Abstract
Purpose
Pulmonary metastases continue to be a significant problem in osteosarcoma. Apoptosis dysfunction is known to influence tumor development. Fas (CD95, APO-1)/FasL is one of the most extensively studied apoptotic pathways. Because FasL is constitutively expressed in the lung, cells that express Fas should be eliminated by lung endothelium. Cells with low or no cell surface Fas expression may be able to evade this innate defense mechanism. The purpose of these studies was to evaluate Fas expression in osteosarcoma lung metastases and the effect of gemcitabine on Fas expression and tumor growth.
Experimental Design and Results
Using the K7M2 murine osteosarcoma model, Fas expression was quantified using immunohistochemistry. High levels of Fas were present in primary tumors, but no Fas expression was present in actively growing lung metastases. Blocking the Fas pathway using Fas-associated death domain dominant-negative delayed tumor cell clearance from the lung and increased metastatic potential. Treatment of mice with aerosol gemcitabine resulted in increased Fas expression and subsequent tum or regression.
Conclusions
We conclude that corruption of the Fas pathway is critical to the ability of osteosarcoma cells to grow in the lung. Agents such as gemcitabine that up-regulate cell surface Fas expression may therefore be effective in treating osteosarcoma lung metastases. These data also suggest that an additional mechanism by which gemcitabine induces regression of osteosarcoma lung metastases is mediated by enhancing the sensitivity of the tumor cells to the constitutive FasL in the lung.
Osteosarcoma is the most common malignant bone tumor in both adults and children with peak incidence in the adolescent years. The lungs are the most common site of metastasis. Despite aggressive chemotherapy and successful control of the primary tumor, metastasis to the lungs continues to be a challenge resulting in the death of ∼30% of these patients (1 – 6). Understanding the mechanisms involved in the metastatic spread of osteosarcoma to the lungs may uncover new therapeutic targets.
Apoptosis dysfunction is known to influence tumor development. The Fas (CD95, APO-1)/FasL pathway is one of the most extensively studied apoptotic pathways. Fas (CD95/APO-1) and FasL are complementary receptor ligand proteins that induce cell death in many cells. Recently, Fas-mediated cell death has been implicated as a regulator of tumor development, outgrowth, and progression (7 – 11). Previous studies have shown that when Fas is down-regulated or Fas signaling is impaired, Fas-mediated cell death can be lost during malignant progression (7, 11). Fas is expressed in many tissues, whereas FasL is restricted to certain organs, such as the lungs. Cells that express Fas are therefore most likely to be eliminated when they enter the lung by the FasL-expressing lung endothelium. Cells with low or no surface Fas expression may be able to evade this FasL-induced cell death.
We have shown previously that the metastatic potential of human osteosarcoma cells is inversely correlated with Fas expression and that up-regulating this receptor in metastatic cells with low Fas expression changes their phenotype to nonmetastatic (12, 13). These data suggest that identifying agents that can up-regulate Fas expression may be a new and unique therapeutic approach for patients with osteosarcoma lung metastases.
To study the status of Fas expression in osteosarcoma lung metastasis and its role as a therapeutic target, we used a murine model of osteosarcoma characterized by orthotopic primary tumor growth, a period of minimal residual disease, and spontaneous lung metastasis (14). Here, we confirmed that Fas is a critical determinant of the ability of osteosarcoma cells to grow in the lung. We further showed that blocking Fas signaling by transfecting the cells with Fas-associated death domain (FADD) dominant-negative (FDN) plasmid, a truncated FADD lacking the procaspase-8 binding domain, resulted in delayed clearance of cells from the lung and increased metastatic potential. These data support our hypothesis that corruption of the Fas pathway influences the ability of osteosarcoma cells to metastasize to the lungs. We further showed that aerosol administration of gemcitabine, a nucleoside analogue known to up-regulate Fas expression, increased Fas expression in osteosarcoma pulmonary metastases and induced tumor regression.
Materials and Methods
Cell lines
The K7M3 cell line, a subline of K7M2 murine osteosarcoma cells (14 – 16), was developed by injecting K7M2 cells i.v. into mice, harvesting the lung metastases, and growing these metastatic cells in culture as described previously (12). All murine osteosarcoma cells were maintained in vitro using complete culture medium (DMEM supplemented with nonessential amino acids, sodium pyruvate, l-glutamine, and 10% FCS) at 37°C and 5% CO2. Cells used for in vivo and in vitro studies were from the 3rd and 10th passages, respectively, and verified to be negative for Mycoplasma species using the Mycoplasma Plus PCR Primer set (Stratagene, Inc.). For all in vitro assays, viability was assessed using trypan blue.
Plasmid construct for mouse FDN and control plasmids (neo) were a gift from Dr. A. Winoto (University of California, Berkeley, CA; ref. 17). Stable clones expressing FDN and neo were established in K7M3 cells by transfection using nonviral Fugene 6 transfection reagent (Roche Applied Biosciences) and were further selected and maintained in 1.0 mg/mL hygromycin-containing DMEM.
Animal model
BALB/c mice were purchased from the National Cancer Institute and Charles River Breeding Laboratories and housed five per cage in an animal facility approved by the American Association of Laboratory Animal Care in accordance with the current regulations and standards of the U.S. Department of Agriculture, Department of Health and Human Services, and the NIH. Mice were held for 2 weeks before being used in experiments. BALB/c-gld/gld (CPt.C3-Tnfsf6gld) mice carrying homozygous loss-of-function gld mutations of FasL (hereafter referred to as gld mice) were purchased from The Jackson Laboratory and housed in the same conditions described above.
