Abstract
Outdoor ambient weather has been hypothesized to be responsible for the seasonal distribution of cardiac arrhythmias. Because people spend most of their time indoors, we hypothesized that weather-related arrhythmia risk would be better estimated using an indoor measure or an outdoor measure that correlates well with indoor conditions, such as absolute humidity. The clinical records of 203 patients in eastern Massachusetts, USA, with an implantable cardioverter-defibrillator were abstracted for arrhythmias between 1995 and 2002. We used case-crossover methods to examine the association between weather and ventricular arrhythmia (VA). Among 84 patients who experienced 787 VAs, lower estimated indoor temperature (odds ratio (OR) = 1.16, 95% confidence interval (CI) 1.05–1.27 for a 1 °C decrease in the 24-h average) and lower absolute humidity (OR = 1.06, 95% CI 1.03–1.08 for a 0.5 g/m3 decrease in the 96-h average) were associated with increased risk. Lower outdoor temperature increased risk only in warmer months, likely attributable to the poor correlation between outdoor and indoor temperature during cooler months. These results suggest that lower temperature and drier air are associated with increased risk of VA onset among implantable cardioverter-defibrillator patients.
Keywords: arrhythmia, humidity, implantable cardioverter-defibrillator, temperature, weather
INTRODUCTION
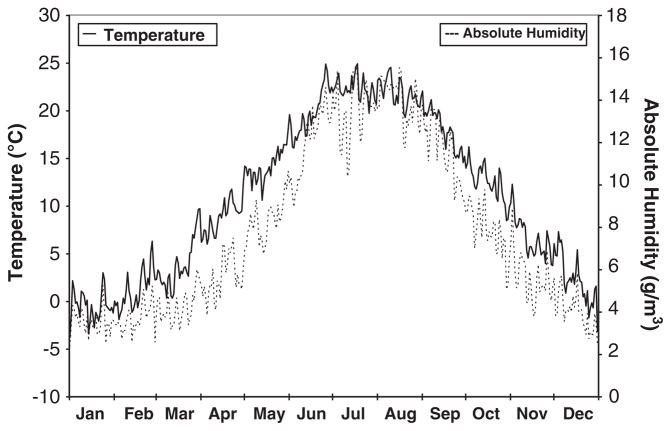
In temperate climates, cardiovascular diseases, such as cardiac arrhythmias,1–4 sudden cardiac death (SCD),5 and death due to ischemic heart disease,6 follow a seasonal pattern and peak in the wintertime. The mechanism for this seasonality is not understood. One proposed explanation is the seasonal pattern of outdoor weather,5,7,8 as the coldest and driest conditions occur during winter months in temperate climates (Figure 1). Several studies have examined the association between outdoor ambient weather conditions and ventricular arrhythmia (VA) with mixed results. Studies have reported positive9,10 and negative1 as well as linear and J-shaped11 associations with outdoor temperature.
Figure 1.
Seasonal variation of outdoor ambient weather. Plot of the average daily outdoor temperature and absolute humidity from measurements taken August 1995–June 2002 at Boston Logan International Airport measured by the National Weather Service.
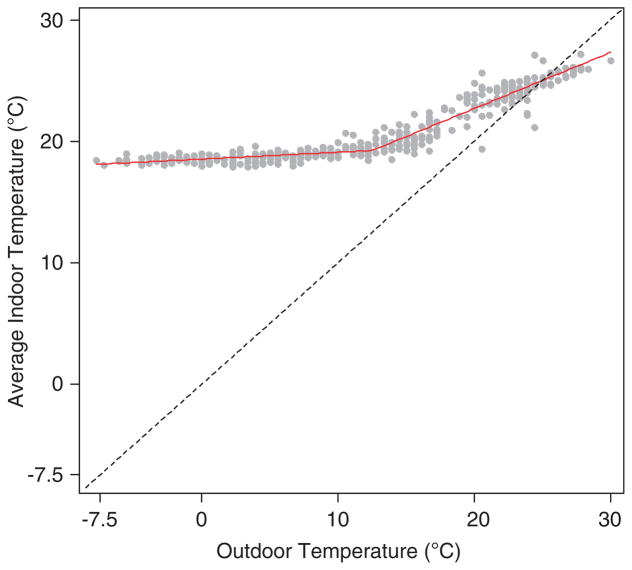
Studies of how weather relates to health have generally considered only outdoor ambient temperature. However, there is often a very weak link between outdoor and indoor temperature because indoor temperature can be controlled by heating and cooling systems. In the Greater Boston, Massachusetts area, there is a weak correlation between outdoor and indoor temperatures when outdoor temperatures are low (Figure 2). Because of this weak correlation at cooler temperatures, and because people in industrialized countries spend ~90% of their time indoors,12 we hypothesized that the association between weather and health would be better estimated using indoor measures, or an outdoor measure that correlates well with indoor conditions. Absolute humidity (AH), the mass of water vapor in a volume of air, is a measure of the actual water content of the air.13 Because AH is generally conserved as air moves indoors, it may be a better indicator of weather exposure than ambient outdoor temperature. In Boston, outdoor and indoor AH levels correlate highly year-round (r = 0.96) and also during the summer months of June–September (r = 0.90) when air conditioning use is expected to be highest (which lowers the moisture content of indoor air).14 A majority of the literature on humidity-related health concerns focus on how humidity influences human health indirectly through biological pollutants (e.g., dust mites, fungi, bacteria, viruses);15 few studies have examined whether humidity is directly related to adverse health outcomes.
Figure 2.
Scatterplot and piecewise regression line relating daily outdoor temperature measured at Boston Logan International Airport to average daily indoor temperature in 16 homes from January to December 2011, Greater Boston, MA, USA. Knot location is at 12.5 °C. For temperatures <12.5 °C, Pearson’s correlation coefficient, r = 0.61; β = 0.06, standard error (SE) = 0.008. For temperatures ≥12.5 °C, r = 0.91; β = 0.41, SE = 0.016. Dashed line, y = x (45°) line.
