were obtained from Stressgen Biotechnologies (San Diego, CA). IgG isotype, β1, VLA4, VLA5 antibodies were purchased from BD Biosciences (San Jose, CA). Specific band was developed by chemiluminescence (Amersham, Piscataway, NJ). To confirm equal loading, membrane was reprobed with monoclonal β-actin antibody (Sigma).
Preparation of RNAi/HSP70 retrovirus
For construction of HSP70/RNAi1 we used 5′- GAT CCG CCA AGC AGA CGC AGA TCT TTT CA-3′ as a forward strand and 5′- AAT TCA AAA AAG CCA AGC AGA CGC AGA TC-3′ as reverse strand were used. For construction of HSP70/RNAi2 we used 5′- GAT CCG AGT GTC AAG AGG TCA TCT CTT CA-3′ as a forward strand and 5′- AAT TCA AAA AAG AGT GTC AAG AGG TCA TC as reverse strand were used. Forward and reverse strands were annealed and cloned into the BamH1 and Hind 111 sites in pSlincer™ 5.1 Si RNA expression vector (Ambion, Austin TX). Puromycin resistance gene was used to select for packaging cells (PT67) that stably express the introduced DNA. Mock virus was created by transfecting vector without the sequence. The viral supernatant was collected and mixed with polybrene (4 µg/ml) to infect the myeloma cells.
Isolation of CD138 positive plasma cells from bone marrow
A 30 ml aliquot of bone aspirate was collected from 23 myeloma patients with informed consent. Of these 23 patients, 12 were newly diagnosed and 11 patients had relapsed disease. Mononuclear cells were separated by ficoll-hypaque gradient centrifugation and washed twice with cold PBS. After red blood cell lyses of bone marrow with 0·86% ammonium chloride, plasma cells were positively selected using CD138 micro beads and magnetic-activated cell sorting (MACS; Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer’s instructions. Purity was assessed by morphology and flow cytometry (FACScan; BD Biosciences, San Jose, CA). If the purity of the plasma cells was >95% as measured by morphology (haematoxylin & eosin [H&E] stain), and >90% by flow cytometry, those cells we used directly in the experiments without further purification. These cells were maintained in culture in alpha minimal essential medium (α-MEM) supplemented with 15% fetal bovine serum.
Results
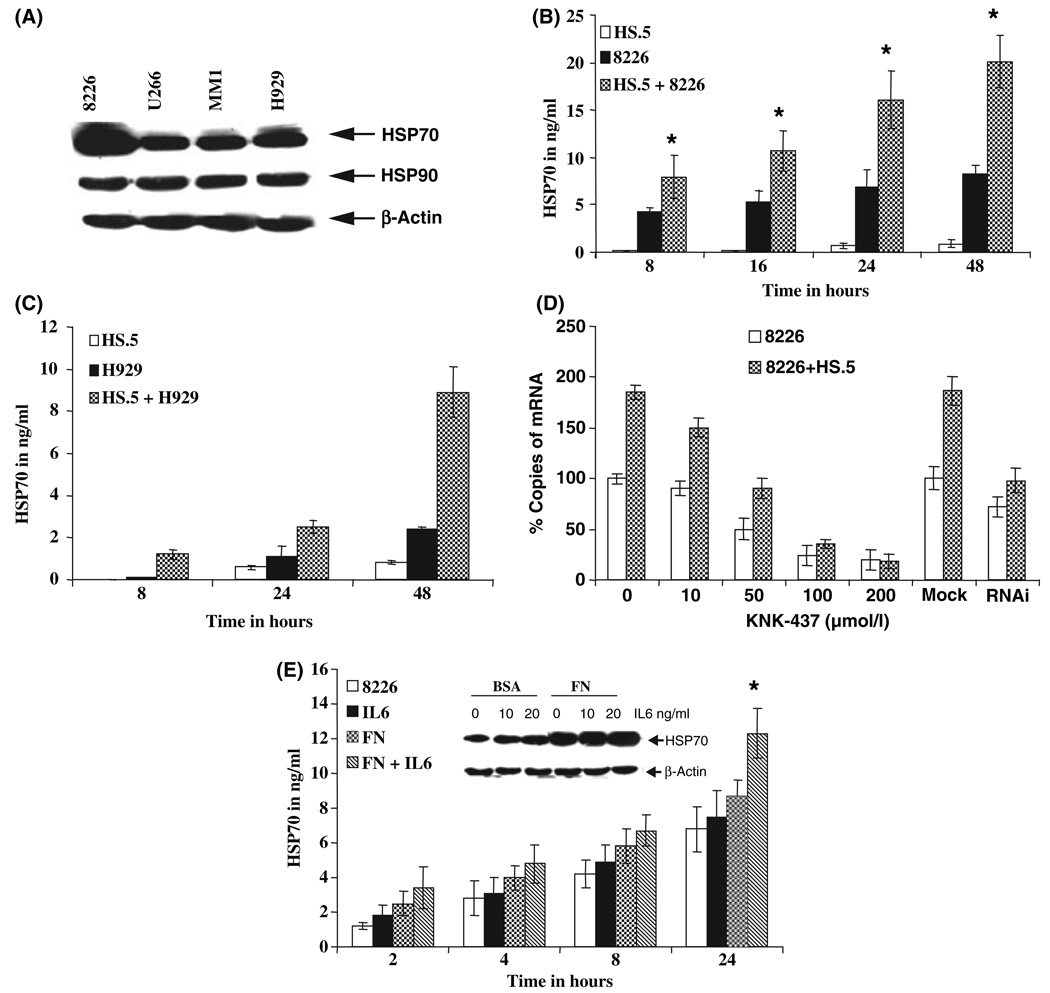
Adhesion of myeloma cells enhances HSP70 expression
We hypothesized that HSP70 may play a critical role in interaction of myeloma cells with bone marrow stroma and hence in tumor survival and progression. In order to study the role of HSP70 in myeloma stromal cell interaction, we initially determined the expression patterns of HSP70 in several MM cell lines. As shown in Fig 1(A), HSP70 is expressed in all four commonly used MM cell lines, namely, 8226, U266, MM1, and H929. For these cell lines, when protein intensity in the immunoblots was measured, 8226 cells expressed the highest amount of the intracellular HSP70 (2·68), whereas U266 had the lowest (1·16), MM1 (1·28) and H929 (1·45) had intermediate levels. Stromal cells (HS.5) did not express intracellular HSP70 levels (data not shown). To determine whether adhesion to stromal cells enhances extra-cellular expression of HSP70 in myeloma cells, 8226 cells were exposed to either BSA or stromal cells and measured HSP70 by enzyme-linked immunosorbent assay (ELISA). As shown in Fig 1(B), adhesion of 8226 cells to stromal cells enhanced