To assess the importance of constitutive FasL in the metastatic process, K7M3 cells (2.5 × 105) were injected in the tail vein of wild-type BALB/c and gld mice. To assess the role of the Fas signaling pathway in the metastatic process, 5 × 105 nontransfected K7M3 cells, control-transfected K7M3 cells (K7M3/neo), or K7M3 cells stably transfected with FDN (K7M3/FDN) were injected i.v. into wild-type BALB/c mice. To induce a primary bone tumor, 2 × 104 K7M3 cells were injected into the tibia of wild-type BALB/c mice. At the end of each experiment (∼2.5 to 3 weeks for the i.v. injected groups and ∼5 to 6 weeks for the intrabone injected group), mice were sacrificed. Their lungs were extracted, weighed, fixed in formalin, and examined for the presence of metastases. Each experiment was repeated at least twice.
Reagents and drugs
Serum was purchased from Intergen, and the remaining medium supplements were purchased from Whittaker Bioproducts. Gemcitabine HCL was purchased from Eli Lilly.
Flow cytometric analysis of Fas expression
Fas expression in the murine osteosarcoma cell lines K7M3, K7M3/neo, and K7M3/FDN clones was measured by plating 0.8 × 106 cells. After 24 h, cells were trypsinized, suspended in fluorescence-activated cell sorter buffer (PBS containing 2% FCS and 0.1% NaN3), and incubated with either 1 mg/mL phycoerythrin-conjugated hamster anti-mouse Fas monoclonal antibody or isotype-matched, phycoerythrin-conjugated control hamster IgG antibody (PharMingen) for 30 min at 4°C. Samples were washed and analyzed by flow cytometry (FACScan, Becton Dickinson).
Western blot analyses
Cells were lysed using a proteinase inhibitor cocktail (Roche Applied Diagnostics) in radioimmunoprecipitation assay buffer. Protein concentration in lysates was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories). Protein lysate (50 μg) was boiled for 5 min before being loaded into an 18% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane (Amersham Biosciences). Specific protein bands were detected with rabbit polyclonal anti-FADD antibody (Upstate Cell Signaling Solutions) using the electrochemiluminescence Western blotting analysis system (Amersham Biosciences) according to the manufacturer's instructions.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma Chemical) assay was carried out as described previously (18). Briefly, osteosarcoma cells (1 × 104) were incubated overnight in a 96-well plate and treated with FasL and gemcitabine at different doses and kept for 24 and 48 h in a humidified atmosphere of 5% CO2 at 37°C. Wells with no treatment were used as positive control; wells with only medium were used as negative control. Twenty percent (v/v) of 0.42 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was added to each well, and cells were incubated for an additional 2 to 4 h. Medium was then aspirated, cells were lysed with 0.1 mL DMSO, and the absorbance was measured using an automated microplate reader at a wavelength of 570 mm.
Aerosol gemcitabine treatment and dosage
Aerosol treatment with gemcitabine was done as described previously (19, 20). Mice were placed unrestrained in a clear plastic cage with a sealed top and a wire netting floor located in a safety hood. Aerosol was introduced via an accordion tube connected to a nebulizer at one end and discharge at the other end. Time of exposure was 30 min at which time the volume of the liquid from the reservoir was nearly consumed. The total deposited dose of gemcitabine after 30-min inhalation of 1and 5 mg/mL stock solution was ∼1 and 2.5 mg/kg, respectively.
Treatment schedule
Therapeutic efficacy was evaluated by i.v. injecting 2.5 × 105 K7M3 cells into BALB/c and gld mice. For BALB/c mice, aerosol treatment was initiated 2 or 7 days after tumor cell injection and given thrice weekly for 2 weeks.
One extra group was added in which treatment was given once weekly. For gld mice, aerosol treatment was initiated 7 days after tumor cell injection and given thrice weekly for 2 weeks. Control mice did not receive treatment. At the end of treatment, mice were sacrificed. Their lungs were weighed, fixed in formalin, and assessed for the presence of both microscopic and macroscopic metastases.
Toxicity studies
Naive immunocompetent mice were treated with aerosol gemcitabine thrice weekly for 4 weeks at a dose of 0.5 mg/kg or twice weekly for 5 weeks at a dose of 2.5 mg/kg. At the end of treatment, mice were sacrificed. Organs were resected. Five-micrometer sections of lungs, liver, kidney, heart, brain, spleens, and sternum (for bone marrow evaluations) were stained with H&E for analysis by a veterinary pathologist.
