Abstract
Lung sensory receptors with afferent fibers coursing in the vagus nerves are broadly divided into three groups: slowly (SAR) and rapidly (RAR) adapting stretch receptors and bronchopulmonary C fibers. Central terminations of each group are found in largely nonoverlapping regions of the caudal half of the nucleus of the solitary tract (NTS). Second order neurons in the pathways from these receptors innervate neurons located in respiratory-related regions of the medulla, pons, and spinal cord. The relative ease of selective activation of SARs, and to a lesser extent RARs, has allowed for more complete physiological and morphological characterization of the second and higher order neurons in these pathways than for C fibers. A subset of NTS neurons receiving afferent input from SARs (termed pump or P-cells) mediates the Breuer-Hering reflex and inhibits neurons receiving afferent input from RARs. P-cells and second order neurons in the RAR pathway also provide inputs to regions of the ventrolateral medulla involved in control of respiratory motor pattern, i.e., regions containing a predominance of bulbospinal premotor neurons, as well as regions containing respiratory rhythm-generating neurons. Axon collaterals from both P-cells and RAR interneurons, and likely from NTS interneurons in the C-fiber pathway, project to the parabrachial pontine region where they may contribute to plasticity in respiratory control and integration of respiratory control with other systems, including those that provide for voluntary control of breathing, sleep-wake behavior, and emotions.
Keywords: central pathways, C-fiber afferents, lung reflexes, rapidly adapting receptor, respiratory control, slowly adapting pulmonary stretch receptor
The morphology and respiratory reflex effects of activating pulmonary and airway receptors have been the subjects of many excellent reviews (22–24, 32, 83, 84, 87, 88, 95, 105, 125, 132, 137, 138, 141). Within the airways below the larynx, three categories of receptor are typically identified: slowly (SAR) and rapidly (RAR) adapting stretch receptors and bronchopulmonary C fibers. The major cardiorespiratory reflex effects of each of these are listed in Table 1. The present minireview will focus on the central pathways mediating the reflexes evoked by these receptors. Neuroepithelial bodies provide an additional airway receptor type (see reviews in Refs. 24, 137, 141), but little is known about their reflex effects or central pathways, and they will not be considered further. Within the larynx there are several additional receptor types responsive to a range of modalities including pressure, respiratory drive, and cold. Stimulation of these receptors exerts powerful effects on the control of breathing, but there is a limited knowledge of the central processing of input from these afferents (reviewed in Refs. 8, 84, 123, 137).
Table 1.
Major classes of pulmonary receptors and their reflex effects
| Receptor Type | Reflex Effects |
|---|---|
| SAR | Breuer-Hering reflex: inspiratory termination, expiratory facilitation Enhancement of inspiratory effort Bronchodilation Tachycardia |
| RAR | Cough Broncho- and laryngoconstriction Augmented breath/gasp (on stimulation of RAR by a large, rapid lung inflation) Irregular, augmented inspiration and shortened expiration (on maintained airway deflation or inhalation of irritants) Airway mucus secretion |
| Slowly adapting deflation receptors (present in rats and rabbits; some may be classified as RARs based on lung deflation tests) |
Unknown |
| Bronchopulmonary C fibers | Rapid, shallow breathing Apnea (on synchronous chemical stimulation) Broncho- and laryngoconstriction Airway mucus secretion Vasodilatation (pulmonary C fibers only) Bradycardia |
SAR, slowly adapting stretch receptor; RAR, rapidly adapting stretch receptor.
Pulmonary and airway mechanoreceptors are innervated by fast-conducting, myelinated afferent fibers. They are sensitive to both the static and dynamic aspects of lung volume and transmural pressure. Lung inflation has been the most commonly used stimulus to activate mechanoreceptors and identify SARs. RARs, also referred to as irritant receptors because of their activation by inhaled irritants such as ammonia or cigarette smoke, have phasic responses to large lung inflations and deflations. There appear to be notable species differences. Rats have relatively few RARs when defined by their response to lung inflation. In contrast, deflation-activated receptors with either slowly or rapidly adapting properties are relatively common in the rat (47, 52, 109) and rabbit but less apparent in the cat or monkey (136).
Bronchopulmonary C fibers have slowly conducting, non-myelinated axons and encompass all receptors present in the lungs and airways other than the distinctly defined mechanoreceptors. C-fiber receptors are typically polymodal; many are excited by large mechanical deformations, chemical stimuli (e.g., capsaicin, phenylbiguanide, CO2, autacoids, delivered through either the bronchial or pulmonary circulation), increased interstitial fluid volume (lung edema), or by increased temperature. Bronchial and pulmonary C fibers elicit similar effects on breathing (24, 32, 87, 88, 137) but may express different neuropeptides, suggesting that future refinements in experimental design may reveal functional differences (24, 141).
Respiratory reflexes from pulmonary and airway receptors in humans
The reflex control exerted by pulmonary and airway vagal afferents in humans is generally similar to that established in other mammals. This also applies to the Breuer-Hering inspiration-terminating and expiration-lengthening reflexes. These reflexes were initially not believed to modulate breathing at eupneic tidal volumes in adult awake humans (25). However, SAR afferents are active in humans at functional residual capacity (59), and recent data show that these reflexes can be detected when the conscious perception of chest wall movements is suppressed (20, 21). Moreover, the Breuer-Hering reflexes are easy to detect in infants and neonates (94, 120; see Ref. 135 for review) and abnormal development of vagal pathways may play a role in breathing disorders during the early postnatal period (see reviews in Refs. 8, 58). Upper airway mechanoreceptor reflexes have been extensively studied in humans because of their importance for airway protection (e.g., expiration reflex 79, 132) and the maintenance of upper airway motor tone during sleep and its potential dysregulation in obstructive sleep apnea (OSA) patients (64, 67, 85).
NTS SITES OF THE CENTRAL PROJECTIONS FROM LUNG AND AIRWAY VAGAL AFFERENTS
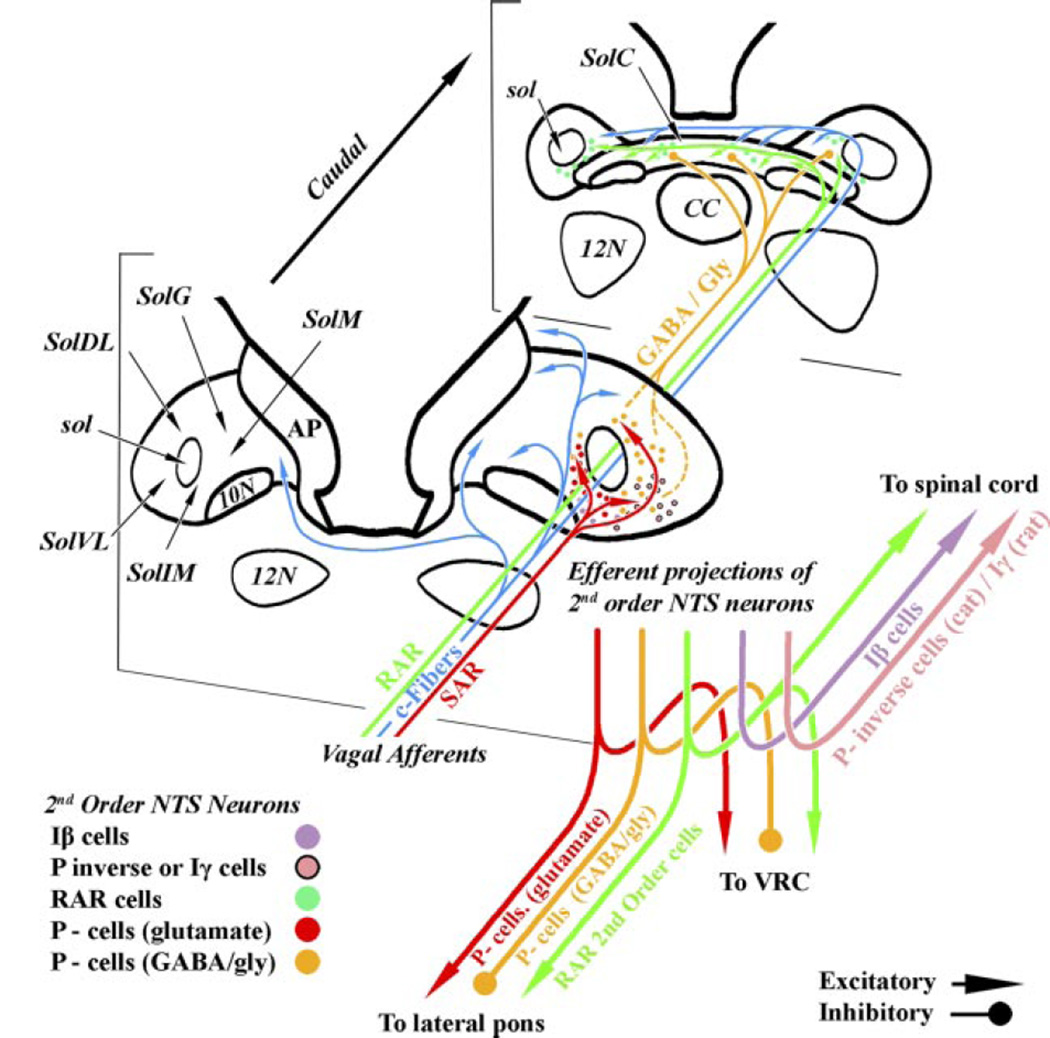
The lung and airway vagal afferents terminate principally in the middle and caudal portions of the nucleus of the solitary tract (NTS) (71, 72). On the basis of cytoarchitecture and, in part, the organization of pulmonary afferent projections, as well as projections from other organ systems, the NTS in the rat has been divided into distinct subnuclei: commissural (SolC), dorsal (SolD), dorsolateral (SolDL), gelatinosus (SolG), interstitial (SolI), intermediate (SolIM), medial (SolM), ventral (SolV), and ventrolateral (SolVL) (Fig. 1 and reviews in Refs. 72 and 84, cat; and Refs. 65, 77, 124, rat; anatomical abbreviations after Ref. 117). Afferents from the laryngeal region course in the superior laryngeal nerves (SLN, a branch of the vagus) and project mainly to the SolI and SolM subnuclei, but intra-axonal labeling shows that individual SLN axons can have additional, less dense terminations in several subnuclei (e.g., SolDL, SolI, SolV, SolVL, and SolC; cat, Ref. 10). The projections of bronchopulmonary afferents of distinct types, especially SARs and RARs, have been studied relatively extensively (34, 39, 73). Fig. 1 shows the distribution of their main termination regions within the NTS.