Implantable cardioverter-defibrillators (ICDs) are devices placed subcutaneously in patients at risk of VA and SCD. These devices continuously monitor the electrical activity of the heart and record VA occurrence. They detect arrhythmias based on beat-to-beat (R-R) intervals (i.e., heart rate), and will then treat the arrhythmias with anti-tachycardiac pacing or cardioverter shock. The occurrence of VA and the associated shocks in patients with ICDs is associated with increased mortality and heart failure hospitalizations despite effective termination of the arrhythmia.16,17 ICD shocks can also damage myocardial cells and induce sympathetic responses.16 Even if arrhythmias are promptly detected and appropriately treated, asystole and pulseless electrical activity can result.18 Thus, identifying triggers of arrhythmias is important for preventing additional morbidity and mortality associated with these events.
ICDs record the onset date and time of arrhythmic events and the associated electrocardiograms (ECGs), which allows for subsequent objective review of the detected cardiac arrhythmias. Using ICD records of the date and time of arrhythmic events, we examined the association between weather and ventricular tachyarrhythmias among patients with an ICD. We focused on three weather measures—outdoor ambient temperature, estimated indoor temperature, and outdoor AH. In sensitivity analyses, we also examined the association with barometric pressure, dew point, and relative humidity.
METHODS
Study Population
The study population consisted of patients implanted with an ICD at Tufts Medical Center (Boston, Massachusetts) between 1 June 1995 and 31 December 1999. These patients were followed until their last clinic visit before 15 July 2002. Date of birth, race/ethnicity, clinic visit dates, prescribed medications (i.e., β-blockers, digoxin, and other anti-arrhythmic medications) and residential zip code were abstracted from patient records. Patients with residential zip codes of >40 km from the air pollution monitoring station at the Harvard School of Public Health were excluded. The Harvard School of Public Health Human Subjects Committee and the Tufts Medical Center Institutional Review Board approved the study.
Outcome
The date and time of ICD-detected arrhythmias were abstracted from patient records, and recorded ECGs and R-R intervals were printed. An electrophysiologist reviewed the associated ECGs and classified the events according to onset rate, regularity, QRS morphology during and before the episode, duration, and response to therapy. Arrhythmias were classified as ventricular tachycardia, ventricular fibrillation, sinus tachycardia, atrial flutter, atrial fibrillation, supraventricular tachycardia, or noise/oversensing. The current analyses were restricted to VAs, defined as ventricular tachycardia and ventricular fibrillation, separated by at least 1 h from the previous arrhythmia. VAs that occurred within 2 weeks following implantation and during inpatient hospital stays were excluded.
Outdoor Weather and Air Pollution Data
Hourly measurements of outdoor temperature, barometric pressure, dew point, and relative humidity reported at Boston Logan International Airport were extracted from National Weather Service records. AH concentrations (g/m3) were calculated from the dew point and temperature using the following formulas:19,20
where e is actual vapor pressure (mb or hPa), Td is dew point (°C), T is temperature (°C), and Rw is the gas constant for water vapor (461.5 J/kg × K).
We adjusted for two air pollutants–particulate matter <2.5 μm in aerodynamic diameter (PM2.5) and ozone—previously found to be associated with VA in this cohort.21 Hourly ambient concentrations of ozone measured at six sites in the greater Boston area were obtained from the Massachusetts Department of Environmental Protection. We calculated the average hourly ozone concentration using all reporting monitors for that hour. Concentrations of PM2.5 were measured using a tapered element oscillating microbalance (model 1400A, Rupprecht and Patashnick, East Greenbush, New York, USA) in south Boston from 1 April 1995 to 20 January 1998, and at the Harvard School of Public Health from 16 March 1999 to 31 July 2002. The PM2.5 values were imputed for the hours (12,204, or 20.1%) when measurements were missing. The imputed values were generated from a regression model that included long-term time trend, day of the week, hour of the day, temperature, relative humidity, barometric pressure, and nitrogen dioxide as predictors (cross-validated R2 = 0.72).
Estimated Indoor Temperature
A separate indoor environment study was carried out in the Boston area to characterize the relationship between indoor and outdoor temperature. The living room temperature was measured in 16 homes located an average of 20.3 km from the Boston Logan airport (range: 6.8 to 42.5 km) from January to December 2011. Further details on the design and homes participating in this study are available elsewhere.14 None of the ICD patients lived in the homes that participated in the indoor environment study. Temperature was measured continuously using HOBO U8 (accuracy of ±0.7 °C at 21 °C) and HOBO U12 (accuracy of ±0.35 °C from 0 °C to 50 °C) Data Loggers (Onset, Bourne, MA, USA). During warmer seasons, there is a strong linear relationship between outdoor and indoor temperature (Figure 2). This relationship is considerably weaker in the colder seasons. Using the relationship found in this survey, we estimated the 24-h moving average indoor temperature using the following equation (model R2 = 0.92):
where Tin is indoor temperature (°C), Tout is airport temperature (°C), and δT = 1 if Tout≥12.5 °C, otherwise δT = 0.
Statistical Analysis
We used case-crossover methods to examine the association between weather and incident VA. Individual arrhythmic events were modeled as separate strata in conditional logistic regression analyses. The time of arrhythmia onset was rounded to the nearest hour and linked to the corresponding hourly weather and air pollution value. Referent periods (3 to 4) were chosen using a time-stratified approach and matched to the case period on the hour of the day, day of the week, and calendar month. This approach intrinsically adjusts for confounding by season, month, day of the week, all interactions between these variables, time trend in the exposure, circadian patterns, and avoids the overlap bias that would be induced if referent periods were conditional on the outcome.22,23 All statistical models adjusted for the 24-h moving average concentrations of PM2.5 and ozone as linear terms.