Immunohistochemical analysis of Fas expression and apoptosis
The resected lungs were washed in saline, fixed in 10% formalin buffer, and embedded in paraffin. Five-micrometer-thick tissue sections were deparaffinized in xylene and rehydrated. Sections were first incubated with 3% hydrogen peroxide for 12 min to block exogenous peroxidase and subsequently incubated with PBS containing 10% normal horse serum and 1% normal goat serum for nonspecific binding. The primary antibody, polyclonal rabbit anti-Fas antibody (Santa Cruz Biotechnology), diluted to 4 mg/mL, was applied to the sections and left overnight at 4°C. The secondary antibody labeled with horseradish peroxidase was then applied for 1h at room temperature. The slides were then developed with 3,3′-diaminobenzidine as a substrate and lightly counterstained with hematoxylin. Sections not exposed to the primary antibody served as negative controls. Because Fas is constitutively expressed in the liver (21), normal human liver tissue was used as a positive control. Apoptosis was measured by terminal deoxynucleotidyl transferase – mediated dUTP nick end labeling (TUNEL) assay. After samples were deparaffinized as described above, tissues were incubated with 20 μg/mL proteinase K for 15 min at room temperature. After two 5-min washes with double-distilled water, tissues were incubated with hydrogen peroxide as described previously, washed again with double-distilled water thrice (3 min each), and incubated with terminal deoxynucleotidyl transferase buffer [30 mmol/L Trizma (pH 7.2), 140 mmol/L sodium cacodylate, 1 mmol/L cobalt chloride in double-distilled water] for 10 min at room temperature. Avoiding light, the reaction buffer was added to the tissue sections, and the slides were incubated with terminal transferase (1:400 diluted in terminal deoxynucleotidyl transferase buffer; Boehringer Mannheim Corp.) and biotin-160 dUTP (1:200 diluted in terminal deoxynucleotidyl transferase buffer; Roche) in a humid atmosphere at 37°C for 1h. The enzymatic reaction was terminated by rinsing the sections twice with TB buffer (300 mmol/L NaCl, 30 mmol/L sodium citrate in double-distilled water). The tissues were washed twice (5 min each) with double-distilled water and then PBS and stained with 3,3′-diamino-benzidine as described above. Fas and TUNEL immunostaining was scored as previously published (22): negative, no expression on tumor cells; weak, 10% to 50% of tumor cells stained positive for Fas or TUNEL; and positive, >50% of tumor cells stained positive for Fas or TUNEL. One to two tumor areas per slide for a total of five different slides were analyzed.
Ex vivo imaging of tumor cell clearance in the lungs
The percentage of tumor cells retained in the lungs after i.v. injection was determined as described previously (23). Briefly, cells were fluorescently labeled with 5 μmol/L 5-chloromethylfluorescein (Molecular Probes) according to the manufacturer's recommendations. BALB/c mice (five mice per cell line per time point) were injected in the tail vein with 0.5 × 106 tumor cells. At 1, 6, 24, and 48 h after injection, mice were euthanized by CO2 inhalation. Lungs were inflated by intratracheal infusion of 0.75 mL PBS and dissected free for ex vivo imaging by inverted fluorescent videomicroscopy (Leica DM IRB, Leica Microsystems) at × 100 magnification. Ten fluorescent images from each lung were randomly selected for analysis, and fluorescent 5-chloromethylfluorescein tumor cell events at 10 pixels or larger were defined and counted using OpenLab software. Five mice per cell line per time point were analyzed, and the total number of events per mouse lung was presented as a mean of the values for the five mice. Percentage metastatic survival was defined by normalizing the mean number of fluorescent cells at 6, 24, and 48 h with the mean number of cells at 1h for each cell line.
Statistical analysis
Statistical analysis in the lung metastases studies was done using an unpaired, two-tailed Student's t test, with P < 0.05 considered statistically significant.
Results
Fas expression in K7M3 primary bone tumor and pulmonary metastases
K7M3 cells are 75% Fas positive in vitro when analyzed by flow cytometry (data not shown). We examined Fas expression in K7M3 bone tumors and pulmonary metastases in BALB/c mice (Fig. 1A and B). The K7M3 bone tumors were positive for Fas expression, whereas K7M3 pulmonary metastases were negative for Fas expression (Fig. 1A and B). To evaluate the contribution of constitutive FasL in the Fas status of osteosarcoma lung metastases, K7M3 cells were injected i.v. into gld mice. The resultant metastases had heterogeneous Fas expression, with some areas Fas positive and others Fas negative (Fig. 1C and D). These data support the concept that constitutive FasL in the lung functions as a clearance mechanism, eliminating the Fas-positive tumor cells when they reach the lung.
Fig. 1.