Fig. 1.
Distribution within the nucleus of the solitary tract (NTS) of terminal regions for slowly and rapidly adapting stretch receptors (SARs and RARs, respectively) and bronchopulmonary C fibers, and major projections of their 2nd order neurons. The 3 afferent systems project to largely nonoverlapping areas. The diagram summarizes studies in cats in which individual vagal afferent fibers were recorded and their central projections determined by antidromic mapping (e.g., Refs. 33, 39, 89) and in rats or cats in which 2nd order neurons activated by these primary afferent fibers were recorded within the NTS (34, 45, 52, 53, 90, 109, 110). Of note in the rat, pump cells (P-cells) are not recorded dorsolateral to the solitary tract, but presumably homologous inhibitory P-cells located in SolVL project caudally to second order RAR neurons in the SolC (48). P-inverse cells (deflation-sensitive neurons) are rare in the cat but have frequently been found by Ezure and Tanaka (48) in more caudal portions of SolVL in rats. P-cells and P-inverse cells may be homologous. Abbreviations generally conform to Paxinos and Watson (117): 10N, dorsal motor nucleus of the vagus; 12N, hypoglossal nucleus; AP, area postrema; CC, central canal; sol, solitary tract; SolDL, dorsolateral subnucleus; SolG, gelatinous subnucleus; SolIM, intermediate subnucleus; SolM, medial subnucleus; SolVL, ventrolateral subnucleus; VRC, ventral respiratory column of the ventrolateral medulla.
CENTRAL PATHWAYS OF THE BREUER-HERING AND OTHER SAR-MEDIATED REFLEXES
SARs project to the ipsilateral SolIM, SolI, SolV, and SolVL subnuclei of the NTS (Fig. 1, Fig. 2) at about the rostrocaudal level of the area postrema (34, 39, 73). Additionally, in cats, projections of SARs to the SolD and medial portion of the SolDL subnuclei have been reported (12, 34, 39). Ultrastructural examination of the terminal boutons of SARs within the SolV and SolVL subnuclei reveals asymmetric synaptic contacts with cell bodies, dendrites, and dendritic spines. SAR terminals also receive axo-axonal synapses from unidentified sources (74).
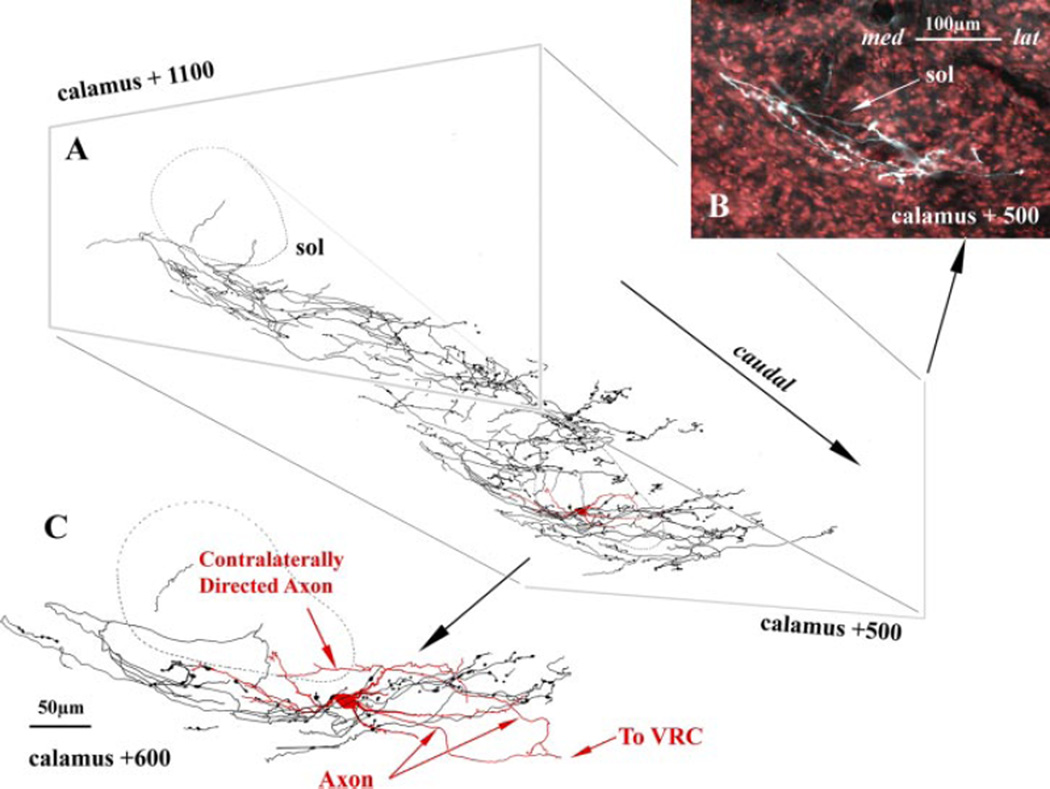
Fig. 2.
Composite image of an intra-axonally labeled rat SAR axon superimposed on an intracellular labeled P-cell (from a separate experiment). A: oblique perspective of an SAR reconstructed over six 100-µm sections; a reconstructed P-cell (red) is inserted within the terminal field of the SAR axon at the approximate level it was located in vivo. B: pseudocolored image of the SAR axon (white) from a single caudal coronal plane of a Nissl (red) counterstained section. Note the involvement of SolIM and SolVL by the medial (med) to lateral (lat) excursion of the SAR. C: enlarged view of the P-cell and the SAR axonal arborization for a single coronal section at the same rostrocaudal level as in B. Note that the horizontal orientation of the P-cell dendrites matches the distribution of the SAR terminals and that the P-cell dendrites may receive terminations within both the SolIM and SolVL. Both the SAR axon and P-cell were filled with neurobiotin.