We first examined moving averages of the three weather measures as linear terms. For outdoor temperature and AH, we examined various lags up to 168 h (i.e., 7 days) before the arrhythmia. For estimated indoor temperature, we examined only the 24-h moving average because the formula provided above applies only to 24-h averages. Moving averages were created if at least 75% of the hourly measurements were available. Otherwise, the exposure was considered missing. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported for a temperature decrease of 1 °C and an AH decrease of 0.5 g/m3 (at 50% relative humidity, a 1 °C drop in temperature from 20 °C to 19 °C lowers AH by 0.494 g/m3). Estimating indoor temperature exposure directly from the outdoor temperature likely underestimates the true variability in exposure. This would result in standard errors that are too small in logistic regression models for indoor temperature. When a tractable variance formula does not exist, bootstrapping may be used to obtain the CIs. To construct CIs for the association with estimated indoor temperature, we generated 2000 bootstrap samples, each formed by resampling 787 strata with replacement from the original data. We ran a conditional logistic regression on each of the 2000 bootstrap samples and took the 2.5th and 97.5th percentiles as the lower and upper CI for the β estimate. All statistical tests were two sided.
We assessed for non-linear exposure–response relationships using penalized regression splines. The number of degree of freedom (d.f.) of the spline was initially chosen to minimize the Generalized Cross Validation criterion, a measure of the model’s goodness of fit. If the best fit model resulted in less smoothed curves than biologically plausible, we assigned 3 d.f. to the penalized spline.24 Only the outdoor temperature association appeared non-linear. To quantify this association, we fit a piecewise linear model, allowing for one knot. To determine the knot location, we fit models placing the knot between 0 and 10 °C in increments of 0.25 °C and used the model-specific log likelihoods to determine the best fitting model.
We examined potential effect modification by gender, race (white vs non-white), age (<65 years vs ≥65 years), diagnosis of coronary artery disease (CAD), low pre-implantation ejection fraction (<35% vs ≥35%), prescription of β-blockers, digoxin, and other anti-arrhythmic medications (i.e., amiodarone, mexiletine, quinidine, or sotalol), duration of the arrhythmia (>30 s vs ≤30 s), the total number of events during follow-up (<30 vs ≥30), and the warm (May–September) vs cold (November–March) season. For each arrhythmic event, prescription drug status was assigned based on a report of prescriptions recorded at the patient’s most recent clinic follow-up visit. As AH25–28 and temperature29 have been linked to the influenza virus, we assessed modification by influenza activity. As an individual can experience multiple arrhythmias during a season but only one influenza infection, we rejected the possibility that influenza is an intermediate between weather and arrhythmias (i.e., weather may influence influenza, which then may trigger arrhythmias). We created an influenza activity score using 1997–2002 weekly surveillance data on influenza A (H1N1 and H3N2) and influenza B virus activity for the New England region.30 The score was created using the following formula:
Flu activity score = Σstrain (% weighted physician visits for influenza-like illness × (number of isolates for a specific strain/all positive isolates) × ( total number of positive isolates/total number tested)).
We defined weeks as having high influenza activity when the weekly score was at the 20th percentile or above of the non-zero scores. Effect modification was assessed using an interaction term between the exposure and potential modifier, and ORs and CIs were obtained from stratified analyses.
In sensitivity analyses, we examined the associations for barometric pressure, dew point, and relative humidity. Barometric pressure levels are very similar indoors and outdoors, and hence outdoor measurements are likely a very good proxy for indoor exposure. Dew point is analogous to absolute humidity and is the most common measure reported by meteorologists to evaluate the actual amount of moisture in the air. In contrast to absolute humidity and temperature, which display a clear seasonal pattern of lows in the winter and highs in summer both indoors and outdoors, outdoor relative humidity fluctuates with no consistent pattern, whereas the indoor levels are generally lowest during the winter.14 Therefore, outdoor relative humidity is likely a poor indicator of indoor exposure, and associations for outdoor relative humidity are difficult to interpret.
SAS version 9.2 (SAS Institute, Cary, NC, USA) was used to construct data sets and to calculate descriptive statistics. R version 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical modeling.
RESULTS
Among 203 patients enrolled in the study, VAs were detected in 84 patients during follow-up. These 84 patients were followed an average of 3.5 years (range: 5 weeks, 6.9 years) and experienced 798 VAs separated by at least 1 h from the previous event. After dropping events with missing exposure or covariate information, analyses were confined to 787 VAs among 84 patients.
Patients with confirmed VAs were predominantly white males (Table 1) with an average age of 64 years (range: 19–90). The most common implantation diagnosis was CAD, and 55 (65%) had a pre-implantation ejection fraction of <35%. At the first clinic visit, over half of the patients were on prescribed β-blockers or digoxin. Most of the events (78%) were episodes of sustained ventricular tachycardia. In all, 19 patients (23%) experienced one VAs, 23 (27%) experienced between 4 and 9 VAS, and 24 (29%) experienced ≥10 VAs during follow-up.
Table 1.