secretory HSP70 in a time-dependent manner. Consistent with 8226 data, H929 cells when adhered to HS.5 cells also enhanced the HSP70 secretion but the level of expression was much less when compared to that of 8226 cells (Fig 1C). To measure adhesion induced intracellular expression of HSP70 in myeloma cells, we exposed 8226 cells to either BSA or stromal cells for 8 h and measured the HSPA4 mRNA copies by real time reverse transcription polymerase chain reaction (RT-PCR). The adhesion of 8226 cells to stromal cells enhanced HSPA4 mRNA (Fig 1D). Induction of HSP70 was not restricted to HS.5 cell-mediated adhesion but was also observed when we exposed 8226 cells to FN (Fig 1E). As we have previously shown that IL-6 enhances adhesion of MM cells to FN, we studied the effect of IL-6 and IL-6 in combination with FN adhesion on HSP70 expression. As shown in Fig 1(E), 8226 cell adhesion to FN, or IL-6 addition increased HSP70 expression in a time-dependent manner. Significant increase in HSP70 expression with a combination of IL-6 and FN adhesion was seen at 24 h (Fig 1E, P = 0·05). To determine the effect of IL-6 and adhesion to FN in myeloma cells on endogenous expression levels of HSP70, 8226 cells were exposed either to BSA or FN and/or IL-6 and HSP70 expression was measured by immunoblotting. Adhesion to FN enhanced HSP70 expression and IL-6 stimulation with FN adhesion enhanced HSP70 expression in a dose-dependent manner (Fig 1E insert).
Fig 1.
Adhesion of myeloma cells to either FN or stromal cells enhances HSP70 expression. (A) Immunoblot analysis of intracellular protein expression levels of HSP70and HSP90 in MM cells using specific antibodies. β-actin was used as loading control. (HS.5 (stromal) cells were plated at concentration of 1 × 105 cells/well in 12-well plates for 16 h). 1 × 1068226 (B), H929 myeloma cells (C) were incubated with stromal cells (HS.5) for 2 h. After 2 h of adhesion, non-adherent cells were aspirated and 1 ml of serum-free medium was added to the wells. Conditioned medium was collected at different times after the addition of the serum-free medium and HSP70 expression was quantitated by ELISA. D). HS.5 (stromal) cells were plated at concentration of 5 × 105 cells/well in 6 well plates for 16 h. 8226 myeloma cells 2 × 106 were incubated with stromal cells (HS.5) for 2 h. After 2 h of adhesion, non-adherent cells were aspirated and 2 ml of medium was added to the wells. Then 8226 cells were treated with 0, 10, 50, 100 and 200 µmol/l KNK-437 for 8 h or infected with retrovirus expressing RNAi or mock infection for 12 h. 8226 cells were striped from stromal cells by gentle washing and total RNA was isolated using Qiagen kit according to the manufacturer’s instructions. Using real time RT-PCR we quantitated the HSPA4 mRNA levels. The values were normalized with 18S as loading control. (E) 8226 cells were incubated in the presence and absence of IL-6 (10 ng/ml) for 2 h then cells were either adhered to FN or kept in suspension. Eight hours after treatment, cell culture supernatant was collected and quantitated for HSP70. *Indicates statistically significant P value ≤0·05. Insert) Cells were lysed and immunoblot analysis of intracellular protein expression levels of HSP70 using specific antibody was conducted. β actin was used as loading control.