Second order SAR neurons
Within the NTS, at least two types of neurons are monosynaptically activated by SAR afferents (Fig. 1). One, referred to as pump cells (P-cells) (11), has activity that parallels the activity of SARs. The second type, inspiratory-β (Iβ) cells, receives an excitatory input from both SARs and the central inspiratory drive (11). P-cells are monosynaptically activated by SARs and are located in the NTS regions (Fig. 2) targeted by SAR terminals (1, 18, 27, 34, 46, 78, 102, 110). Both Iβ neurons and P-cells are found among other respiratory neurons in the SolVL, the site of the classical dorsal respiratory group (DRG).
Like SARs, P-cells exhibit phasic activity that tracks lung volume changes, sometimes superimposed on tonic activity. Although P-cells are not activated by central inspiratory drive in cats, in rats glycinergic inhibition is present during early inspiration and a glutamatergic excitation is present during late inspiration and early expiration. Additionally, their activity is constrained by a tonic GABAergic input (102, 103). Rat P-cells also receive a phasic inhibitory input at the time of transition from inspiration to expiration (103). Similar GABAergic input to bulbospinal respiratory neurons in the dog modulates the gain of other inputs to these neurons (143). At least in cats, many P-cells in the SolVL and some in SolM also receive monosynaptic excitatory input from SLN afferents (11, 110).
As shown in Fig. 1, P-cells project caudally to the SolC and SolM subnuclei, reaching the contralateral side (34, 53). Collaterals of the same axons also target the ipsilateral dorsolateral pons (parabrachial and Kölliker-Fuse regions) and the ventrolateral pontine region (the noradrenergic A5 region) as well as the ipsilateral ventral respiratory column (VRC) (46, 50, 53). In the rat, P-cells in the SolV and SolVL apparently use GABA as a transmitter because about two-thirds of these cells express mRNA for the GABA-synthesizing enzyme GAD, and some also for a glycine transporter (49).
The existence of at least two classes of P-cells is suggested by the observation in cats that projections to contralateral SolC and SolM were identified for most P-cells located dorsal and lateral to the solitary tract in the SolD, SolDL, and SolVL, but not for those located medial to the tract in the SolIM and SolV (34). Furthermore, neither P-cells located within the SolVL nor the projections of P-cells to the SolC appear to mediate the Breuer-Hering reflexes because lesion of the SolVL (96) or the SolC (34, 45, 84) leave these reflexes intact. In contrast, microinjection of an excitatory amino acid into the region of SolIM containing P-cells mimics the Breuer-Hering reflex, whereas blockade of excitatory amino acid transmission in this region induces changes in the respiratory rhythm comparable to those resulting from reductions in the SAR afferent input (16, 18). It is worth noting that in rats and cats, P-cells with intrinsic NTS projections to SolC are found with somewhat different orientations to the solitary tract (SolDL in cat vs. SolV and SolVL in rat). Whether these two zones are homologous between the rat and cat is not clear. If they are (as suggested by the similarity of their functional cell types and caudal NTS projections), the nomenclature used to designate these areas may need to be reevaluated.
Iβ cells are located in the SolVL and, like P-cells, are monosynaptically excited by SARs (1, 2, 13). Iβ cells are inspiratory neurons that show a ramplike increase in firing during inspiration and are silent during expiration. This phasic activity is reduced if lung inflation is withheld during the period of central inspiratory activity (13, 26, 27, 91). In addition to a monosynaptic input from SARs of the ipsilateral vagus nerve, a substantial polysynaptic SAR-related input reaches Iβ cells from SAR afferents of the contralateral vagus nerve. Both excitatory (cat, Ref. 82) and inhibitory (dog, Ref. 3) inputs from SARs of the opposite side were observed, the excitatory or inhibitory effect probably depending on the specific experimental conditions. Because SAR afferents do not cross the midline, the SAR input reaching contralateral NTS neurons, including contralateral Iβ and P-cells, must be mediated by P-cells. In the cat, most Iβ cells have spinal projections and monosynaptically excite phrenic motoneurons (91). Some may also have intramedullary collaterals (43). As with P-cells, Iβ cells are monosynaptically excited by electrical stimulation of myelinated afferents of the SLN (11, 37, 38). Thus they also function as second order neurons in the afferent pathway from laryngeal afferents.
Third and higher order SAR neurons
Electrical stimulation of the vagus nerve has been used to probe pathways arising from SARs. These afferents have large myelinated axons that are preferentially activated by low-stimulus currents. Respiratory responses elicited in this fashion mimic many aspects of SAR-induced reflexes, including the Breuer-Hering reflex (61, 142).
Most Iβ neurons show a linear (i.e., additive) increase in discharge frequency with increasing SAR input frequency (or lung volume) (3). In contrast, SAR activation by either vagal stimulation or lung inflation causes a change in the discharge rate of rostral and caudal VRC bulbospinal neurons such that their resultant discharge pattern is similar to the prestimulus pattern multiplied by a constant “gain” factor (36, 142, 143). The magnitude of the gain is proportional to the SAR discharge frequency and regulated by a GABAergic mechanism (143).
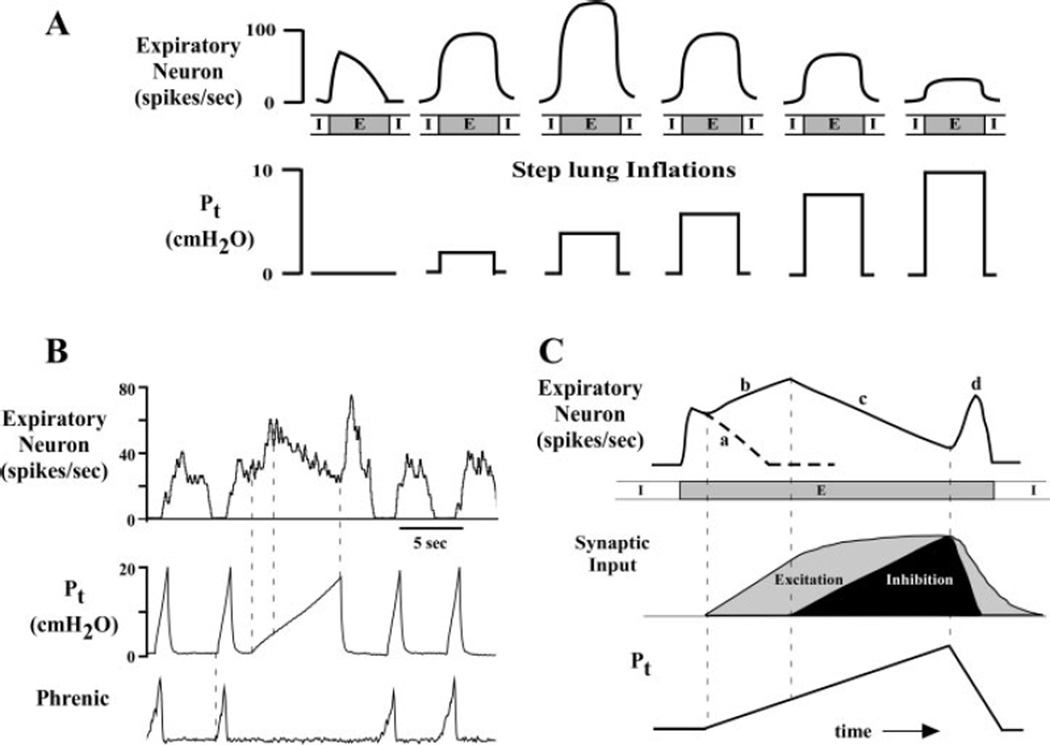
Caudal VRC bulbospinal expiratory premotor neurons in both cats (28) and dogs (4, 134) exhibit a biphasic excitatory and inhibitory response to increasing lung inflation (Fig. 3). Neuronal discharge frequency is increased by small lung inflations in expiration, whereas larger inflations depress activity (Fig. 3A). Because these responses are related to transpulmonary pressure and independent of time within the expiratory phase (Fig. 3B), it suggests the existence of two different SAR pathways to expiratory premotor neurons mediated by different groups of P-cells (one excitatory and another inhibitory, Fig. 3C). Excitatory and inhibitory P-cells could, for example, relay input from SARs with different thresholds of activation. Consistent with this possibility, extrapulmonary SARs have a lower threshold pressure for activation compared with intrapulmonary airways (99). Differences in the forces applied to the intrapulmonary vs. extrapulmonary airways may be important in the regulation of airway defense reflexes and the ventilatory response to pathophysiological situations such as airway obstruction.
Fig. 3.