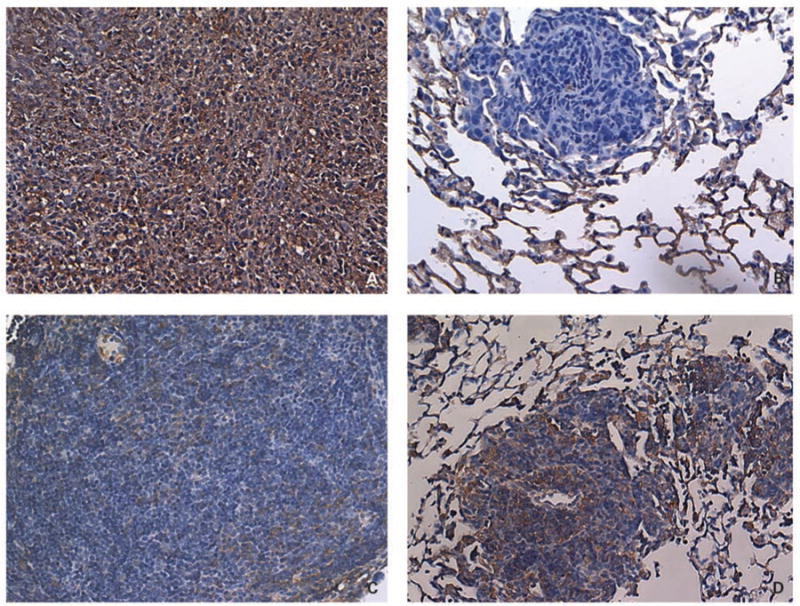
Representative images of Fas expression in K7M3 tumor tissue. A and B, Fas expression in a primary tumor (A) and a pulmonary metastasis (B) from a BALB/c mouse that received intrabone injection of K7M3 cells. Mice were sacrificed 6 wks after tumor injection. C and D, Fas expression in pulmonary metastases from a gld mouse injected i.v. with K7M3 cells. Mice were sacrificed 3 wks after i.v. tumor injection. Lung and bone tissues were processed and stained for Fas by immunohistochemistry. Positive Fas expressionis shown as brown-stained areas and was scoredas described in Materials and Methods. Primary tumorin BALB/c mice showed positive Fas expression (>50% of the tumor cells stained Fas positive; A), whereas pulmonary metastases from the same mouse were Fas negative (no tumor cells stained positive for Fas; B). Pulmonary metastases from gld mice showed areas of Fas-positive (D) and Fas-negative (C) expression within the same lung.
Inhibiting the Fas pathway prolongs the retention of K7M3 cells in the lungs
To investigate the importance of Fas and a functional Fas pathway in the clearance of tumor cells from the lung, K7M3 cells were transfected with FDN plasmid to block Fas signaling (Fig. 2). K7M3/FDN cells were not sensitive to FasL-induced cytotoxicity (data not shown), confirming that the Fas pathway was blocked. A single-cell fluorescent imaging system was used to quantify the number of tumor cells in the lung following i.v. injection (16). When fluorescently labeled K7M3, K7M3/neo, and K7M3/FDN cells were i.v. injected into mice, the retention of K7M3/FDN cells in the lungs was higher than that of K7M3 and K7M3/neo cells (Fig. 3). Two days after injection, the number of K7M3/FDN cells in the lungs was approximately five times the number of K7M3 and K7M3/neo cells. No difference in retention was observed between K7M3 and K7M3/neo cells.
Fig. 2.
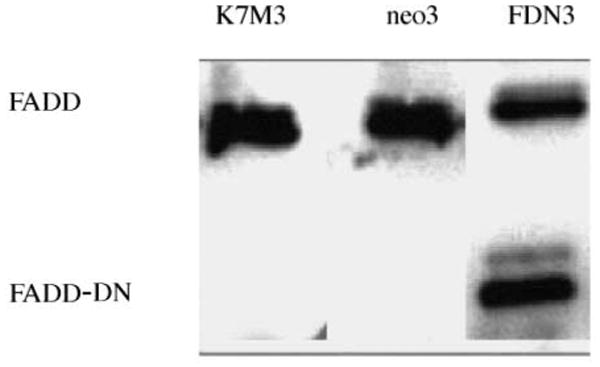
The expression of FDN (FADD-DN) protein in K7M3/FDN-transfected clones. FDN protein was confirmed by Western blot. K7M3 and K7M3/neo cells were used as negative controls.
Fig. 3.
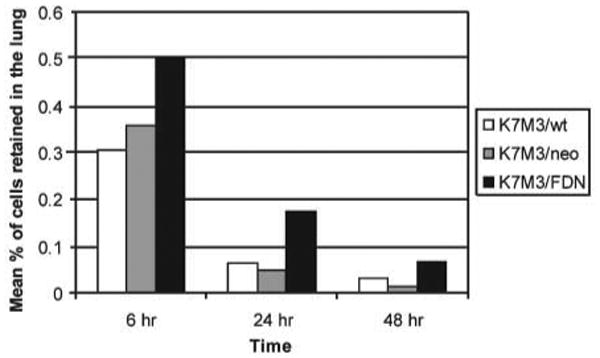
K7M3 cell clearance from the lungs is decreased when the Fas pathway is disrupted. K7M3, K7M3/neo, and K7M3/FDN cells were fluorescently labeled and injected i.v. into BALB/c mice. Mice were killed 1, 6, 24, and 48 h after injection, and lungs were examined under a fluorescent microscope to determine the proportion of fluorescent tumor cells retained. P = 0.005 and 0.02 for K7M3/FDN versus K7M3/neo and K7M3/FDN versus K7M3, respectively.
The metastatic potential of the FDN-transfected cells was also assessed 2 weeks after i.v. injection. The average wet-lung weight of lungs from mice injected with K7M3/FDN cells was significantly higher than that of lungs from mice injected with K7M3/neo cells (Fig. 4; P = 0.03). There was no difference in wet-lung weights between K7M3- and K7M3/neo-injected mice. On immunohistochemical examination, the K7M3/FDN metastases contained both Fas-positive and Fas-negative cells, whereas the K7M3/neo metastases contained only Fas-negative cells (data not shown). Taken together, these data support the hypothesis that the Fas pathway contributes to clearance of K7M3 cells from the lungs and in turn influences metastatic potential.
Fig. 4.