Characteristics of 84 patients with 787 confirmed ventricular arrhythmias, Boston Implantable Cardioverter Defibrillator Study, 1995–2002.
| Characteristic |
Patients
|
Arrhythmias
|
||
|---|---|---|---|---|
| No. | %a | No. | % a | |
| Male gender | 66 | 79% | 671 | 85% |
| Race/ethnicity | ||||
| Caucasian | 70 | 83% | 670 | 85% |
| African American | 4 | 5% | 45 | 6% |
| Hispanic | 7 | 8% | 54 | 7% |
| Asian | 1 | 1% | 11 | 1% |
| Unknown | 2 | 2% | 7 | 1% |
| Age (years) at first event | ||||
| <55 | 17 | 20% | 74 | 9% |
| 55–64 | 21 | 25% | 172 | 22% |
| 65–74 | 29 | 35% | 378 | 48% |
| ≥75 | 17 | 20% | 163 | 21% |
| Diagnosis at implantation | ||||
| Coronary artery disease | 62 | 74% | 689 | 88% |
| Idiopathic cardiomyopathy | 12 | 14% | 52 | 7% |
| Hypertrophic cardiomyopathy | 1 | 1% | 1 | 0.1% |
| Long QT syndrome | 1 | 1% | 11 | 1% |
| Arrhythmogenic right valve dysplasia | 2 | 2% | 8 | 1% |
| Mitral valve prolapse | 1 | 1% | 1 | 0.1% |
| Congenital heart disease | 1 | 1% | 2 | 0.3% |
| Normal | 4 | 5% | 23 | 3% |
| Ejection fraction (%) | ||||
| <25 | 23 | 27% | 207 | 26% |
| 25–34 | 32 | 38% | 297 | 38% |
| 35–49 | 16 | 19% | 221 | 28% |
| ≥50 | 13 | 15% | 62 | 8% |
| Prescribed β-blockers | 57 | 68% | 458 | 58% |
| Prescribed digoxin | 43 | 53% | 471 | 61% |
| Prescribed other antiarrhythmic agents | 21 | 25% | 192 | 24% |
| Type of arrhythmiab | ||||
| Ventricular tachycardia | ||||
| Sustained | 58 | 69% | 613 | 78% |
| Non-sustained (≤30 s) | 39 | 46% | 86 | 11% |
| Ventricular fibrillation | ||||
| Sustained | 20 | 24% | 63 | 8% |
| Non-sustained | 11 | 13% | 25 | 3% |
| No. of events per patient | ||||
| 1 | 19 | 23% | 19 | 2% |
| 2 | 10 | 12% | 20 | 3% |
| 3 | 8 | 10% | 24 | 3% |
| 4–9 | 23 | 27% | 119 | 15% |
| 10–29 | 17 | 20% | 253 | 32% |
| ≥30 | 7 | 8% | 352 | 45% |
Percentages may not sum to 100% because of rounding.
The number of patients does not sum to 84 because some patients experienced multiple types of arrhythmia.
Table 2 presents the daily weather and air pollution distribution in Boston during the study period, as well as the estimated daily average indoor temperature during this time. Event days were, on average, slightly colder and drier than reference days (by 0.5 °C for temperature, 0.1 °C for estimated indoor temperature, 0.3 g/m3 for AH, 0.6 °C for dew point, and 7.1% for relative humidity). The average barometric pressure was the same for event and reference days. Within matched sets, outdoor ambient temperature was moderately correlated with AH (Table 3). Correlations between the weather and air pollution variables were modest to low (r’s ≤0.51; Table 3).
Table 2.
Daily weather and air pollution profile, Boston Implantable Cardioverter Defibrillator Study, August 1995–June 2002.
| Parameter | No. of days | Min | 25th percentile | 50th percentile | 75th percentile | Max |
|---|---|---|---|---|---|---|
| Outdoor temperature (°C) | 2526 | −14.2 | 3.6 | 10.7 | 18.5 | 31.9 |
| Estimated indoor temperature (°C) | 2526 | 17.7 | 18.8 | 19.2 | 22.1 | 28.4 |
| Absolute humidity (g/m3) | 2526 | 0.69 | 4.1 | 6.9 | 11.1 | 21.1 |
| Barometric pressure (hPa) | 2526 | 981.7 | 1008.6 | 1014.1 | 1019.4 | 1041.9 |
| Dew point (°C) | 2526 | −24.7 | −2.4 | 5.1 | 12.8 | 23.7 |
| Relative humidity (%) | 2526 | 24.0 | 56.9 | 69.2 | 81.8 | 100 |
| PM2.5 (μg/m3) | 2524 | 1.5 | 7.2 | 10.2 | 14.7 | 53.2 |
| Ozone (p.p.b.) | 2525 | 1.2 | 15.2 | 22.6 | 30.9 | 77.5 |
Abbreviations: PM2.5, particulate matter <2.5 μm in aerodynamic diameter; p.p.b., parts per billion.
Table 3.
Pearson’s correlation coefficients for the matched differences in 24 h weather and air pollution levels in Boston, Massachusetts, August 1995–June 2002.
| Toutdoor | Tindoor | AH | BP | Dew point | RH | PM2.5 | Ozone | |
|---|---|---|---|---|---|---|---|---|
| Toutdoor (°C) | — | 0.76 | 0.65 | − 0.10 | 0.76 | 0.06 | 0.37 | 0.22 |
| Tindoor (°C) | — | 0.55 | − 0.15 | 0.45 | − 0.14 | 0.45 | 0.44 | |
| AH (g/m3) | — | − 0.14 | 0.89 | 0.64 | 0.51 | 0.23 | ||
| BP (hPa) | — | − 0.11 | − 0.07 | 0.03 | − 0.10 | |||
| Dew point (°C) | — | 0.69 | 0.43 | 0.08 | ||||
| RH (%) | — | 0.24 | − 0.11 | |||||
| PM2.5 (μg/m3) | — | 0.21 | ||||||
| Ozone (p.p.b.) | — |
Abbreviations: AH, absolute humidity; BP, barometric pressure; PM2.5, particulate matter <2.5 μm in aerodynamic diameter; p.p.b., parts per billion; RH, relative humidity; Tindoor, estimated indoor temperature; Toutdoor, outdoor temperature.