Biphasic responses of a caudal VRC expiratory bulbospinal neuron to lung inflations. A: diagram illustrating the responses of an expiratory neuron to a series of increasing lung inflations delivered during the expiratory (E) phase. Without inflation, the neuron has a decrementing pattern typical of canine expiratory bulbospinal neuron. Transpulmonary pressures (Pt) below ∼5 cmH2O produce a graded increase in neuronal discharge frequency, whereas Pt above this threshold level produce a graded decrease in activity. The threshold level closely approximates the Pt at functional residual capacity. B: the biphasic response of a canine E bulbospinal neuron is evoked within a single expiratory phase by a slow ramp inflation. In an anesthetized, paralyzed dog the onset of phrenic activity was used to synchronize inflations during control cycles, whereas the offset was used to trigger a slow ramp inflation during the E phase. In B, note the increase in expiratory neuron activity at Pt in the low range and the progressive linear decrease in unit discharge for Pt at higher pressures. When the inflation is abruptly terminated, unit activity increases. C: schematic of the neuronal response shown in B illustrating the response characteristics and the hypothesized mechanisms. The dashed line in part a of the curve indicates the intrinsic decrementing discharge pattern without inflation. In part b the excitatory portion of the inflation response shown by gray shading is hypothesized to be due to excitatory P-cell inputs. In part c of the response, the ongoing excitation appears to be masked by inflation-mediated inhibition. This may result from inhibitory P-cell inputs (shown by black shading). In part d, the more rapid decline in inhibition unmasks the excitation.
SAR inputs also influence the activity of subsets of propriobulbar VRC neurons likely to have central roles in respiratory rhythm generation. Neurons with decrementing patterns (either inspiratory or expiratory) are affected by lung inflation in a manner consistent with their postulated central roles in the control of inspiratory and expiratory durations (54, 60, 81, 92, 142). Specifically, expiratory-decrementing neurons respond to lung inflation with a slowed rate of decline in their discharge rate and their prolonged discharge period correlates with the lengthening of expiration (54, 60, 81, 92, 111). This strongly implicates these neurons in the control of expiratory duration.
With respect to the control of inspiratory duration, lung inflation accelerates the decline in the discharge rate of propriobulbar inspiratory-decrementing neurons, thereby decreasing their period of activity in proportion to the amount inspiration is shortened (81). A subset of inspiratory-augmenting neurons (late-inspiratory neurons) have a nonlinear response to lung inflation (29). They begin to fire late in inspiration and exhibit a peak in firing at the end of inspiration. Lung inflation has little effect on their peak firing rate but advances the onset of their discharge proportionally to the magnitude of inspiratory shortening. The fixed relationship between the peak firing rate of these neurons and inspiratory termination has lead to the suggestion that they contribute to the termination of inspiration, i.e., provide the inspiratory off-switch (6, 29).
SAR activation by either low-intensity electrical stimulation of the vagus nerve or lung inflation also modulates the activity of neurons in the upper cervical spinal cord (C1–C3) (35), pontine parabrachial region (30, 31, 44, 55, 119, 127, 131), and midbrain (42). Inspiratory modulation of many neurons located in the dorsolateral pons is suppressed, or abolished, by lung inflations delivered in inspiration (40, 55, 70, 127, 130, 131), possibly by presynaptic inhibition (55). Pontine influences may contribute to short-term plasticity in respiratory reflexes, including accommodation in the Breuer-Hering reflex (129).
Neurons in the hypothalamus, posterior thalamus, amygdala, basal forebrain, medial and lateral frontal cortices, and cerebellum respond to low-intensity electrical vagal stimulation, implying that they may modulate behavioral or emotive responses to SAR activation (7, 9, 68, 118, 121, 122, 133). Inputs to areas such as the hypothalamus are likely to contribute to metabolic and neuroendocrine homeostasis, whereas those to the posterior thalamus, amygdala, and medial and lateral frontal cortices may interactively provide emotional (e.g., air hunger, dyspnea) and behavioral responses to challenges to respiratory homeostasis. Neurons in these regions may also contribute to the voluntary control of lung volume.
Central interactions between SAR and chemoreceptors
At the reflex level, there is convincing evidence for a central interaction between inputs from SARs and CO2-sensitive chemoreceptors. The lengthening of expiratory duration by lung inflation during expiration, known as the Breuer-Hering expiratory facilitatory reflex, is markedly attenuated by hypercapnia (100, 101). The central pathways underlying this phenomenon are not known, but the arterial PCO2 effect must impinge on neurons generating rhythm. This may include the expiratory-decrementing neurons (described above) excited by lung inflation.
Studies of the interaction between SARs and chemoreceptors at the neuronal level are limited in number. These, however, indicate that activation of carotid body or central chemoreceptors via hypercapnia excites most medullary respiratory neurons, whereas lung inflation causing activation of SARs either excites or inhibits tertiary neurons depending on the type of respiratory cells studied. The interaction between carotid body chemoreceptor and SAR inputs is for the most part linear (additive) for canine DRG Iβ neurons, VRC inspiratory-decrementing, and caudal VRC expiratory-augmenting neurons (5). Increases in arterial PCO2 produce an upward shift in the discharge rate of these neurons at all levels of lung inflation. Interestingly, the interaction of chemoreceptor and SAR inputs at the level of caudal VRC expiratory-decrementing neurons differs between carotid body and central chemoreceptor activation. Activation mediated by stimulation of central chemoreceptors generally produces an additive effect on VRC expiratory-decrementing neurons (134). In contrast, the interaction between carotid chemoreceptor and SAR input in these neurons is nonadditive (5). Thus the direction and type of interaction depends on neuron type and functional role, such as motor pattern generation vs. rhythm control.
CENTRAL PATHWAYS OF THE REFLEXES FROM RARS
The central projection sites of RAR primary afferent fibers are strikingly different from those of SARs (Fig. 1). Individual RARs consistently project to multiple sites within the NTS (33, 75), suggesting that each afferent may contribute to several distinct components of the respiratory and cardiovascular effects ascribed to these receptors (Table 1). Individual RAR afferents project to caudal levels of the ipsilateral NTS, with a smaller projection to comparable contralateral NTS areas. The SolC and caudal SolM are the primary termination subnuclei, with additional terminations in the SolD, SolDL, and SolIM (33, 75, 76, 83). At least in cats, projections to the inspiratory region of the SolVL are weak or absent, suggesting that inspiratory neurons of the DRG are not directly excited by RARs (33, 75). In rats, however, an additional projection to inspiratory neurons in the SolV regions has been implied from the activation patterns of second order neurons receiving input from deflation-sensitive receptors, suggested to be RARs (48).
Neurons, referred to as “RAR cells,” in the caudal SolM and SolC in both cats and rats receive monosynaptic EPSPs in response to low-intensity electrical stimulation of both vagi, are excited by ammonia inhalation, and adapt rapidly to hyperinflations of the lungs (51, 52, 83, 90). The excitatory input from RARs is mediated, at least in part, by non-NMDA glutamate receptors (52). RAR cells are unlikely to be inhibitory interneurons because they do not express mRNA markers for GABA or glycine (49). Lesions of the SolC abolish the reflex excitatory responses of the phrenic nerve to ammonia inhalation, rapid hyperinflations of the lungs, and mechanical stimulation of the mucosa near the tracheal bifurcation, without impairing the inspiratory inhibitory Breuer-Hering reflex (45, 84).
In rats, as noted above, lung deflation also activates significant numbers of inspiratory neurons in SolV (47). Ezure and Tanaka (47) termed these cells Iγ neurons and postulated that these deflation-activated neurons receive input from RARs and hence are RAR second order neurons. However, whether receptors identified by their response to lung deflation are the same as RAR receptors activated by lung inflation has yet to be established. Many of these neurons were minimally activated by even large lung inflations but received input originating in slowly adapting lung-deflation receptors, which are relatively numerous in the rat (14). Antidromic stimulation of single axons indicated that, similar to Iβ cells, almost all Iγ neurons had axons descending in the spinal cord (47). RAR cells located in the SolC and SolM are distinct from Iγ cells in that they do not exhibit a strong inspiratory modulation in either cats or rats.
Many NTS RAR cells exhibit spontaneous activity (51, 90), unlike RAR afferent fibers, which tend to be silent in the absence of a stimulus (33). RAR cell activity patterns include modulation with ventilator-induced lung volume changes, central respiratory drive, and cardiac rhythm. They are also phasically inhibited during inspiration by glycine, possibly from P-cells (53), and receive a tonic inhibition mediated by GABAA receptors (52).