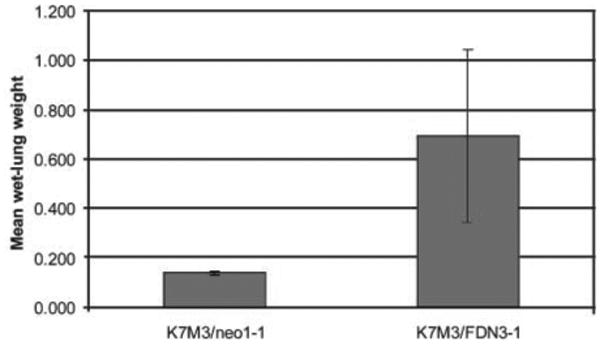
Inhibition of the Fas pathway increases the metastatic potential of K7M3 cells. K7M3, K7M3/neo, and K7M3/FDN cells were injected into the tail vein of BALB/c mice. The mean wet-lung weight was significantly higher in mice injected with K7M3/FDN cells than in mice injected with K7M3 or K7M3/neo cells (P = 0.03).
Effects of aerosol gemcitabine on K7M3 lung metastases
Flow cytometric analysis of K7M3 cells before and after treatment with gemcitabine in vitro showed that Fas expression was significantly increased following incubation with gemcitabine (mean fluorescence intensity, 75 pretreatment versus 137 posttreatment; P = 0.003). In addition, gemcitabine-treated cells showed increased sensitivity to superFasL in vitro (data not shown).
To assess whether gemcitabine also induced up-regulation of Fas in vivo, mice were injected i.v. with K7M3 cells. Aerosol gemcitabine therapy was initiated on either day 2 or 7. Therapy was given either thrice weekly or once weekly for 2 weeks. Mice were killed at the end of therapy. Lungs were examined by immunohistochemistry for Fas expression and TUNEL. Fas expression was scored as weak to negative in the untreated mice. By contrast, Fas expression was positive following aerosol gemcitabine, with 50% of tumor cells expressing Fas (Fig. 5). Apoptosis, indicated by TUNEL staining, was also increased in the gemcitabine-treated group (Fig. 5).
Fig. 5.
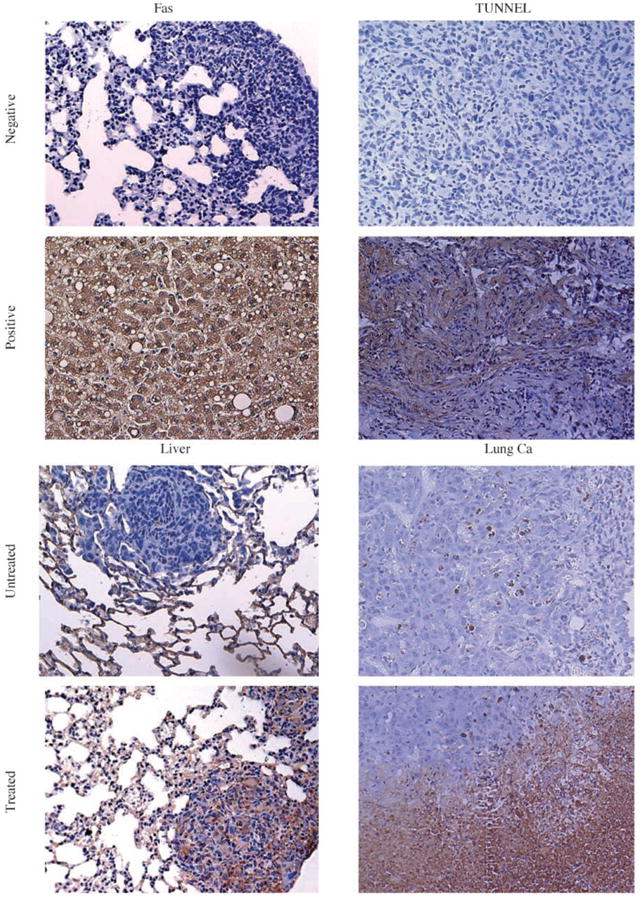
Representative images of the effect of aerosol gemcitabine on K7M3 pulmonary metastases. BALB/c mice with established lung metastases were sacrificed after treatment with gemcitabine for 2 wks. Sections were evaluated by immunohistochemistry for Fas and apoptosis. Positivity for Fas and apoptosis is depicted as a brown staining and scored as mentioned in Materials and Methods. Normal liver and lung cancer (Ca) tissues were used as the positive controls for Fas expression and apoptosis, respectively. Untreated pulmonary metastases were Fas negative (no tumor cells stained for Fas) with little or no apoptosis (weak staining). Gemcitabine-treated pulmonary metastases were Fas positive with increased apoptosis (>50% of the tumor cells stained positive for Fas and TUNEL).
We assessed the therapeutic effect of aerosol gemcitabine on pulmonary metastases by quantifying wet-lung weights. Gemcitabine significantly inhibited the growth of pulmonary metastases (P < 0.02; Fig. 6A). Initiating therapy on day 7 was as effective as initiating therapy on day 2. We also compared the efficacy of 1 and 5 mg/mL gemcitabine doses. Although both doses were effective in inhibiting lung metastases (P < 0.001), the 5 mg/mL dose was more effective than the 1 mg/mL dose (P < 0.01; Fig. 6B) and was not associated with increased toxicity. (Mean whole-body weight was 24.2 g for untreated mice versus 23.5 and 24.3 g for mice treated with the 1 and 5 mg/mL doses, respectively). We have shown previously that aerosol saline has no effect on Fas expression, TUNEL, or tumor growth (19).