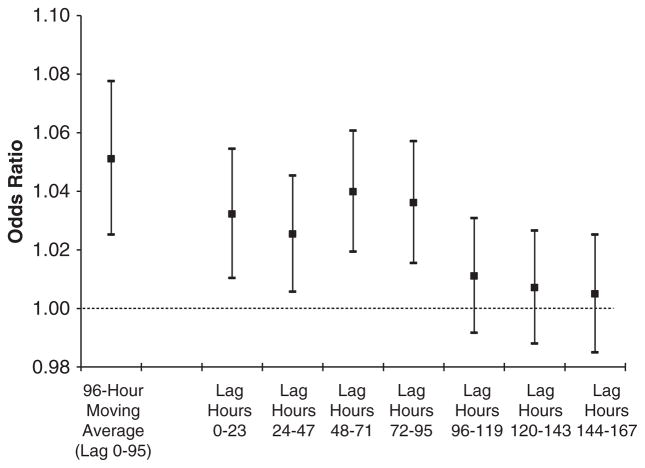
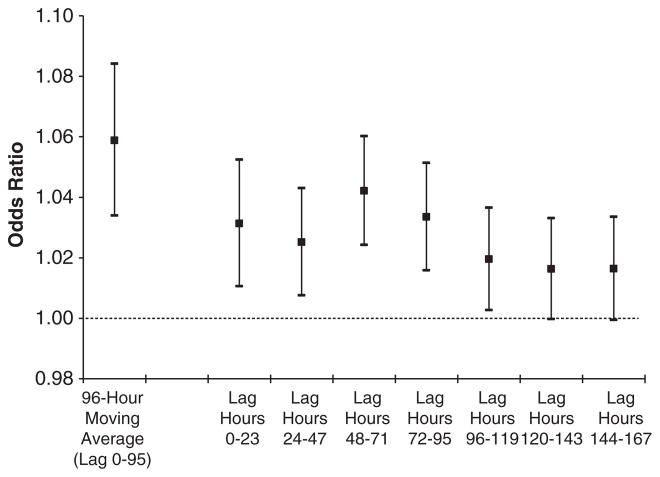
There were significant associations with lower outdoor temperature and lower AH for all moving averages in the previous week. When lag hours were examined in separate models, we observed associations up to 96 h (4 days) following exposure for both outdoor temperature (Figure 3) and AH (Figure 4). Lower estimated indoor temperature in the previous 24 h was also significantly associated with VA.
Figure 3.
Odds ratios for ventricular arrhythmias associated with a 1 °C decrease in outdoor temperature, Boston Implantable Cardioverter Defibrillator Study, 1995–2002. Odds ratios were estimated using separate models, adjusted for the 24 h moving average concentrations of particulate matter <2.5 μm in aerodynamic diameter (PM2.5), and matched on month, day of the week, and hour. Bars, 95% confidence interval.
Figure 4.
Odds ratios for ventricular arrhythmias associated with a 0.5 g/m3 decrease in outdoor absolute humidity, Boston Implantable Cardioverter Defibrillator Study, 1995–2002. Odds ratios were estimated using separate models, adjusted for the 24 h moving average concentrations of particulate matter <2.5 μm in aerodynamic diameter (PM2.5) and ozone, and matched on month, day of the week, and hour. Bars, 95% confidence interval.
We then examined the shape of the exposure–response relationships. For outdoor temperature and AH, we used the 96-h moving average, and for estimated indoor temperature, we used the 24-h moving average. Although a likelihood ratio test indicated that a linear fit was adequate (P = 0.53), graphical inspection of the outdoor temperature relationship (Figure 5a) suggested that a non-linear fit was more appropriate. Piecewise linear models indicated an adverse association with cooler temperatures at a threshold of ≥4.5 °C (OR = 1.09, 95% CI 1.03–1.16 for a 1 °C decrease in the 96-h average outdoor temperature; Table 4).
Figure 5.
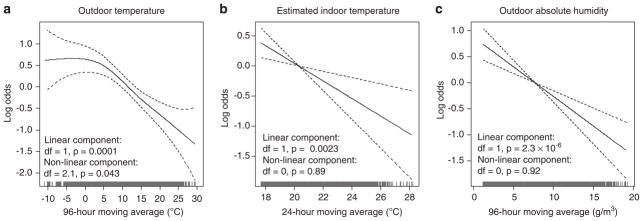
Log odds of ventricular arrhythmias associated with (a) outdoor temperature, (b) estimated indoor temperature, and (c) outdoor absolute humidity, Boston Implantable Cardioverter Defibrillator Study, 1995–2002. Plots were generated using penalized regression splines. Results are adjusted for the 24 h moving average concentrations of particulate matter <2.5 μm in aerodynamic diameter (PM2.5) and ozone, and matched on month, day of the week, and hour. Dashed lines, 95% confidence intervals.
Table 4.
Odds ratios for ventricular arrhythmias associated with a 1 °C decrease in outdoor temperature (96 h average), 1 °C decrease in estimated indoor temperature (24 h average), and 0.5 g/m3 decrease in outdoor absolute humidity (96 h average) among 84 patients (N = up to 787 arrhythmias), Boston Implantable Cardioverter Defibrillator Study, 1995–2002.
| Parameters included in model | ORa | 95% CI | P-value |
|---|---|---|---|
| Outdoor temperatureb | |||
| ≤4.5 °C | 1.00 | (0.96–1.05) | 0.90 |
| >4.5 °C | 1.08 | (1.02–1.14) | 0.01 |
| + PM2.5 | |||
| ≤ 4.5 °C | 1.00 | (0.96–1.05) | 0.90 |
| >4.5 °C | 1.08 | (1.02–1.14) | 0.01 |
| + Ozone | |||
| ≤4.5 °C | 1.00 | (0.96–1.04) | 0.98 |
| >4.5 °C | 1.09 | (1.03–1.16) | 0.004 |
| + PM2.5 + ozone | |||
| ≤4.5 °C | 1.00 | (0.96–1.04) | 0.999 |
| >4.5 °C | 1.09 | (1.03–1.16) | 0.004 |
| Estimated indoor temperature | 1.08 | (1.01–1.17) | 0.03 |
| + PM2.5 | 1.12 | (1.03–1.21) | 0.01 |
| + Ozone | 1.12 | (1.03–1.22) | 0.006 |
| + PM2.5 + ozone | 1.16 | (1.05–1.27) | 0.003 |
| Absolute humidity | 1.06 | (1.03–1.08) | 4.0 × 10−6 |
| + PM2.5 | 1.06 | (1.03–1.08) | 2.3 × 10−6 |
| + Ozone | 1.06 | (1.03–1.08) | 3.2 × 10−6 |
| + PM2.5 + ozone | 1.06 | (1.03–1.08) | 2.3 × 10−6 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Matched on month, day of the week, and hour.