Cat RAR cells in the SolC have axonal ramifications in the ipsilateral pontine parabrachial region and Kölliker-Fuse nucleus (45). Additionally in the rat, but less so in the cat, RAR neurons project to the VRC, with the densest projections to its caudal aspects where bulbospinal inspiratory and expiratory neurons are concentrated, and may have only sparse projections to the more rostral pre-Bötzinger and Bötzinger regions (45, 109). This is consistent with their prominent effects on respiratory motor pattern and suggests that some of these can be mediated by direct inputs to the bulbospinal premotor neurons, bypassing the rhythm generating circuitry concentrated in the pre-Bötzinger complex (56).
CENTRAL PROJECTIONS AND PATHWAYS OF BRONCHOPULMONARY C FIBERS
In the cat, bronchial and pulmonary C fibers have similar projection patterns; they terminate bilaterally (with an ipsilateral predominance) in the medial NTS, in the parvicellular subnucleus, in the dorsal aspects of the SolC, and in the area postrema (Fig. 1) (86).
Glutamate, acting on non-NMDA receptors, appears likely to mediate synaptic transmission from pulmonary C-fiber afferents in the SolC (140). Consistent with dorsal SolC being an important site of bronchopulmonary C-fiber terminations, activation of neurons within this subnucleus by microinjection of an excitatory amino acid mimics the respiratory response evoked by C-fiber stimulation, and interruption of synaptic transmission in this same region by cobalt ions impairs the respiratory effects of intra-atrial phenylbiguanide injections (17). The projection patterns of NTS neurons receiving bronchopulmonary C-fiber input have not been determined, but bronchopulmonary C-fiber activation by capsaicin or phenylbiguanidine injections excites VRC expiratory-decrementing cells and suppresses the firing of inspiratory neurons (112, 139).
CONVERGENCE ON SECOND ORDER NEURONS AND MODALITY-SPECIFIC ORGANIZATION OF THE NTS
There is significant support for the concept that NTS second order neurons occur in clusters, or groups, that receive their main sensory input from only one type of vagal afferent (86) and then project to distinct tertiary target neurons (65, 66). Afferent fibers arising from SAR, RAR, and bronchopulmonary C fibers largely terminate in different regions of the NTS (Fig. 1). Lesions or pharmacological manipulations within the SolC abolish reflexes that are characteristic of RARs without impairing the Breuer-Hering reflex (45, 84). Reversible lesions medial to the solitary tract at the level of the area postrema abolish the Breuer-Hering reflex (18), and blockade of synaptic transmission in the dorsomedial SolC subnucleus impairs the respiratory effects of bronchopulmonary C-fiber stimulation without affecting the blood pressure response (17). A relative segregation within the NTS can be extended to other afferent systems. NTS cells excited by stimulation of bronchopulmonary C fibers, for example, are rarely responsive to inputs from arterial baroreceptors or cardiac mechanoreceptors (115), and cells affected by the latter afferents rarely receive input from the subdiaphragmatic vagus nerve (113). One functional advantage of such an organization is that it allows for selectivity in modulation of transmission in pathways from distinct peripheral receptors before they become integrated with other central or peripheral inputs.
There is also convergence of afferent inputs onto NTS neurons; one (or few) of such converging inputs may originate directly from primary afferents and others may reach the same cell by polysynaptic pathways. SAR and SLN inputs converge on most P-cells and Iβ cells (discussed in Second order SAR neurons), and some Iβ cells are excited by RARs (11, 93). Convergence of pharyngeal mechanoreceptors and peripheral chemoreceptor afferents has been demonstrated for the adult rat “in situ” (114, 116), and a relatively high proportion of convergence has been observed in the cat SolI for SLN and glossopharyngeal afferents, or SLN and carotid sinus nerve afferents (98, 107). However, Ootani et al. (107) note that monosynaptic innervation by both afferents is relatively rare.
EFFECT OF SLEEP ON RESPIRATORY VAGAL REFLEXES
Attenuation of respiratory reflexes during sleep has been reported (see reviews, Refs. 15, 63, 85), but it is often not possible to distinguish between a genuine state-dependent central suppression of reflex transmission and attenuation secondary to sleep-related decrements in the excitability of motoneurons. The average diaphragmatic response to airway occlusion is reduced during rapid-eye-movement (REM) sleep compared with slow-wave sleep (128), but the reductions may coincide with clusters of respiratory cycles in which the activity of respiratory premotor neurons is depressed by REM sleep-specific central processes related to certain phasic phenomena of REM sleep, rather than to the state per se (108).
Monoaminergic influences may contribute to the state-dependent modulation of respiratory reflexes. Serotonergic and noradrenergic neurons have maximal activity during active wakefulness and minimal activity during REM sleep (57, 69), and they innervate the NTS. Receptors commonly associated with presynaptic effects, such as adrenergic α2 and serotonergic 1B, are present in the NTS. They may contribute to suppression of reflexes during wakefulness and their facilitation during sleep, but it remains to be determined whether such a modulation affects the input from pulmonary and/or airway vagal receptors. Data show that serotonergic type 4 receptors presynaptically suppress input from C fibers (41), and presynaptic actions of substance P (possibly released from C-fiber terminals or serotonergic neurons) may both enhance and suppress transmission of vagal afferent inputs within the NTS (104, 126).
CONCLUSIONS
Separate termination regions within the NTS have been identified for each of the major afferent systems from the lower airways and lungs (SAR, RAR, and C fiber). Emanating from the NTS, these afferent systems provide divergent pathways that send inputs to a variety of central respiratory neurons. Included among the targets are neurons involved in rhythm generation (e.g., those in the pre-Bötzinger and Bötzinger regions) as well those involved in respiratory pattern formation (e.g., cranial motoneurons and bulbospinal premotor neurons in the VRC). This input to both rhythm- and pattern-generating regions is consistent with parallel control of these two components of breathing (89). Additionally, projections to areas such as the parabrachial and Kölliker-Fuse nuclei within the rostral dorsolateral pons may have influences on both rhythm and pattern but be of particular importance for the integration of the reflex control of breathing with other systems such as cardiovascular control, orofacial ingestive behaviors, airway protective reflexes, and both ascending and descending pain pathways (44, 70).
Our understanding of these important concepts is rapidly evolving, aided by the incorporation of novel technologies. These include the ability to study reflexes using extensive in vitro preparations, such as the adult and juvenile working heart-brain stem preparations (112, 116) and the isolated neonatal rodent brain stem with intact, innervated lungs connected to the medulla via the vagus nerve (97). Advances in in vitro and in vivo imaging make it possible to observe multineuronal circuit activity at improved levels of resolution. This will assist observation of ongoing respiratory neuronal activity and its perturbation by reflex activation (62, 80, 106). A combination of molecular biological techniques with studies of the development of the respiratory system provides a powerful tool with which to identify the neurobiochemical substrates specific to SAR and other vagal reflex pathways (19).
Acknowledgments
GRANTS
The authors’ studies were supported by National Heart, Lung, and Blood Institute Grants HL-36621 (L. Kubin), HL-72415, and HL-73474 (D. R. McCrimmon) and a Department of Veterans Affairs Medical Research Grant (E. J. Zuperku).