Fig. 6.
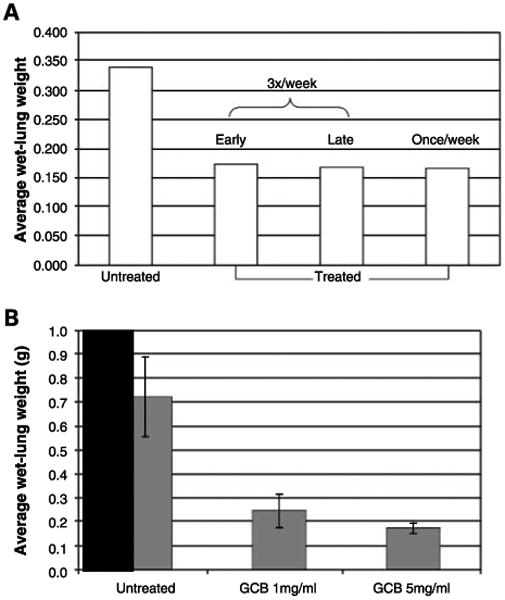
Aerosol gemcitabine (GCB) significantly inhibited the growth of pulmonary metastases. A, mice were injected with K7M3 cells and then treated with aerosol gemcitabine once or thrice weekly and starting 2 or 7 d after tumor cell injection, as indicated. Mice were sacrificed at the end of therapy. The average wet-lung weight in the untreated group was significantly higher than the mean wet-lung weight in the three treatment groups. (P = 0.018, 0.016, and 0.017 for thrice weekly start of day 2, thrice weekly start of day 7, and once weekly, respectively). B, the average wet-lung weight of mice with K7M3 lung metastases treated with the 1or 5 mg/mL dose was significantly less (P = 0.000009 and 0.000008) than the mean wet-lung weight of the untreated control mice. The higher dose (5 mg/mL) resulted in a significantly better response than the lower dose (1 mg/mL; P = 0.009).
To determine if the Fas/FasL pathway is implicated in the mechanism of action of aerosol gemcitabine and plays a role in its therapeutic efficacy, gld mice were injected with K7M3 cells and treated with aerosol gemcitabine thrice weekly for 2 weeks, starting 7 days after tumor injection. Mice were sacrificed at the end of therapy. Lungs were examined by immunohistochemistry for Fas expression. Homogeneous positive Fas expression was seen in the lung nodules of the treated mice. Fas expression was negative in the untreated control mice. Therapeutic effect of the drug was determined by average wet-lung weight, quantitative analyses of micrometastases and macrometastases and by mean tumor size. Average wet-lung weight for the control group was 0.26 versus 0.38 g for the untreated group (P = 0.5). The median number of lung nodules was 17 for the control group versus 32 for the treated group (P = 0.52). The median size of the nodules was 1.67 mm for the control versus 1.60 mm for the treated group (P = 0.81). The median number of micro-metastases was 23 for the control group versus 28 for the treated group (P = 0.84). These findings indicated that aerosol gemcitabine had no therapeutic effect on pulmonary metastases in the absence of FasL in the lung.
Toxicity of aerosol gemcitabine
Toxicity studies were done in immunocompetent naive mice. Aerosol gemcitabine was well tolerated. As seen in the mice with pulmonary metastases treated with gemcitabine, there was no difference in animal weights between the treated and control mice at the end of therapy (P > 0.05; data not shown). Histologic examination of lungs, bone marrow, livers, kidneys, spleens, and hearts from mice treated with aerosol gemcitabine at 1 mg/mL thrice weekly for 4 weeks showed no abnormalities. In addition, there were no signs of inflammation or fibrosis in the lungs. Mice receiving the higher dose of 5 mg/mL gemcitabine by inhalation twice weekly for 5 weeks also did not have any signs of toxicity.
Discussion
The role of the Fas/FasL pathway in the metastatic potential of osteosarcoma has been a major focus of our laboratory investigations (13, 22, 24). We showed previously that Fas expression correlates inversely with the metastatic potential of osteosarcoma cells and that altering Fas expression changes their ability to form lung metastases (13, 24). These prior investigations were done using an experimental metastasis mouse model in which tumor cells were injected i.v. Here, we extend the studies using an orthotopic mouse osteosarcoma model. K7M3 mouse osteosarcoma cells form a primary tumor when injected intratibially and spontaneously metastasize to the lung. Whereas the K7M3 cells were 90% Fas positive in vitro and the primary tumors were highly positive for Fas expression (Fig. 1A), the pulmonary metastases were all Fas negative (Fig. 1B). These data suggest that down-regulation occurred during the metastatic process or, alternatively, that there was a selection process that eliminated the Fas-positive cells, allowing only Fas-negative cells to remain and proliferate. Because FasL is constitutively expressed in the lung, we favored the later explanation, hypothesizing that Fas-positive K7M3 cells are eliminated by the constitutive FasL following entry into the lung.