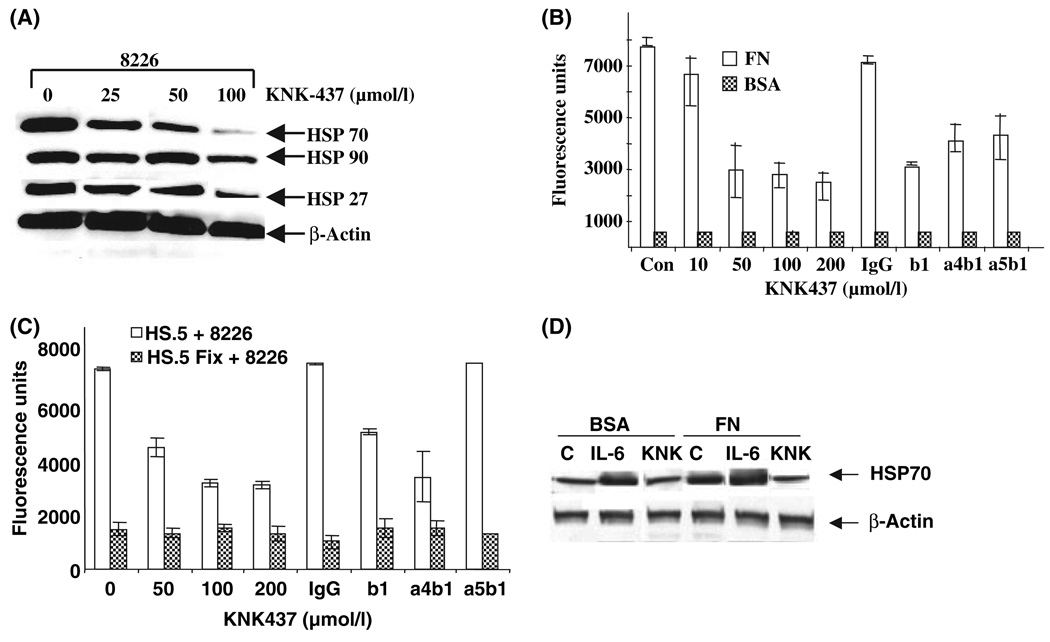
KNK-437 attenuated the adhesion of myeloma cells and induced apoptosis
We have shown that adhesion of myeloma cells enhanced HSP70 expression and it has also been demonstrated that myeloma cells adhered to FN and/or stromal cells, showed cell adhesion mediated drug resistance (CAM-DR) (Damiano, et al 1999, Damiano and Dalton 2000). Hence, we investigated the effect of HSP70 inhibition on adhesion of myeloma cells to FN or bone marrow stromal cell line (HS.5). To inhibit endogenous expression of HSP70, we used a specific HSF inhibitor, KNK-437, which interferes with the formation of the complex between HSE (Heat shock elements) and heat shock factor (HSF), inhibits inducible HSPA4 mRNA and is a specific inhibitor of HSF (Koishi, et al 2001, Yokota, et al 2000). This inhibitor at 25 to 100 lmol/l reduced expression of HSPs with maximum effect on HSP70 (Fig 2A). This decline was associated with a decrease in the adhesion of 8226 cells to FN (Fig 2B) as well as HS.5 (Fig 2C) in a dose-dependent manner. Inhibitions of myeloma cell adhesion to patient-derived primary stromal cells were also observed (data not shown). As reported earlier, β1, β4α1 (VLA-4) and β5α1 (VLA-5) antibodies blocked adhesion of 8226 cells to FN (Fig 2B). In contrast, only b1 and α5β1 (VLA5) could block the 8226 adhesions to stromal cells (HS.5) (Fig 2C). Cells were pretreated with KNK-437 for 2 h and adhered for 2 h. At this time point there was no cell death as measured by annexin V-PI (data not shown). To measure whether KNK437 could reduce intracellular levels of HSP70 induced due to FN adhesion or IL6 stimulation or both, cells were adhered either to FN or kept in plates coated with BSA. KNK-437 inhibited the FN and or IL-6 induced HSP70 expression as measured by immunoblotting (Fig 2D). KNK437 also inhibited the stromal cell adhesion-induced mRNA expression in 8226 cells in a dose-dependant manner (Fig 1D). Previous reports suggest that HSP70 is an anti-apoptotic protein and modulation of its intracellular levels could influence apoptosis caused by chemotherapeutic drugs. However, the anti-apoptotic role of HSP70 in MM was not studied. Treatment of 8226 cells with KNK-437 for 24 h caused apoptosis in a dose-dependent manner when cells were either adhered or in suspension (Fig 2E). KNK-437 caused significantly more apoptosis when cells were adhered to FN compared to suspension. This could be due to the fact that, when cells were adhered to FN HSP70, expression was increased rendering these cells more sensitive to the inhibitor.
Fig 2.
KNK-437 reduced the myeloma cell adhesion to both FN and stromal cells and induced apoptosis. (A) 8226 cells were treated with 0, 25, 50 and 100 µmol/l KNK-437 for 24 h. Cells were lysed and immunoblotted for intracellular protein expression levels of HSP70, HSP90 and HSP27 using specific antibodies. β-actin was used as loading control. (B & C) Cell adhesion was determined using a CFMDA cell tracker reagent in conjunction with integrin-blocking antibodies. 8226 cells were pretreated for 1 h with either blocking monoclonal antibody or with 10, 50, 100 and 200 µmol/l KNK-437 prior to application to either fibronectin (FN)-coated wells (B) or wells with stromal cells (C). Absorbance values show mean of the three replicates with background binding either with bovine serum albumin (BSA) or Paraformaldehyde-fixed HS.5. (D) 8226/s cells were either kept in suspension or adhered to FN for 2 h. Non-adherent cells were aspirated and adhered cells were induced with IL-6 10 ng/ml for 2 h or treated with KNK-437 for 8 h and then cells were lysed and immunoblot analysis of intracellular protein expression levels of HSP70 using specific antibody was conducted. β-actin was used as loading control. (E) 8226/s cells were either kept in suspension or adhered to FN for 2 h. Non-adherent cells were aspirated and adhered cells were treated with 10, 50, 100 and 200 µmol/l KNK437 for 24 h and the percentage of apoptotic cells was measured by Flow cytometry using AnnexinV and 7-AAD. *Statistically significant P value ≤0·05. β1 blocking antibody (b1) α4β1 blocking antibody (a4b1), α5β1 blocking antibody (a5b1).