REFERENCES
- 1.Averill DB, Cameron WE, Berger AJ. Monosynaptic excitation of dorsal medullary respiratory neurons by slowly adapting pulmonary stretch receptors. J Neurophysiol. 1984;52:771–785. doi: 10.1152/jn.1984.52.4.771. [DOI] [PubMed] [Google Scholar]
- 2.Backman SB, Anders C, Ballantyne D, Röhrig N, Camerer H, Mifflin S, Jordan D, Dickhaus H, Spyer KM, Richter DW. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflügers Arch. 1984;402:129–136. doi: 10.1007/BF00583324. [DOI] [PubMed] [Google Scholar]
- 3.Bajic J, Zuperku EJ, Hopp FA. Processing of pulmonary afferent input patterns by respiratory I-beta neurons. Am J Physiol Regul Integr Comp Physiol. 1989;256:R379–R393. doi: 10.1152/ajpregu.1989.256.2.R379. [DOI] [PubMed] [Google Scholar]
- 4.Bajic J, Zuperku EJ, Tonkovic-Capin M, Hopp FA. Expiratory bulbospinal neurons of dogs I. Control of discharge patterns by pulmonary stretch receptors. Am J Physiol Regul Integr Comp Physiol. 1992;262:R1075–R1086. doi: 10.1152/ajpregu.1992.262.6.R1075. [DOI] [PubMed] [Google Scholar]
- 5.Bajic J, Zuperku EJ, Tonkovic-Capin M, Hopp FA. Interaction between chemoreceptor and stretch receptor inputs at medullary respiratory neurons. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1951–R1961. doi: 10.1152/ajpregu.1994.266.6.R1951. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barone FC, Armstrong DL, Wayner MJ, Zarco De Coronado I. Effects of neurotransmitters and vagus nerve stimulation on diencephalic and mesencephalic neuronal activity. Brain Res Bull. 1984;13:565–571. doi: 10.1016/0361-9230(84)90039-x. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett D., Jr Respiratory functions of the larynx. Physiol Rev. 1989;69:33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- 9.Bassal M, Bianchi AL. Effets de la stimulation des structures nerveuses centrales sur les activités respiratoires efférentes chez le chat I. Réponses á la stimulation corticale. J Physiol (Paris) 1981;77:741–757. [PubMed] [Google Scholar]
- 10.Bellingham MC, Lipski J. Morphology and electrophysiology of superior laryngeal nerve afferents and postsynaptic neurons in the medulla oblongata of the cat. Neuroscience. 1992;48:205–216. doi: 10.1016/0306-4522(92)90349-7. [DOI] [PubMed] [Google Scholar]
- 11.Berger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977;135:231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- 12.Berger AJ, Averill DB. Projection of single pulmonary stretch receptors to solitary tract region. J Neurophysiol. 1983;49:819–830. doi: 10.1152/jn.1983.49.3.819. [DOI] [PubMed] [Google Scholar]
- 13.Berger AJ, Dick TE. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neurons. J Neurophysiol. 1987;58:1259–1274. doi: 10.1152/jn.1987.58.6.1259. [DOI] [PubMed] [Google Scholar]
- 14.Bergren DR, Peterson DF. Identification of vagal sensory receptors in the rat lung: Are there subtypes of slowly adapting receptors? J Physiol. 1993;464:681–698. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 16.Bonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol. 1993;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonham AC, Joad JP. Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol. 1991;441:95–112. doi: 10.1113/jphysiol.1991.sp018740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borday C, Wrobel L, Fortin G, Champagnat J, Thaeron-Antono C, Thoby-Brisson M. Developmental gene control of brainstem function: views from the embryo. Prog Biophys Mol Biol. 2004;84:89–106. doi: 10.1016/j.pbiomolbio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.BuSha BF, Judd BG, Manning HL, Simon PM, Searle BC, Daubenspeck JA, Leiter JC. Identification of respiratory vagal feedback in awake normal subjects using pseudorandom unloading. J Appl Physiol. 2001;90:2330–2340. doi: 10.1152/jappl.2001.90.6.2330. [DOI] [PubMed] [Google Scholar]
- 21.BuSha BF, Stella MH, Manning HL, Leiter JC. Termination of inspiration by phase-dependent respiratory vagal feedback in awake normal humans. J Appl Physiol. 2002;93:903–910. doi: 10.1152/japplphysiol.00153.2002. [DOI] [PubMed] [Google Scholar]
- 22.Canning BJ. Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:33S–47S. doi: 10.1378/chest.129.1_suppl.33S. [DOI] [PubMed] [Google Scholar]
- 23.Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol. 2001;125:113–127. doi: 10.1016/s0034-5687(00)00208-5. [DOI] [PubMed] [Google Scholar]
- 24.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 25.Clark FJ, Euler von C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen MI, Feldman JL. Models of respiratory phase-switching. Fed Proc. 1977;36:2367–2374. [PubMed] [Google Scholar]
- 27.Cohen MI, Feldman JL. Discharge properties of dorsal medullary inspiratory neurons: relation to pulmonary afferent and phrenic efferent discharge. J Neurophysiol. 1984;51:753–776. doi: 10.1152/jn.1984.51.4.753. [DOI] [PubMed] [Google Scholar]
- 28.Cohen MI, Feldman JL, Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MI, Huang WX, Barnhardt R, See WR. Timing of medullary late-inspiratory neuron discharges: vagal afferent effects indicate possible off-switch function. J Neurophysiol. 1993;69:1784–1787. doi: 10.1152/jn.1993.69.5.1784. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MI, Shaw CF, Barnhardt R. Connectivity of rostral pontine inspiratory-modulated neurons as revealed by responses to vagal and superior laryngeal afferent stimulation. In: Speck DF, Dekin MS, Revelette WR, Frazier DT, editors. Respiratory Control: Central and Peripheral Mechanisms. Lexington, KY: The University Press of Kentucky; 1993. pp. 91–94. [Google Scholar]
- 31.Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol. 2004;143:127–140. doi: 10.1016/j.resp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Coleridge HM, Coleridge JC, Schultz HD. Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther. 1989;42:1–63. doi: 10.1016/0163-7258(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 33.Davies RO, Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol. 1986;373:63–86. doi: 10.1113/jphysiol.1986.sp016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies RO, Kubin L, Pack AI. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol. 1987;383:571–585. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawkins MA, Foreman RD, Farber JP. Short latency excitation of upper cervical respiratory neurons by vagal stimulation in the rat. Brain Res. 1992;594:319–322. doi: 10.1016/0006-8993(92)91143-3. [DOI] [PubMed] [Google Scholar]
- 36.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol. 1998;80:2368–2377. doi: 10.1152/jn.1998.80.5.2368. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly DF, Sica AL, Cohen MI, Zhang H. Dorsal medullary inspiratory neurons: effects of superior laryngeal afferent stimulation. Brain Res. 1989;491:243–252. doi: 10.1016/0006-8993(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly DF, Sica AL, Cohen MI, Zhang H. Effects of contralateral superior laryngeal nerve stimulation on dorsal medullary inspiratory neurons. Brain Res. 1989;505:149–152. doi: 10.1016/0006-8993(89)90127-3. [DOI] [PubMed] [Google Scholar]
- 39.Donoghue S, Garcia M, Jordan D, Spyer KM. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol. 1982;322:353–363. doi: 10.1113/jphysiol.1982.sp014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutschmann M, Morschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kölliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Edwards E, Paton JFR. Glutamate stimulation of raphe pallidus attenuates the cardiopulmonary reflex in anaesthetised rats. Autonom Neurosci. 2000;82:87–96. doi: 10.1016/S0165-1838(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 42.Eldridge FL, Chen Z. Respiratory-associated rhythmic firing of midbrain neurons is modulated by vagal input. Respir Physiol. 1992;90:31–46. doi: 10.1016/0034-5687(92)90132-g. [DOI] [PubMed] [Google Scholar]
- 43.Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- 44.Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Ezure K, Otake K, Lipski J, Wong She RB. Efferent projections of pulmonary rapidly adapting receptor relay neurons in the cat. Brain Res. 1991;564:268–278. doi: 10.1016/0006-8993(91)91463-b. [DOI] [PubMed] [Google Scholar]
- 46.Ezure K, Tanaka I. Pump neurons of the nucleus of the solitary tract project widely to the medulla. Neurosci Lett. 1996;215:123–126. [PubMed] [Google Scholar]