To address this hypothesis, we did an i.v. tumor cell clearance assay (23). Twenty-four hours after i.v. injection, 80% of K7M3 cells had been cleared from the lung, and by 48 h, <10% of the cells remained (Fig. 3). These remaining cells presumably were the Fas-negative population that were able to circumvent FasL-induced clearance. Further support for this hypothesis came from our experiments in FasL-deficient gld mice. If constitutive FasL is absent in the lung, then both Fas-positive and Fas-negative cells should be able to grow in the lung, causing lung metastases. Indeed, when K7M3 cells were injected i.v. into gld mice, the resulting lung metastases were heterogeneous, that is, Fas-positive, Fas-negative, and mixed tumor nodules were present within the same lung (Fig. 1C and D).
Fas-mediated apoptosis is a complex process that requires not only sufficient levels of Fas to ensure optimal receptor cross-linking in the presence of FasL but also the assembly of the critical intracellular Fas-associated proteins necessary to initiate the apoptotic cascade. Blocking the signaling pathway should, therefore, prevent FasL-mediated tumor cell clearance.
To determine the significance of the Fas pathway, we blocked Fas signaling by overexpressing FDN. Ligation of Fas by FasL triggers aggregation of the receptors. The clustering of the death domains in the intracellular portion of the receptors recruits FADD, which interacts with a death domain of procaspase-8 to form the death-inducing signal complex. Caspase-8 activation subsequently leads to apoptosis. Two different Fas apoptosis signaling pathways have been delineated. One involves caspase-8 activation and the other involves activation of a mitochondrial pathway. At present, we do not know which pathway is primarily used by K7M3 cells. However, FDN blocks both of the known pathways by inhibiting caspase-8 at the death-inducing signal complex (25). We transfected K7M3 osteosarcoma cells with FDN plasmid as described previously (26). This plasmid contains the death domain but does not contain the death effector domain and therefore cannot bind procaspase-8. Transfection of FDN into K7M3 cells resulted in decreased sensitivity to superFasL in vitro, increased cellular retention in the lung by fivefold, and enhanced metastatic potential (Figs. 3 and 4). Furthermore, the lung metastases formed by the K7M3/FDN cells were both Fas negative and Fas positive. Taken together, these data support our hypothesis that the Fas pathway and FasL-induced tumor cell clearance play an important role in controlling metastatic growth of osteosarcoma cells in the lung.
We recognize that the metastatic process is complicated and that multiple steps and factors contribute to the ability of tumor cells to (a) break through the extracellular matrix into the circulation, (b) travel from the local primary site to a distant organ, (c) survive in that new organ, and (d) initiate the growth and proliferation of vascular structures that support tumor growth. Other factors have certainly been shown to be important in the metastatic process of osteosarcoma cells. For example, ezrin, an actin filament plasma membrane linker that is an important determinant of the ability of cell to tether to the surrounding extracellular matrix, has also been shown to correlate with the metastatic potential of osteosarcoma cells. Down-regulation of ezrin decreased the ability of osteosarcoma cells to form pulmonary metastases (15, 16). Vascular endothelial growth factor is another crucial protein that is essential for tumor vascular expansion, and matrix metalloproteinase-9 (MMP-9), a family of Zn-dependent enzymes, plays a role in the ability of cells to adhere to the microenvironment. MMPs are able to disrupt the extracellular matrix and basement membrane, essential steps for the process of invasion and metastases. MMP-2 and MMP-9 are the gelatinases most consistently expressed in malignant tissues. MMP-9 is expressed in primary tumors and metastases in children with osteosarcoma. (27 – 31). Putting our data on Fas in context with the data of other investigators (15, 16), we hypothesize that Fas may be an early defense mechanism to clear invading tumor cells from the lung. Fas-negative cells or cells with a blocked or nonfunctional Fas pathway can evade FasL-induced cell death. Once the Fas-negative tumor cells escape other factors such as ezrin, vascular endothelial growth factor and MMP-9 become critical in the ability of tumor cells to form lung metastases. These factors allow the tumor cells to anchor to the extracellular matrix and alter the microenvironment.
If one accepts that Fas expression is a critical determinant of the ability of tumor cell to survive in the lung environment, then targeting Fas is a therapeutic opportunity. Identifying agents that up-regulate Fas and testing their use in a clinical trial may expand treatment options for patients with relapsed osteosarcoma in the lung in whom standard front-line and salvage therapy regimens have failed to control disease. To this end, we showed that gemcitabine up-regulated Fas expression in vivo when administered by aerosol. K7M3 lung nodules showed up-regulation of Fas expression following aerosol therapy, along with increased tumor cell apoptosis and subsequent tumor regression (Figs. 5 and 6). Aerosol gemcitabine had no effect in gld mice, which lack constitutive FasL in the lung. These data indicate that the efficacy of aerosol gemcitabine may be closely linked to the status of the microenvironment in the lung. Taken together, our data suggest that aerosol gemcitabine may be a novel therapeutic approach for the treatment of osteosarcoma lung metastases.
In summary, the data presented confirm that the Fas/FasL pathway plays a critical role in the metastatic potential of osteosarcoma cells. Our data also suggest that targeting this pathway has therapeutic potential, even against established metastases.
Acknowledgments
Grant support: National Cancer Institute grant CA42992 (E.S. Kleinerman) and NIH core grant CA16672.