The cutpoint for outdoor temperature was chosen based on model-specific goodness-of-fit tests.
The associations were linear for estimated indoor temperature and outdoor AH (Figures 5b and c). A 1 °C decrease in the 24-h average estimated indoor temperature increased risk by 16% (95% CI 5–27%). A decrease of 0.5 g/m3 in the 96-h average AH increased risk by 6% (95% CI 3–8%).
There was a stronger association with AH among patients with CAD (OR = 1.07, 95% CI 1.04–1.09) compared with patients without CAD (OR = 1.00, 95% CI 0.94–1.07; P for interaction = 0.07). Otherwise, there was no significant evidence of effect modification of the AH association by patient characteristics, arrhythmia characteristics, medical history, season, or influenza (all P-values for interaction >0.25, data not shown).
For barometric pressure, none of the moving averages were significant, and only lag hours 96–119 were negatively associated with VAs. All moving averages of dew point were significantly negatively associated with VAs; in separate lag analyses, dew point exposure up to 96 h before the event was significant. The 3-day through 7-day moving averages of relative humidity were significantly negatively associated with VAs. However, in lag analyses, only the previous 48–71 h were significantly associated, suggesting that the moving average associations were driven by these lag hours.
Ozone was marginally associated with VA in the fully adjusted models for outdoor temperature (P = 0.08) and estimated indoor temperature (P = 0.08), but not in the AH model (P = 0.35). PM2.5 was not associated with VA in any of the models (all P-values >0.23).
DISCUSSION
In this cohort of patients at high risk for VA, cooler and drier weather days before an event were associated with increased risk of VA. Lower indoor temperature and lower outdoor AH were linearly associated with increased risk. The association with outdoor temperature was non-linear, with higher temperatures associated with decreased risk of VA only for temperatures >4.5 °C. This non-linear association is likely attributable to the weak relationship between outdoor and indoor temperatures during colder months in Boston (Figure 2). We observed a stronger association for estimated indoor temperature than for outdoor temperature. This stronger health effect is likely because of decreased exposure misclassification, as people spend most of their time indoors.
VAs exhibit a seasonal pattern, peaking in the winter months.1,2 Winter weather is characterized by low temperatures and low AH. Previous studies have reported a correlation between low outdoor temperatures and ventricular tachyarrhythmias, but these studies were limited to univariate analyses.1,10,11 Koken et al.31 reported no association between outdoor maximum temperature and hospitalization for cardiac arrhythmias, but their study was restricted to the warm weather months of July and August. Čulić et al.9 reported positive associations between ventricular tachycardia and temperature, which conflicts with our findings. One possible explanation for the conflicting results is confounding by air pollution. Several studies have reported associations between air pollution exposure and the onset of VA,21,32,33 indicating that statistical analyses should consider and possibly adjust for confounding due to air pollution. Our study examined the association between weather conditions and VA while also adjusting for air pollution. McGuinn et al.34 also found a negative association between outdoor temperature and VA among ICD patients; adjusting for PM10, each 1 °C decrease in outdoor temperature increased risk by 1.8% (95% CI −0.5–4.1%). The authors focused on the cumulative effect summed over lags 0 to 7. For comparison, in our study, adjusting for PM2.5 and ozone, the 7-day moving average outdoor temperature (considered as a linear term) increased risk by 4.3% (95% CI 1.4–7.2%).
The physiologic adjustments made by the human body to preserve body temperature could increase cardiac work and sympathoadrenergic activation.11 Consistent with this hypothesis, norepinephrine and epinephrine levels in plasma and urine, markers for a sympathetic reaction in the nervous system, are higher in winter,35 and air temperature has been associated with heart rate variability measures.36,37 Cold weather conditions could contribute to the occurrence of arrhythmias through activation of both the sympathetic nervous system and the coagulation system. Vasoconstriction induced by cold weather increases blood pressure, heart rate, and left ventricular end-diastolic pressure and volume. These changes increase heart workload and may reduce the ischemic threshold, especially among individuals with compromised coronary circulation,38,39 and in turn trigger arrhythmia onset. This mechanism may explain why we observed increased sensitivity to drier air among patients with CAD as compared with patients without CAD.