- 47.Ezure K, Tanaka I. Identification of deflation-sensitive inspiratory neurons in the dorsal respiratory group of the rat. Brain Res. 2000;883:22–30. doi: 10.1016/s0006-8993(00)02871-7. [DOI] [PubMed] [Google Scholar]
- 48.Ezure K, Tanaka I. Lung inflation inhibits rapidly adapting receptor relay neurons in the rat. Neuroreport. 2000;11:1709–1712. doi: 10.1097/00001756-200006050-00023. [DOI] [PubMed] [Google Scholar]
- 49.Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 50.Ezure K, Tanaka I, Miyazaki M. Pontine projections of pulmonary slowly adapting receptor relay neurons in the cat. Neuroreport. 1998;9:411–414. doi: 10.1097/00001756-199802160-00010. [DOI] [PubMed] [Google Scholar]
- 51.Ezure K, Tanaka I, Miyazaki M. Inspiratory inhibition of pulmonary rapidly adapting receptor relay neurons in the rat. Neurosci Lett. 1998;258:49–52. doi: 10.1016/s0304-3940(98)00858-1. [DOI] [PubMed] [Google Scholar]
- 52.Ezure K, Tanaka I, Miyazaki M. Electrophysiological and pharmacological analysis of synaptic inputs to pulmonary rapidly adapting receptor relay neurons in the rat. Exp Brain Res. 1999;128:471–480. doi: 10.1007/s002210050870. [DOI] [PubMed] [Google Scholar]
- 53.Ezure K, Tanaka I, Saito Y, Otake K. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J Comp Neurol. 2002;446:81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- 54.Feldman JL, Cohen MI. Relation between expiratory duration and rostral medullary expiratory neuronal discharge. Brain Res. 1978;141:172–178. doi: 10.1016/0006-8993(78)90627-3. [DOI] [PubMed] [Google Scholar]
- 55.Feldman JL, Cohen MI, Wolotsky P. Powerful inhibition of pontine respiratory neurons by pulmonary afferent activity. Brain Res. 1976;104:341–346. doi: 10.1016/0006-8993(76)90629-6. [DOI] [PubMed] [Google Scholar]
- 56.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- 58.Haddad GG, Farber JP. Developmental Neurobiology of Breathing. New York: Marcel Dekker; 1991. [Google Scholar]
- 59.Hamilton RD, Horner RL, Winning AJ, Guz A. Effect on breathing of raising end-expiratory lung volume in sleeping laryngectomized man. Respir Physiol. 1990;81:87–98. doi: 10.1016/0034-5687(90)90072-7. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi F, McCrimmon DR. Respiratory motor responses to cranial nerve afferent stimulation in rats. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1054–R1062. doi: 10.1152/ajpregu.1996.271.4.R1054. [DOI] [PubMed] [Google Scholar]
- 62.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Med. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 63.Henke KG, Badr MS, Skatrud JB, Dempsey JA. Load compensation and respiratory muscle function during sleep. J Appl Physiol. 1992;72:1221–1234. doi: 10.1152/jappl.1992.72.4.1221. [DOI] [PubMed] [Google Scholar]
- 64.Henke KG, Sullivan CE. Effects of high-frequency oscillating pressures on upper airway muscles in humans. J Appl Physiol. 1993;75:856–862. doi: 10.1152/jappl.1993.75.2.856. [DOI] [PubMed] [Google Scholar]
- 65.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 66.Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 67.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito S. Multiple projection of vagal non-myelinated afferents to the anterior insular cortex in rats. Neurosci Lett. 1992;148:151–154. doi: 10.1016/0304-3940(92)90827-t. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 70.Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol. 2004;143:215–233. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 71.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- 72.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- 73.Kalia M, Richter D. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat I. A light microscopic analysis. J Comp Neurol. 1985;241:503–520. doi: 10.1002/cne.902410409. [DOI] [PubMed] [Google Scholar]
- 74.Kalia M, Richter D. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat II. An ultrastructural analysis. J Comp Neurol. 1985;241:521–535. doi: 10.1002/cne.902410410. [DOI] [PubMed] [Google Scholar]
- 75.Kalia M, Richter D. Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius. J Comp Neurol. 1988;274:560–573. doi: 10.1002/cne.902740406. [DOI] [PubMed] [Google Scholar]
- 76.Kalia M, Richter D. Rapidly adapting pulmonary receptor afferents: II. Fine structure and synaptic organization of central terminal processes in the nucleus of the tractus solitarius. J Comp Neurol. 1988;274:574–594. doi: 10.1002/cne.902740407. [DOI] [PubMed] [Google Scholar]
- 77.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- 78.Koga T, Fukuda H. Neurons in the nucleus of the solitary tract mediating inputs from emetic vagal afferents and the area postrema to the pattern generator for the emetic act in dogs. Neurosci Res. 1992;14:166–179. doi: 10.1016/0168-0102(92)90078-q. [DOI] [PubMed] [Google Scholar]
- 79.Korpas J, Jakus J. The expiration reflex from the vocal folds. Acta Physiol Hung. 2000;87:201–215. doi: 10.1556/APhysiol.87.2000.3.1. [DOI] [PubMed] [Google Scholar]
- 80.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 81.Krolo M, Tonkovic-Capin V, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Subtype composition and responses of respiratory neurons in the pre-Bötzinger region to pulmonary afferent inputs in dogs. J Neurophysiol. 2005;93:2674–2687. doi: 10.1152/jn.01206.2003. [DOI] [PubMed] [Google Scholar]
- 82.Kubin L, Davies RO. Bilateral convergence of pulmonary stretch receptor inputs on I beta-neurons in the cat. J Appl Physiol. 1987;62:1488–1496. doi: 10.1152/jappl.1987.62.4.1488. [DOI] [PubMed] [Google Scholar]
- 83.Kubin L, Davies RO. Sites of termination and relay of pulmonary rapidly adapting receptors as studied by spike-triggered averaging. Brain Res. 1988;443:215–221. doi: 10.1016/0006-8993(88)91615-0. [DOI] [PubMed] [Google Scholar]
- 84.Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Dekker; 1995. pp. 219–284. [Google Scholar]
- 85.Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep Apnea Pathogenesis, Diagnosis, and Treatment. New York: Dekker; 2002. pp. 99–154. [Google Scholar]
- 86.Kubin L, Kimura H, Davies RO. The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J Physiol. 1991;435:207–228. doi: 10.1113/jphysiol.1991.sp018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee LY, Lin YS, Gu QH, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec. 2003:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- 88.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 89.Lindsey BG, Morris KF, Segers LS, Shannon R. Respiratory neuronal assemblies. Respir Physiol. 2000;122:183–196. doi: 10.1016/s0034-5687(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 90.Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lipski J, Kubin L, Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 1983;288:105–118. doi: 10.1016/0006-8993(83)90085-9. [DOI] [PubMed] [Google Scholar]
- 92.Manabe M, Ezure K. Decrementing expiratory neurons of the Botzinger complex I. Response to lung inflation and axonal projection. Exp Brain Res. 1988;72:150–158. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- 93.Marino PL, Davies RO, Pack AI. The responses of Iβ cells to increases in the rate of lung inflation. Brain Res. 1981;219:289–305. doi: 10.1016/0006-8993(81)90292-4. [DOI] [PubMed] [Google Scholar]
- 94.Matsuoka T, Mortola JP. Effects of hypoxia and hypercapnia on the Hering-Breuer reflex of the conscious newborn rat. J Appl Physiol. 1995;78:5–11. doi: 10.1152/jappl.1995.78.1.5. [DOI] [PubMed] [Google Scholar]
- 95.McCrimmon DR, Alheid GF, Zuperku EJ. Reflexes from the lungs and chest wall. In: Laurent GJ, Shapiro SD, editors. Encyclopedia of Respiratory Medicine. Oxford, UK: Elsevier Academic; 2006. pp. 618–625. [Google Scholar]
- 96.McCrimmon DR, Speck DF, Feldman JL. Role of the ventrolateral region of the nucleus of the tractus solitarius in processing respiratory afferent input from vagus and superior laryngeal nerves. Exp Brain Res. 1987;67:449–459. doi: 10.1007/BF00247278. [DOI] [PubMed] [Google Scholar]
- 97.Mellen NM, Roham M, Feldman JL. Afferent modulation of neonatal rat respiratory rhythm in vitro: cellular and synaptic mechanisms. J Physiol. 2004;556:859–874. doi: 10.1113/jphysiol.2004.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol. 1996;271:R870–R880. doi: 10.1152/ajpregu.1996.271.4.R870. [DOI] [PubMed] [Google Scholar]