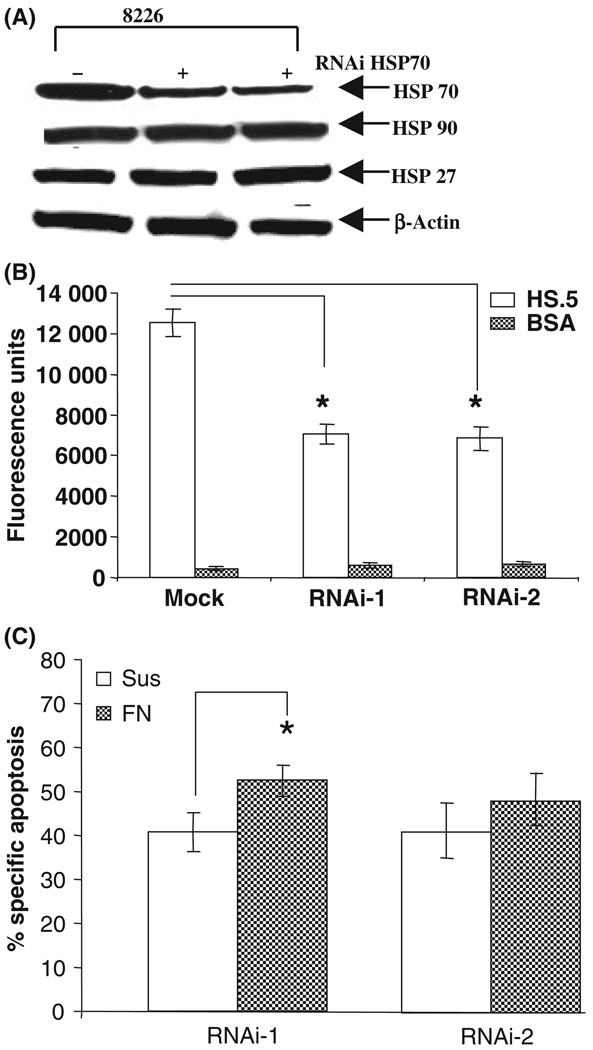
RNAi/HSP70 attenuated the adhesion of myeloma cells and induced apoptosis
To confirm the above results, we infected 8226 cells with retrovirus expressing RNAi to HSP70 (two different RNAi constructs as well as vector only control). RNAi to HSP70 reduced expression of HSP70 specifically without changing the proteins levels of other heat shock proteins (Fig 3A). RNAi to HSP70 also inhibited the adhesion of myeloma cells to stromal cells (Fig 3B). This reduction in adhesion correlated with reduction in HSP70 expression levels (compare 3B with 3A). At this time point there was no cell death as measured by annexin V-PI (data not shown). These changes in HSP70 mediated by the RNAi were also later associated with induction in apoptosis of these cells when cultured in suspension or on stroma for 24 h (Fig 3C). Statistical analysis showed significantly increased apoptosis in FN-adhered cells using clone 1 of RNAi but the difference in apoptosis was less compared to the KNK-437-induced apoptosis, as shown in Fig 2(A). This might be because KNK-437 affects other HSPs, such as HSP27 and HSP90, whereas RNAi was specific to HSP70. These results indicate that HSP70 plays a critical role in adhesion of myeloma cells to FN and stromal cells and survival of these cells.
Fig 3.
RNAi/HSP70 reduced the myeloma cell adhesion to both FN and stromal cells and induced apoptosis. (A) 8226 cells were infected either with two different clones of retrovirus expressing RNAi or Mock transfection for 12 h and immunoblot analysis of intracellular HSP70, HSP27 and HSP90 was performed. (B) CFMDA-treated 8226 cells were infected either with two different clones of retrovirus expressing RNAi or with mock transfected vector alone for 12 h cells were then incubated either with wells containing HS.5 cells or coated with BSA. After 2 h of adhesion, adherent cells were measured by absorbance. The mean value of three different experiments was shown. (C) 8226/s cells were either kept in suspension or adhered to FN for 2 h. Non-adherent cells were aspirated and adhered cells were infected with RNAi to HSP70 for 24 h. After 24 h of infection all the cells were collected and apoptosis was analysed by Annexin V and 7-AAD. *Statistically significant P value ≤0·05.
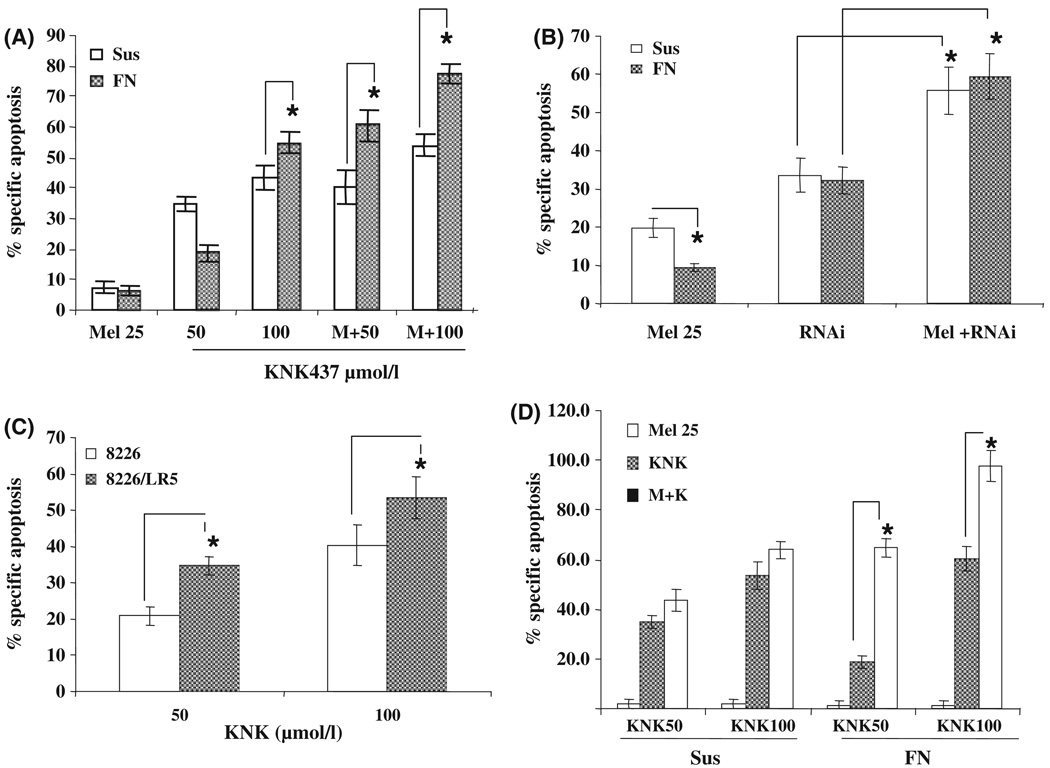
HSP70 inhibition enhances melphalan-induced apoptosis in 8226 cells
To determine if HSP70 inhibition would sensitize MM cells to chemotherapeutic agent, 8226 cells were treated with melphalan (25 lmol/l) with or without KNK-437 or RNAi to HSP70. As shown in Fig 4(A) and (B), the combination of drugs resulted in an increase in apoptosis, which was more significant in cells that were maintained on FN. As HSP70 inhibition enhanced melphalan-induced apoptosis and reversed CAMDR, i.e. de novo drug resistance, we investigated whether HSP70 inhibition causes apoptosis of acquired drug resistance in cells. For this purpose, we utilized melphalan-acquired drug resistance (8226/LR5) and wild type sensitive 8226 cells. KNK437 as well as RNAi to HSP70 caused apoptosis in these cells. Interestingly, 8226/LR5 cells were more sensitive to HSP70 inhibition than the wild type 8226 cells. Further, HSP70 inhibition sensitized melphalan-resistant 8226 cells to melphalan (Fig 4D). This was not due to increased expression of HSP70 as there was no difference in the intracellular HSP70, HSP90 and HSP27 protein levels in the 8226/s and 8226/LR5 cells (data not shown). These data demonstrated that inhibition of HSP70 causes apoptosis of acquired drug-resistant cells as well as de novo drug-resistant cells.