References
- 1.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 2.Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–6. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Goorin AM, Shuster J, Baker A, Horowitz ME, Meyer WH, Link MP. Changing pattern of pulmonary metastases with adjuvant chemotherapy in patients with osteosarcoma: results from the multiinstitutional osteosarcoma study. J Clin Oncol. 1991;9:600–5. doi: 10.1200/JCO.1991.9.4.600. [DOI] [PubMed] [Google Scholar]
- 4.Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer. 1997;33:1725–31. doi: 10.1016/s0959-8049(97)00252-9. [DOI] [PubMed] [Google Scholar]
- 5.Bruheim S, Bruland OS, Breistol K, Maelandsmo GM, Fodstad O. Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol Oncol Res. 2004;10:133–41. doi: 10.1007/BF03033741. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- 7.Owen-Schaub LB, van Golen KL, Hill LL, Price JE. Fas and Fas ligand interactions suppress melanoma lung metastasis. J Exp Med. 1998;188:1717–23. doi: 10.1084/jem.188.9.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller P, Koretz K, Leithauser F, et al. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994;57:371–7. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 9.Hill LL, Ouhtit A, Loughlin SM, et al. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- 10.Zornig M, Grzeschiczek A, Kowalski MB, Hartmann KU, Möröy T. Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in E mu L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene. 1995;10:2397–401. [PubMed] [Google Scholar]
- 11.Owen-Schaub L, Chan H, Cusack JC, Roth J, Hill LL. Fas and Fas ligand interactions in malignant disease. Int J Oncol. 2000;17:5–12. [PubMed] [Google Scholar]
- 12.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–6. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 13.Worth LL, Lafleur EA, Jia SF, Kleinerman ES. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol Rep. 2002;9:823–7. [PubMed] [Google Scholar]
- 14.Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–71. doi: 10.1023/a:1006767007547. [DOI] [PubMed] [Google Scholar]
- 15.Khanna C, Khan J, Nguyen P, et al. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–9. [PubMed] [Google Scholar]
- 16.Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrinis necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–6. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Winoto A. A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Mol Cell Biol. 1996;16:2756–63. doi: 10.1128/mcb.16.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Koshkina NV, Kleinerman ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer. 2005;116:458–63. doi: 10.1002/ijc.21011. [DOI] [PubMed] [Google Scholar]
- 20.Koshkina NV, Kleinerman ES, Waidrep C, et al. 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin Cancer Res. 2000;6:2876–80. [PubMed] [Google Scholar]
- 21.Lafleur EA, Jia SF, Worth LL, et al. Interleukin (IL)-12 and IL-12 gene transfer up-regulate Fas expression in human osteosarcoma and breast cancer cells. Cancer Res. 2001;61:4066–71. [PubMed] [Google Scholar]
- 22.Gordon N, Arndt CA, Hawkins DS, et al. Fas expression in lung metastasis from osteosarcoma patients. J Pediatr Hematol Oncol. 2005;27:611–5. doi: 10.1097/01.mph.0000188112.42576.df. [DOI] [PubMed] [Google Scholar]
- 23.Ivanova ST, Dazri AW, Peck D. The Fas/FasL system potentiates invasive phenotype via transcription factor dependent upregulation of metalloproteases. Proceedings of the 97th American Association for Cancer Research Annual Meeting. 2006 abstract 3453. [Google Scholar]
- 24.Lafleur EA, Koshkina NV, Stewart J, et al. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res. 2004;10:8114–9. doi: 10.1158/1078-0432.CCR-04-0353. [DOI] [PubMed] [Google Scholar]
- 25.Guseva NV, Taghiyev AF, Rokhlin OW, Cohen MB. Death receptor-induced cell death in prostate cancer. J Cell Biochem. 2004;91:70–99. doi: 10.1002/jcb.10707. [DOI] [PubMed] [Google Scholar]
- 26.Koshkina NV, Kleinerman ES. The Fas pathway contributes to the metastatic potential of osteosarcoma to the lungs. Proceedings of the 97th American Association of Cancer Research Annual Meeting. 2006 abstract 738. [Google Scholar]
- 27.Bjornland K, Flatmark K, Pettersen S, Aaasen AO, Fodstad O, Maelandsmo GM. Matrix metalloproteinases participate in osteosarcoma invasion. J Surg Res. 2005;127:151–6. doi: 10.1016/j.jss.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari C, Benassi S, Ponticelli F, et al. Role of MMP-9 and its tissue inhibitor TIMP-1 in human osteosarcoma: findings in 42 patients followed for 1-16 years. Acta Orthop Scand. 2004;75:487–91. doi: 10.1080/00016470410001295-1. [DOI] [PubMed] [Google Scholar]
- 29.Himelstein BP, Asada N, Carlton MR, Collins MH. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediatr Oncol. 1998;31:471–4. doi: 10.1002/(sici)1096-911x(199812)31:6<471::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima A, Nakanishi I, Tsuchiya H, Roessner A, Obata K, Okada Y. Expression of matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) induced by tumour necrosis factor α correlates with metastatic ability in a human osteosarcoma cell line. Virchows Arch. 1994;424:547–52. doi: 10.1007/BF00191442. [DOI] [PubMed] [Google Scholar]
- 31.Kido A, Tsutsumi M, Iki K, et al. Overexpression of matrix metalloproteinase (MMP)-9 correlates with metastatic potency of spontaneous and 4-hydroxyaminoquinoline 1-oxide (4-HAQO)-induced transplantable osteosarcomas in rats. Cancer Lett. 1999;137:209–16. doi: 10.1016/s0304-3835(98)00368-1. [DOI] [PubMed] [Google Scholar]