The clinical relevance of our study likely extends beyond patients with an ICD. Many patients who have indications for an ICD for primary or secondary prevention do not receive one.40 In addition, therapies initiated in response to arrhythmias may cause adverse effects. For example, amiodarone is a common pharmacological option in patients with significant structural heart disease, but is known to have non-cardiac toxicities and is associated with increased mortality in patients with class III heart failure who do not have ICDs.16 More broadly, although left ventricular ejection fraction is currently the main risk stratification tool used to select patients for ICD therapy,41 the majority of SCD occurs in individuals with no known cardiac disease,42,43 with ventricular fibrillation as the major underlying mechanism.40
Several potential limitations of this study should be noted. Study participants likely spent the majority of their time indoors, and hence outdoor weather conditions may be a poor indicator of actual exposure.14 Non-differential misclassification of exposure is therefore of concern in our study and likely attenuated the associations toward the null. We did not have air conditioning information for the ICD patients, so the estimates of indoor temperature may be prone to measurement error. The resulting error is expected to be small; in the sample of 16 homes from which the equation was derived, the predicted indoor temperature was, on average, only 0.1 °C higher for homes with any air conditioning and only 0.2 °C lower for homes with no air conditioning. The equation used to estimate indoor temperature was based on living room measurements, but people spend their time in other environments (e.g., indoor offices, outdoors, other rooms in the home), resulting in exposure misclassification. Differences in air conditioning usage between the ICD patients and the occupants of the 16 homes in the indoor environment study might also increase the likelihood and magnitude of measurement error. Despite these limitations, we think estimated indoor temperature better reflects the variation in temperatures actually experienced than does outdoor temperature. The day-today temperature variation is expected to be much larger than the temperature variation between indoor environments. The case-crossover design inherently adjusts for characteristics that do not vary or vary slowly over time (e.g., gender, age, socioeconomic status), but confounding by factors that change over time may still occur. Confounding by the presence of transient myocardial ischemia may be possible, but unfortunately data on ECG markers of myocardial ischemia, such as the presence of ST-segment depression, were not available. The results of this study may not be applicable to regions that have a smaller range of outdoor temperature and humidity, especially subtropical and tropical areas, or to areas with different patterns of air conditioning and heating use. However, examination of these associations in other climatic regions would be interesting and could help clarify the mixed results reported for weather-related arrhythmia risk.
In a cohort of patients at high risk for VA, cooler and drier conditions were associated with increased risk of VA. These findings support the hypothesis that the seasonality of VA is partly attributable to the weather. As extreme weather patterns become more common,44,45 a better understanding of how weather affects health will be necessary to develop strategies to reduce morbidity and mortality. In future studies of weather and health, investigators should consider examining how outdoor absolute humidity and, when possible, indoor conditions relate to human health.
Acknowledgments
This study was funded in part by grants from the Health Effects Institute (grant 98-14), the National Institute of Environmental Health Sciences (NIEHS) (grants ES09825 and R21ES020194), and the Harvard-NIEHS Center for Environmental Health (NIEHS ES00002). Particulate air pollution measurements at the Harvard School of Public Health were funded in part by the Environmental Protection Agency (EPA)-Harvard Center on Ambient Particle Health Effects (EPA grants R827353 and RD83479801), and measurements in South Boston were funded by contracts with the Boston Edison and Gillette companies (Boston, MA, USA). JLN received support from the Benjamin Greely Ferris, Jr. Fellowship Fund, and NIEHS grant T32ES007069. JS received support from NIEHS R21ES020695 and NIA R21AG040027. We thank the fellows and researchers who abstracted the ICD data. We especially thank David Q. Rich, who organized the abstraction of data and processed these data.
ABBREVIATIONS
- AH
absolute humidity
- CAD
coronary artery disease
- d.f
degree of freedom
- ECG
electrocardiogram
- ICD
implantable cardioverter-defibrillator
- PM2.5
particulate matter <2.5 μm in aerodynamic diameter
- SCD
sudden cardiac death
- VA
ventricular arrhythmia
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Anand K, Aryana A, Cloutier D, Hee T, Esterbrooks D, Mooss AN, et al. Circadian, daily, and seasonal distributions of ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Am J Cardiol. 2007;100:1134–1138. doi: 10.1016/j.amjcard.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Muller D, Lampe F, Wegscheider K, Schultheiss HP, Behrens S. Annual distribution of ventricular tachycardias and ventricular fibrillation. Am Heart J. 2003;146:1061–1065. doi: 10.1016/S0002-8703(03)00426-5. [DOI] [PubMed] [Google Scholar]
- 3.Frost L, Johnsen SP, Pedersen L, Husted S, Engholm G, Sorensen HT, et al. Seasonal variation in hospital discharge diagnosis of atrial fibrillation: a population-based study. Epidemiology. 2002;13:211–215. doi: 10.1097/00001648-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe E, Kuno Y, Takasuga H, Tong M, Sobue Y, Uchiyama T, et al. Seasonal variation in paroxysmal atrial fibrillation documented by 24-hour holter electrocardiogram. Heart Rhythm. 2007;4:27–31. doi: 10.1016/j.hrthm.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, 1979 to 2002. J Am Coll Cardiol. 2006;48:287–292. doi: 10.1016/j.jacc.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 6.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 7.Mercer JB. Cold—an underrated risk factor for health. Environ Res. 2003;92:8–13. doi: 10.1016/s0013-9351(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 8.Dilaveris P, Synetos A, Giannopoulos G, Gialafos E, Pantazis A, Stefanadis C. CLi-mate Impacts on Myocardial infarction deaths in the Athens TErritory: the CLIMATE study. Heart. 2006;92:1747–1751. doi: 10.1136/hrt.2006.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Čulić V, Eterovic D, Miric D, Giunio L, Lukin A, Fabijanic D. Triggering of ventricular tachycardia by meteorologic and emotional stress: protective effect of betablockers and anxiolytics in men and elderly. Am J Epidemiol. 2004;160:1047–1058. doi: 10.1093/aje/kwh335. [DOI] [PubMed] [Google Scholar]
- 10.Pimental M, Grüdtner L, Zimerman LI. Seasonal variation of ventricular tachycardia assessed by 24-hour holter monitoring. Arq Bras Cardiol. 2006;87:362–365. doi: 10.1590/s0066-782x2006001700002. [DOI] [PubMed] [Google Scholar]
- 11.Fries RP, Heisel AG, Jung JK, Schieffer HJ. Circannual variation of malignant ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators and either coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1194–1197. doi: 10.1016/s0002-9149(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe P, Martinac I. Indoor climate and air quality: review of current and future topics in the field of ISB study group 10. Int J Biometeorol. 1998;42:1–7. doi: 10.1007/s004840050075. [DOI] [PubMed] [Google Scholar]
- 13.Ahrens CD. Meteorology Today: An Introduction to Weather, Climate, and the Environment. West Publishing Company; St. Paul, MN: 1991. [Google Scholar]
- 14.Nguyen JL, Schwartz J, Dockery DW. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air. 2013 doi: 10.1111/ina.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baughman A, Arens EA. Indoor humidity and human health—Part I: Literature review of health effects of humidity-influenced indoor pollutants. ASHRAE Trans. 1996;102(Pt. 1):193–211. [Google Scholar]
- 16.Stevenson WG, John RM. Ventricular arrhythmias in patients with implanted defibrillators. Circulation. 2011;124:e411–e414. doi: 10.1161/CIRCULATIONAHA.111.064816. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LR, Daubert JP, Thomas KL. Expanding the benefits of implantable cardioverter-defibrillator therapy: “is less more”? Prog Cardiovasc Dis. 2012;54:372–378. doi: 10.1016/j.pcad.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Pires LA, Lehmann MH, Steinman RT, Baga JJ, Schuger CD. Sudden death in implantable cardioverter-defibrillator recipients: clinical context, arrhythmic events and device responses. J Am Coll Cardiol. 1999;33:24–32. doi: 10.1016/s0735-1097(98)00519-1. [DOI] [PubMed] [Google Scholar]
- 19.National Weather Service Western Region Headquarters. [Accessed June 15, 2011];Vapor pressure. http://www.wrh.noaa.gov/slc/projects/wxcalc/formulas/vaporPressure.pdf.