Fig 4.
Inhibition of HSP70 enhanced melphalan-induced apoptosis and sensitized 8226/LR5 cells to melphalan. (A) 8226/s cells were either kept in suspension (Sus) or adhered to FN for 2 h. Non-adherent cells were aspirated and cells were treated either with 25 µmol/l melphalan, KNK-437 (50 or 100 µmol/l), or combination of both for 24 h. (B) 8226/s cells were either kept in suspension or adhered to FN for 2 h. Non-adherent cells were aspirated and adhered cells were treated with either 25 µmol/l melphalan alone or infected with RNAi/HSP70 for 24 h or combination of both. (C) 8226/LR5 and 8226/s cells were treated with KNK-437 (50 or 100 µmol/l), for 24 h and apoptosis was measured. (D) 8226/LR5 cells were either kept in suspension or adhered to FN for 2 h. Non-adherent cells were aspirated and adhered cells were treated with either KNK-437 (50 and 100 µmol/l) or melpahalan (25 µmol/l), or combination of both. After 24 h of drug treatments all the cells were collected and apoptosis was analysed by Annexin V and 7AAD. *Statistically significant P value ≤0·05.
Primary MM (CD138 positive plasma cells) expresses HSP70
To determine if our data obtained in cell lines could be translated to clinical practice, we isolated CD138-positive plasma cells from bone marrow aspirates of the eight newly diagnosed as well as eight relapsed patients with MM. Whenever the percentage of CD138 positive cells was less than 90%, CD138 positive cells were isolated using CD138 micro beads. Samples that contained more than more than 90% of CD138 positive cells were used for further analysis. HSPA4 mRNA expression levels were measured using gene expression profiles (GEP). As shown in Table I, all the patients expressed HSPA4 and most of the relapsed patients (seven out of eight) showed enhanced expression due to FN adhesion compared to the newly diagnosed patients where only one of eight showed increased expression due to FN. The basal level of HSPA4 expression was high in relapsed patients compared to that in newly diagnosed patients.
Table I.
HSPA4 mRNA expression levels in patient derived plasma cells CD138-positive cells were isolated from bone marrow sample of the myeloma patient as described before and cells were either kept in suspension (SUS) or adhered to FN for 2 h in serum-free medium. After 2 h, non-adherent cells were aspirated and adherent cells were kept in 15% αMEM. The total RNA was extracted using RNA easy kit and RT-PCR analysis was conducted for HSPA4 RNA.
| Disease status |
mRNA copies of HSPA4 |
HSPA4 | % Myeloma cells | |
|---|---|---|---|---|
| SUS | FN | |||
| ND | 2380 | 990 | D | 90 |
| ND | 2121 | 740 | D | 60 |
| ND | 411 | 504 | NC | 45–50 |
| ND | 649 | 668 | NC | 40 |
| ND | 522 | 487 | NC | 40 |
| ND | 276 | 287 | NC | 99 |
| ND | 414 | 406 | NC | 50 |
| ND | 2177 | 2476 | I | 65–70 |
| R | 395 | 504 | I | 50 |
| R | 5852 | 10 379 | I | 85 |
| R | 8602 | 2443 | D | 30–40 |
| R | 958 | 1406 | I | 90 |
| R | 4434 | 4818 | I | 65 |
| R | 1978 | 11 925 | I | 33 |
| R | 1453 | 2092 | I | 25 |
| R | 1913 | 3204 | I | 50 |
ND, newly diagnosed; R, relapsed; D, decreased; NC, no change; I, increased.