- 99.Miserocchi G, Sant’Ambrogio G. Responses of pulmonary stretch receptors to static pressure inflations. Respir Physiol. 1974;21:77–85. doi: 10.1016/0034-5687(74)90009-7. [DOI] [PubMed] [Google Scholar]
- 100.Mitchell GS. Phrenic nerve responses to lung inflation and hypercapnia in decerebrate dogs. Pflugers Arch. 1990;416:580–585. doi: 10.1007/BF00382693. [DOI] [PubMed] [Google Scholar]
- 101.Mitchell GS, Cross BA, Hiramoto T, Scheid P. Interactions between lung stretch and PaCO2 in modulating ventilatory activity in dogs. J Appl Physiol. 1982;53:185–191. doi: 10.1152/jappl.1982.53.1.185. [DOI] [PubMed] [Google Scholar]
- 102.Miyazaki M, Arata A, Tanaka I, Ezure K. Activity of rat pump neurons is modulated with central respiratory rhythm. Neurosci Lett. 1998;249:61–64. doi: 10.1016/s0304-3940(98)00402-9. [DOI] [PubMed] [Google Scholar]
- 103.Miyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp Brain Res. 1999;129:191–200. doi: 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- 104.Mutoh T, Bonham AC, Joad JP. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1215–R1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- 105.Nishino T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn J Physiol. 2000;50:3–14. doi: 10.2170/jjphysiol.50.3. [DOI] [PubMed] [Google Scholar]
- 106.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull. 1995;37:397–404. doi: 10.1016/0361-9230(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 108.Orem J. Central respiratory activity in rapid eye movement sleep: augmenting and late inspiratory cells. Sleep. 1994;17:665–673. doi: 10.1093/sleep/17.8.665. [DOI] [PubMed] [Google Scholar]
- 109.Otake K, Nakamura Y, Tanaka I, Ezure K. Morphology of pulmonary rapidly adapting receptor relay neurons in the rat. J Comp Neurol. 2001;430:458–470. doi: 10.1002/1096-9861(20010219)430:4<458::aid-cne1043>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 110.Pantaleo T, Corda M. Respiration-related neurons in the medial nuclear complex of the solitary tract of the cat. Respir Physiol. 1986;64:135–148. doi: 10.1016/0034-5687(86)90037-x. [DOI] [PubMed] [Google Scholar]
- 111.Parkes MJ, Lara-Munoz JP, Izzo PN, Spyer KM. Responses of ventral respiratory neurones in the rat to vagus stimulation and the functional division of expiration. J Physiol. 1994;476:131–139. [PMC free article] [PubMed] [Google Scholar]
- 112.Paton JFR. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. J Physiol. 1997;502:623–639. doi: 10.1111/j.1469-7793.1997.623bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paton JFR. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol. 1998;79:2365–2373. doi: 10.1152/jn.1998.79.5.2365. [DOI] [PubMed] [Google Scholar]
- 114.Paton JFR. Nucleus tractus solitarii: integrating structures. Exp Physiol. 1999;84:815–833. [PubMed] [Google Scholar]
- 115.Paton JFR, Li YW, Deuchars J, Kasparov S. Properties of solitary tract neurons receiving inputs from the sub-diaphragmatic vagus nerve. Neuroscience. 2000;95:141–153. doi: 10.1016/s0306-4522(99)00416-9. [DOI] [PubMed] [Google Scholar]
- 116.Paton JFR, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 117.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic; 2005. [Google Scholar]
- 118.Perrin J, Crousillat J. The projection of vagal afferents on the cerebellar vermis of the cat. J Auton Nerv Syst. 1985;13:175–177. doi: 10.1016/0165-1838(85)90034-7. [DOI] [PubMed] [Google Scholar]
- 119.Poon CS. Organization of central pathways mediating the Hering-Breuer reflex and carotid chemoreflex. Adv Exp Med Biol. 2004;551:95–100. doi: 10.1007/0-387-27023-x_15. [DOI] [PubMed] [Google Scholar]
- 120.Rabbette PS, Stocks J. Influence of volume dependency and timing of airway occlusions on the Hering-Breuer reflex in infants. J Appl Physiol. 1998;85:2033–2039. doi: 10.1152/jappl.1998.85.6.2033. [DOI] [PubMed] [Google Scholar]
- 121.Radna RJ, MacLean PD. Vagal elicitation of respiratory-type and other unit responses in striopallidum of squirrel monkeys. Brain Res. 1981;213:29–44. doi: 10.1016/0006-8993(81)91246-4. [DOI] [PubMed] [Google Scholar]
- 122.Radna RJ, MacLean PD. Vagal elicitation of respiratory-type and other unit responses in basal limbic structures of squirrel monkeys. Brain Res. 1981;213:45–61. doi: 10.1016/0006-8993(81)91247-6. [DOI] [PubMed] [Google Scholar]
- 123.Sant’Ambrogio G, Tsubone H, Sant’Ambrogio FB. Sensory information from the upper airway: role in the control of breathing. Respir Physiol. 1995;102:1–16. doi: 10.1016/0034-5687(95)00048-i. [DOI] [PubMed] [Google Scholar]
- 124.Saper CB. Central autonomic system. In: Paxinos G, editor. The Rat Nervous System. San Diego, CA: Elsevier Academic; 2004. pp. 761–796. [Google Scholar]
- 125.Schelegle ES. Functional morphology and physiology of slowly adapting pulmonary stretch receptors. Anat Rec. 2003;270A:11–16. doi: 10.1002/ar.a.10004. [DOI] [PubMed] [Google Scholar]
- 126.Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol. 2003;552:547–559. doi: 10.1113/jphysiol.2003.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shaw CF, Cohen MI, Barnhardt R. Inspiratory-modulated neurons of the rostrolateral pons: effects of pulmonary afferent input. Brain Res. 1989;485:179–184. doi: 10.1016/0006-8993(89)90681-1. [DOI] [PubMed] [Google Scholar]
- 128.Smith CA, Henderson KS, Xi L, Chow CM, Eastwood PR, Dempsey JA. Neural-mechanical coupling of breathing in REM sleep. J Appl Physiol. 1997;83:1923–1932. doi: 10.1152/jappl.1997.83.6.1923. [DOI] [PubMed] [Google Scholar]
- 129.Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 130.Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.St. John WM. Influence of pulmonary inflations on discharge of pontile respiratory neurons. J Appl Physiol. 1987;63:2231–2239. doi: 10.1152/jappl.1987.63.6.2231. [DOI] [PubMed] [Google Scholar]
- 132.Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;111(Suppl 8A):69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- 133.Tong G, Robertson LT, Brons J. Vagal and somatic representation by the climbing fiber system in lobule V of the cat cerebellum. Brain Res. 1991;552:58–66. doi: 10.1016/0006-8993(91)90660-n. [DOI] [PubMed] [Google Scholar]
- 134.Tonkovic-Capin M, Zuperku EJ, Stuth EA, Bajic J, Dogas Z, Hopp FA. Effect of central CO2 drive on lung inflation responses of expiratory bulbospinal neurons in dogs. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1606–R1618. doi: 10.1152/ajpregu.2000.279.5.R1606. [DOI] [PubMed] [Google Scholar]
- 135.Trippenbach T. Pulmonary reflexes and control of breathing during development. Biol Neonate. 1994;65:205–210. doi: 10.1159/000244054. [DOI] [PubMed] [Google Scholar]
- 136.Wei JY, Shen E. Vagal expiratory afferent discharges during spontaneous breathing. Brain Res. 1985;335:213–219. doi: 10.1016/0006-8993(85)90472-x. [DOI] [PubMed] [Google Scholar]
- 137.Widdicombe J. Airway receptors. Respir Physiol. 2001;125:3–15. doi: 10.1016/s0034-5687(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 138.Widdicombe J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs) Anat Rec. 2003;270A:2–10. doi: 10.1002/ar.a.10003. [DOI] [PubMed] [Google Scholar]
- 139.Wilson CG, Bonham AC. Effect of cardiopulmonary C fibre activation on the firing activity of ventral respiratory group neurones in the rat. J Physiol. 1997;504:453–466. doi: 10.1111/j.1469-7793.1997.453be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilson CG, Zhang Z, Bonham AC. Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii in rats. J Physiol. 1996;496:773–785. doi: 10.1113/jphysiol.1996.sp021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu J. Airway mechanosensors. Respir Physiol Neurobiol. 2005;148:217–243. doi: 10.1016/j.resp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 142.Zuperku EJ, Hopp FA. Control of discharge patterns of medullary respiratory neurons by pulmonary vagal afferent inputs. Am J Physiol Regul Integr Comp Physiol. 1987;253:R809–R820. doi: 10.1152/ajpregu.1987.253.6.R809. [DOI] [PubMed] [Google Scholar]
- 143.Zuperku EJ, McCrimmon DR. Gain modulation of respiratory neurons. Respir Physiol Neurobiol. 2002;131:121–133. doi: 10.1016/s1569-9048(02)00042-3. [DOI] [PubMed] [Google Scholar]