- 20.National Aeronautics and Space Administration. Equations for the determination of humidity from dewpoint and psychrometric data. Washington, DC: National Aeronautics and Space Administration; 1977. [Accessed June 15, 2011]. NASA Technical Note D-8401. http://www.nasa.gov/centers/dryden/pdf/87878main_H-937.pdf. [Google Scholar]
- 21.Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- 22.Lumley T, Levy D. Bias in the case-crossover design: implication for studies of air pollution. Environmetrics. 2000;11:689–704. [Google Scholar]
- 23.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 25.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci USA. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8:e1000316. doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoji M, Katayama K, Sano K. Absolute humidity as a deterministic factor affecting seasonal influenza epidemics in Japan. Tohoku J Exp Med. 2011;224:251–256. doi: 10.1620/tjem.224.251. [DOI] [PubMed] [Google Scholar]
- 28.van Noort SP, Aguas R, Ballesteros S, Gomes MG. The role of weather on the relation between influenza and influenza-like illness. J Theor Biol. 2011;298C:131–137. doi: 10.1016/j.jtbi.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. [Accessed November 20, 2011];United States surveillance data: 1997–1998 through 2009–2010 seasons. http://www.cdc.gov/flu/weekly/ussurvdata.htm.
- 31.Koken PJ, Piver WT, Ye F, Elixhauser A, Olsen LM, Portier CJ. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in Denver. Environ Health Perspect. 2003;111:1312–1317. doi: 10.1289/ehp.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljungman PL, Berglind N, Holmgren C, Gadler F, Edvardsson N, Pershagen G, et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–2901. doi: 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- 33.Rich DQ, Kim MH, Turner JR, Mittleman MA, Schwartz J, Catalano PJ, et al. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occup Environ Med. 2006;63:591–596. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuinn L, Hajat S, Wilkinson P, Armstrong B, Anderson HR, Monk V, et al. Ambient temperature and activation of implantable cardioverter defibrillators. Int J Biometeorol. 2012;57:655–662. doi: 10.1007/s00484-012-0591-1. [DOI] [PubMed] [Google Scholar]
- 35.Schneider A, Schuh A, Maetzel FK, Ruckerl R, Breitner S, Peters A. Weather-induced ischemia and arrhythmia in patients undergoing cardiac rehabilitation: another difference between men and women. Int J Biometeorol. 2008;52:535–547. doi: 10.1007/s00484-008-0144-9. [DOI] [PubMed] [Google Scholar]
- 36.Ren C, O’Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am J Epidemiol. 2011;173:1013–1021. doi: 10.1093/aje/kwq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Lian Z, Liu Y. Heart rate variability at different thermal comfort levels. Eur J Appl Physiol. 2008;103:361–366. doi: 10.1007/s00421-008-0718-6. [DOI] [PubMed] [Google Scholar]
- 38.Abrignani MG, Corrao S, Biondo GB, Renda N, Braschi A, Novo G, et al. Influence of climatic variables on acute myocardial infarction hospital admissions. Int J Cardiol. 2009;137:123–129. doi: 10.1016/j.ijcard.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Meyer P, Guiraud T, Curnier D, Juneau M, Gayda M, Nozza A, et al. Exposure to extreme cold lowers the ischemic threshold in coronary artery disease patients. Can J Cardiol. 2010;26:e50–e53. doi: 10.1016/s0828-282x(10)70007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Estes NA. Predicting and preventing sudden cardiac death. Circulation. 2011;124:651–656. doi: 10.1161/CIRCULATIONAHA.110.974170. [DOI] [PubMed] [Google Scholar]
- 41.Haugaa KH, Edvardsen T, Amlie JP. Prediction of life-threatening arrhythmias— still an unresolved problem. Cardiology. 2011;118:129–137. doi: 10.1159/000327093. [DOI] [PubMed] [Google Scholar]
- 42.Malik M. Ventricular gradient and cardiac risk. Europace. 2011;13:605–607. doi: 10.1093/europace/eur095. [DOI] [PubMed] [Google Scholar]
- 43.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 44.Peterson TC, Zhang XB, Brunet-India M, Vázquez-Aguir JL. Changes in North American extremes derived from daily weather data. J Geophys Res. 2008;113:D07113. [Google Scholar]
- 45.Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, et al., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK, and New York, NY: 2007. [Google Scholar]