KNK-437 induces apoptosis in primary MM (CD138 positive plasma cells)
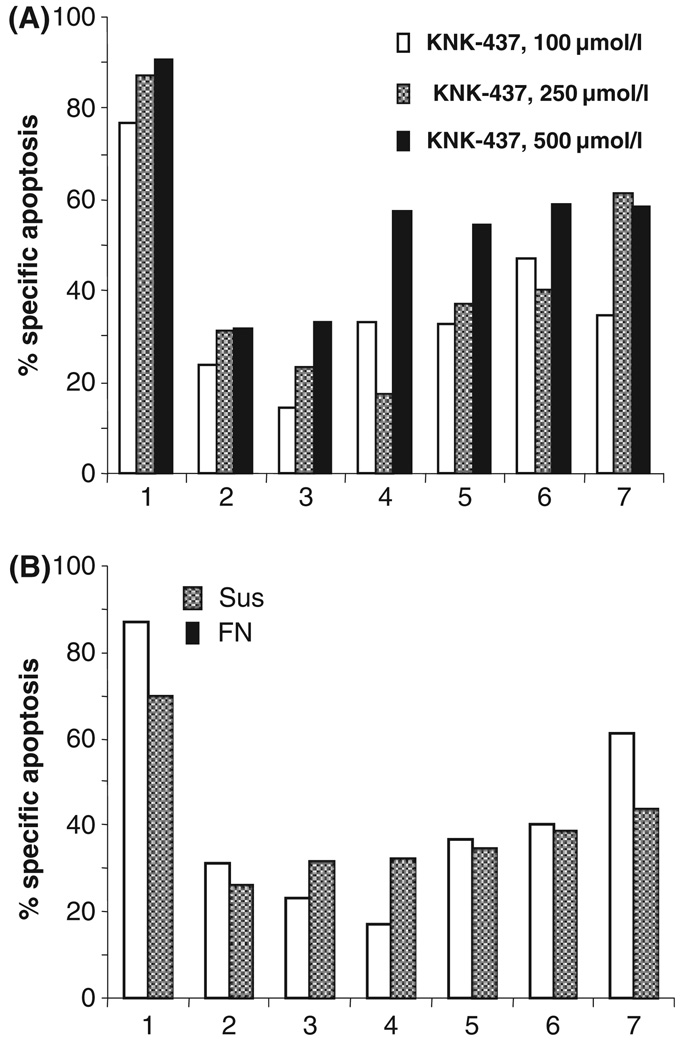
Patient plasma cells (four newly diagnosed and three relapsed) were kept either in suspension or adhered to FN in serum-free medium for 2 h, then the non-adherent cells were aspirated and adherent cells were treated with different doses of KNK-437 in medium containing 15% serum for 24 h. KNK-437 induced apoptosis in all patient samples when cultured in suspension (Fig 5A) or when they were adhered to FN (Fig 5B). The CAM-DR phenotype was observed in two out of seven samples (Patients 1 and 7); three out of seven samples (Patients 2, 5 and 6) showed no significant difference in apoptosis levels whether cells were adhered to FN or kept in suspension and two out of seven samples (Patients 3 and 4) showed reversal of CAM-DR phenotype.
Fig 5.
KNK-437 caused apoptosis in CD138-positive cells derived from bone marrow of patients with MM. (A) CD138 positive cells isolated from seven myeloma patients using CD138 magnetic beads were treated with KNK-437 (100, 250, 500 µmol/l). Apoptosis was measured at the end of 24 h of treatment using Annexin V/7-AAD by flow cytometry. (B) Patient-derived CD138-positive myeloma cells were either kept in suspension (Sus) or adhered to FN for 2 h in serum-free medium. After 2 h non-adherent cells were aspirated and adhered cells were kept in 15% α-MEM and treated with 250 µmol/l KNK-437. Apoptosis was measured at the end of 24 h of treatment using Annexin V/7-AAD. All the samples were tested in duplicate and averages of the duplicates are shown in the figure.
Discussion
In addition to the chaperone and anti-apoptotic roles of HSP70, its role on the cell surface is becoming better appreciated following work on HSP70 receptor interactions (Lipsker, et al 2002). HSP70 has been shown to activate the interleukin-1 (IL-1) receptor-signaling pathway (Vabulas, et al 2002). Previous studies have shown that over-expression of HSP70 causes resistance to many chemotherapeutic agents and inhibits apoptosis by preventing the recruitment of procaspase- 9 to the Apaf-1 and apoptosome (Beere, et al 2000). Though there is plenty of evidence for HSP70 role in apoptosis, drug resistance, receptor binding and cytokine stimulation, information on its role in tumor microenvironment and cell adhesion-mediated drug resistance in cancer therapy is lacking. Increasing evidence suggests that the bone marrow microenvironment strongly influences growth, survival, and drug response of myeloma cells (Thomas, et al 1998, Vidriales and Anderson 1996). Adhesion of myeloma cells to bone marrow stromal cells induces the secretion and up regulation of several cytokines such as IL-1β, TNFα and IL-6 (Chauhan, et al 1995). Adhesion-mediated drug resistance has been attributed to both soluble cytokines and cell-to-cell contact factors that result from the interactions of the myeloma cells with the bone marrow microenvironment. CAM-DR has been extensively investigated and shown to be involved in MM biology, hence, we hypothesized that HSP70 may play a critical role in interaction of myeloma cells with bone marrow stroma and hence in tumor survival and progression.
In order to establish the role of HSP70 protein in myeloma stromal cell interaction, we initially determined the endogenous expression patterns of HSP70 in several MM cell lines. Our results suggest an inherent presence of inducible HSP70 in all four commonly used MM cell lines studied, namely, H929, MM1/S, U266 and 8226. Additionally, there was a correlation with the adhesion ability of these cells to FN and stromal cells and levels of HSP70 protein expression (data not shown).
In addition to endogenous expression of HSP70, our data demonstrated that adhesion to either FN or stromal cells enhanced extra-cellular expression or secretion of HSP70 (Fig 1B–E). As it has previously been shown that IL-6 enhanced adhesion to FN, it was important to determine whether the combination of FN adhesion and IL-6 could enhance HSP70 secretion by MM cells. In fact, our data suggest that this combination significantly increased HSP70 secretion by MM cells (Fig 1E) and, though the quantity of HSP70 secreted is slightly less than the amount secreted when MM cells were attached to stromal cells, considering the complexity of the stromal cell system and presence of multiple soluble factors and their influence on adhesion, we think the data are comparable. Finally, when tested using primary MM cells, our results suggested that malignant plasma cells of patients have high expression of HSP70 (Table I). Collectively, these data demonstrate expression of HSP70 in MM cells and induction of this protein by microenvironment. The secreted HSP70 has been shown to act as chaperokine (Asea, et al 2000). We postulate that, in our model system, HSP70 increases IL-6 secretion by stromal cells and then IL-6 augments survival of myeloma cells by enhancing STAT and ERK1/2 activation. Hence HSP70 inhibition induces apoptosis in cells because survival pathways, such as STAT and ERK1/2, are inhibited and also recruitment of procaspase-9 to the Apaf-1 apopto-some is prevented.
Because HSP70 acts as an anti-apoptotic and survival factor (Beere, et al 2000, Guo, et al 2005, Jaattela, et al 1998), and it is present in MM cells, we postulated that this molecule could be targeted for therapeutic inventions in MM. Two approaches-were used: inhibiting HSP70 pharmacologically using KNK-437 as well as biologically by RNAi/HSP70. KNK-437 has previously been used either to inhibit acquiring the thermo tolerance (Yokota, et al 2000) or to inhibit induction of HSP70 protein due to HSP90 inhibitor 17AAG (Guo, et al 2005). Use of these agents clearly demonstrated that inhibition of HSP70 expression in 8266 cells attenuated not only adhesion to both FN and bone marrow stromal cells but also caused apoptosis of myeloma cells (Fig 2). Surprisingly, FN-adhered cells underwent more apoptosis than cells in suspension i.e reversal of CAM-DR phenotype (Fig 2E). This might be because adhesion enhances HSP70 expression (which causes drug resistance); removing this block by treating the cells with HSF-1 inhibitor reduces the effect of adhesion and the HSP70-enhanced cells will be more sensitive with this drug. The concentrations used in our study were similar to the previously published data using this drug (Yokota, et al 2000) (Guo, et al 2005).
Previous studies have shown that inhibition of HSP70 induction can enhance 17-AAG induced apoptosis in leukemic cells (Guo, et al 2005). As melphalan is the more commonly used drug of choice, we investigated whether inhibition of HSP70 could enhance melphalan-induced apoptosis. Inhibition of HSP70 not only increased the apoptosis induced by melphalan but also reversed the CAM-DR phenotype (Fig 4A and B). Our data are consistent with the previous observation in leukemic cells regarding enhancement of chemotherapyinduced apoptosis (Guo, et al 2005) by HSP70 inhibition. These results demonstrated that inhibition of HSP70 can cause apoptosis in de novo drug-resistant MM cells
Because KNK-437 enhanced melphalan-induced apoptosis, we determined whether this drug could also cause apoptosis in cells with acquired melphalan resistance. Inhibition of HSP70 caused significantly more apoptosis in 8226/LR5 (acquired melphalan-resistance) than sensitive wild type 8226 cells. Further, KNK-437 sensitized 8226/LR5 cells to melphalan. Although there was no significant difference in HSP70, 27 or 90 protein expression levels in resistant versus sensitive cells, resistant cells (8226/LR5) were more sensitive to HSP70 inhibition. This might be due to the fact that drug resistant cells depend more on HSP70 for survival and proliferation than drug sensitive cells. These results indicate that HSP70 could be targeted in MM and that inhibition of this protein causes apoptosis in cells with either acquired or de novo drug resistance.
Previous reports suggest that HSP70 is expressed only in malignant plasma cells but not in normal plasma cells (Munshi, et al 2004). GEP data indicated that HSPA4 mRNA expression is seen in all patient bone marrow-derived purified CD138 plasma cells and is at a greater level with relapsed disease compared to de novo disease. As shown in the Table I, plasma cells from all patients expressed HSPA4 and almost all of the patients with relapsed disease (seven out of eight) showed enhanced expression due to FN adhesion. As in the cell lines, our data demonstrated that inhibition of HSP70 caused apoptosis in patient-derived plasma cells in both culture conditions; adhered to FN or in suspension.
Our results with HSP70 inhibitor as single agent and in combination with melphalan suggest the feasibility of targeting this survival factor in MM and encourage the use of such agents in the clinic. Though currently specific HSP70 inhibitors are not available for human use, HSP70-targeted strategies are likely to reduce the influence of microenvironment on drug resistance and enhance the anti myeloma effects of melphalan.
Overall, our data provide evidence that a novel target (HSP70) mediates myeloma stromal cell interactions and cell adhesion-mediated drug resistance. A corollary to this observation is that inhibition of HSP70 enhanced apoptosis induced by other chemotherapeutic drugs and also inhibited the acquired drug resistance. Collectively, we have provided strong preclinical evidence for the development of HSP70 inhibitors for clinical use in MM.
Acknowledgement
This work was supported in part by Multiple Myeloma Research Foundation (MMRF) fellowship.
References
- Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biology. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bellamy WT, Dalton WS, Gleason MC, Grogan TM, Trent JM. Development and characterization of a melphalan-resistant human multiple myeloma cell line. Cancer Research. 1991;51:995–1002. [PubMed] [Google Scholar]
- Bhalla K, Huang Y, Tang C, Self S, Ray S, Mahoney ME, Ponnathpur V, Tourkina E, Ibrado AM, Bullock G. Characterization of a human myeloid leukemia cell line highly resistant to taxol. Leukemia. 1994;8:465–475. [PubMed] [Google Scholar]
- Chant ID, Rose PE, Morris AG. Analysis of heat-shock protein expression in myeloid leukaemia cells by flow cytometry. British Journal of Haematology. 1995;90:163–168. doi: 10.1111/j.1365-2141.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Uchiyama H, Urashima M, Yamamoto K, Anderson KC. Regulation of interleukin 6 in multiple myeloma and bone marrow stromal cells. Stem Cells. 1995;13(Suppl 2):35–39. [PubMed] [Google Scholar]
- Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Research. 1986;46:5125–5130. [PubMed] [Google Scholar]
- Damiano JS, Dalton WS. Integrin-mediated drug resistance in multiple myeloma. Leukemia and Lymphoma. 2000;38:71–81. doi: 10.3109/10428190009060320. [DOI] [PubMed] [Google Scholar]
- Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- Geginat G, Heine L, Gunther E. Effect of heat shock on susceptibility of normal lymphoblasts and of a heat shock protein 70-defective tumour cell line to cytotoxic T lymphocytes in vitro. Scandinavian Journal of Immunology. 1993;37:314–321. doi: 10.1111/j.1365-3083.1993.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Guo F, Rocha K, Bali P, Pranpat M, Fiskus W, Boyapalle S, Kumaraswamy S, Balasis M, Greedy B, Armitage ES, Lawrence N, Bhalla K. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Research. 2005;65:10536–10544. doi: 10.1158/0008-5472.CAN-05-1799. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Reviews. 2001;20:43–50. doi: 10.1023/a:1013156407224. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, Saporta S, Boulware D, Moscinski L, Alsina M, Dalton WS. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Research. 2003;63:7900–7906. [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. The EMBO Journal. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clinical Cancer Research. 2001;7:215–219. [PubMed] [Google Scholar]
- Lipsker D, Ziylan U, Spehner D, Proamer F, Bausinger H, Jeannin P, Salamero J, Bohbot A, Cazenave JP, Drillien R, Delneste Y, Hanau D, de la Salle H. Heat shock proteins 70 and 60 share common receptors which are expressed on human monocyte-derived but not epidermal dendritic cells. European Journal of Immunology. 2002;32:322–332. doi: 10.1002/1521-4141(200202)32:2<322::AID-IMMU322>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, Kato J, Hirayama Y, Sakamaki S, Kohda K, Miyake K, Niitsu Y. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nature Medicine. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- Munshi NC, Hideshima T, Carrasco D, Shammas M, Auclair D, Davies F, Mitsiades N, Mitsiades C, Kim RS, Li C, Rajkumar SV, Fonseca R, Bergsagel L, Chauhan D, Anderson KC. Identification of genes modulated in multiple myeloma using genetically identical twin samples. Blood. 2004;103:1799–1806. doi: 10.1182/blood-2003-02-0402. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- Preisler HD, Anderson K, Rai K, Cuttner J, Yates J, DuPre E, Holland JF. The frequency of long-term remission in patients with acute myelogenous leukaemia treated with conventional maintenance chemotherapy: a study of 760 patients with a minimal follow-up time of 6 years. British Journal of Haematology. 1989a;71:189–194. doi: 10.1111/j.1365-2141.1989.tb04253.x. [DOI] [PubMed] [Google Scholar]
- Preisler HD, Raza A, Larson R, LeBeau M, Browman G, Goldberg J, Grunwald H, Volger R, Verkh L, Singh P. Protooncogene expression and the clinical characteristics of acute nonlymphocytic leukemia: a Leukemia Intergroup pilot study. Blood. 1989b;73:255–262. [PubMed] [Google Scholar]
- Ralhan R, Kaur J. Differential expression of Mr 70,000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clinical Cancer Research. 1995;1:1217–1222. [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nature Cell Biology. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14:467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. European Journal of Cancer. 1997;33:873–877. doi: 10.1016/s0959-8049(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Shain KH, Landowski TH, Dalton WS. The tumor microenvironment as a determinant of cancer cell survival: a possible mechanism for de novo drug resistance. Current Opinion in Oncology. 2000;12:557–563. doi: 10.1097/00001622-200011000-00008. [DOI] [PubMed] [Google Scholar]
- Sliutz G, Karlseder J, Tempfer C, Orel L, Holzer G, Simon MM. Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: implication of quercetin as sensitiser in chemotherapy. British Journal of Cancer. 1996;74:172–177. doi: 10.1038/bjc.1996.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas X, Anglaret B, Magaud JP, Epstein J, Archimbaud E. Interdependence between cytokines and cell adhesion molecules to induce interleukin-6 production by stromal cells in myeloma. Leukemia and Lymphoma. 1998;32:107–119. doi: 10.3109/10428199809059251. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. Journal of Biological Chemistry. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Vidriales MB, Anderson KC. Adhesion of multiple myeloma cells to the bone marrow microenvironment: implications for future therapeutic strategies. Molecular Medicine Today. 1996;2:425–431. doi: 10.1016/1357-4310(96)84846-5. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiological Reviews. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Research. 2000;60:2942–2948. [PubMed] [Google Scholar]