Abstract
This study aimed to develop a three dimensional culture platform for aggregates of human embryonic stem cell (hESC)-derived pancreatic progenitors that enables long-term culture, maintains aggregate size and morphology, does not adversely affect differentiation and provides a means for aggregate recovery. A platform was developed with poly(ethylene glycol) hydrogels containing collagen type I, for cell-matrix interactions, and peptide crosslinkers, for facile recovery of aggregates. The platform was first demonstrated with RIN-m5F cells, showing encapsulation and subsequent release of single cells and aggregates without adversely affecting viability. Aggregates of hESC-derived pancreatic progenitors with an effective diameter of 82 (15) μm were either encapsulated in hydrogels or cultured in suspension for 28 days. At day 14, aggregate viability was maintained in the hydrogels, but significantly reduced (88%) in suspension culture. However by day 28, viability was reduced in both culture conditions. Aggregate size was maintained in the hydrogels, but in suspension was significantly higher (~2-fold) by day 28. The ability to release aggregates followed by a second enzyme treatment to achieve single cells enabled assessment by flow cytometry. Prior to encapsulation, there were 39% Pdx1+/Nkx6.1+ cells, key endocrine markers required for β-cell maturation. The fraction of doubly positive cells was not affected in hydrogels but was slightly and significantly lower in suspension culture by 28 days. In conclusion, we demonstrate that a MMP-sensitive PEG hydrogel containing collagen type I is a promising platform for hESC-derived pancreatic progenitors that maintains viable aggregates, aggregate size, and progenitor state and offers facile recovery of aggregates.
Keywords: Poly(ethylene glycol) hydrogels, Human embryonic stem cells, Pancreatic precursor cells, Long-term culture platforms, Controlled release
1. Introduction
The dimensionality of culture platforms is increasingly being recognized as an important factor for the in vitro culture of cells [1–3]. In three dimensional (3D) culture, cells are often embedded within a material where they can migrate and experience cell-matrix interactions and cell-cell contacts in all directions. On the contrary, two dimensional (2D) cultures polarize these interactions, restricting movement and cell interactions to a single plane. The difference between 2D and 3D cultures is striking, leading to very different cell morphologies [4], gene expression profiles [5], and tissue production [6]. These differences have been highlighted in 3D cancer cell models [7], the maintenance of pluripotency for embryonic stem cells [8,9], and the differentiation of stem cells [10]. For these reasons, 3D culture platforms represent a promising and important approach to in vitro cell culture that better captures the native tissue environment.
In their native environment, β-cells are located within aggregates known as the islets of Langerhans, which are comprised of endocrine cells and extracellular matrix (ECM) molecules and are found embedded in pancreatic tissue. Within this 3D environment, β-cells experience cell-matrix and cell-cell interactions. In 3D cultures within a biomaterial, extracellular matrix (ECM) molecules can readily be incorporated to facilitate cell-matrix interactions. For β-cells isolated from islets and other insulin producing cells (e.g., pancreatic precursor cells and β-cell lines), the inclusion of ECM proteins has been shown to be important for their survival and insulin secretion [11]. For example, Mason et al. [12] showed that the incorporation of collagen into a poly(ethylene glycol) hydrogel supported differentiation of encapsulated rat pancreatic precursors into glucose responsive β-like cells. Culturing cells within aggregates in 3D culture platforms enables cell-cell contacts, which has also been shown to be important for survival and insulin secretion of β-cells and β-like cells [13], but depends on aggregate size [14]. There have been a few studies investigating 3D culture platforms for human embryonic stem cell (hESC) derived pancreatic precursors with the most common being suspension cultures (e.g., spinner flasks). While suspension cultures support cellular aggregates, they do not afford tight control over aggregate size and are not able to easily provide ECM cues. Alternative strategies such as microwells have been used to control aggregate size [15], but long-term culture and incorporating ECM molecules are more challenging. Given the clinical potential of hESC derived pancreatic precursors [16] and the recent evidence demonstrating for the first time the ability to achieve hESC derived insulin producing cells [17,18], there is a need to establish improved 3D culture platforms for hESC derived pancreatic precursors.
The goal of this study was therefore to develop a biomaterial-based 3D culture platform for aggregates of hESC-derived pancreatic progenitor cells, which meet the following criteria: (a) enable their long-term culture, (b) maintain aggregate size and morphology, (c) not adversely affect differentiation and (d) provide a means for aggregate recovery. The ability to recover aggregates enables this system to serve as a temporary 3D culture platform for in vitro differentiation from which aggregates could be recovered and subsequently analyzed or implanted following established clinical protocols. Previous works have demonstrated that hydrogels formed with protease sensitive crosslinks are promising for the culture and release of murine cardioprogenitor clusters [19] and MIN6 β-cell spheroids [20]. This study, therefore, builds off of this prior work, but for the clinically relevant cell population – hESC-derived pancreatic progenitor cells. A platform was chosen based on a poly(ethylene glycol) (PEG) hydrogel containing entrapped collagen type 1 [12] to support cell-matrix interactions and matrix metalloproteinase (MMP)-sensitive crosslinks to enable facile recovery of the encapsulated aggregates using an enzyme suitable for islet isolation. The utility of this hydrogel platform to recover encapsulated single cells and aggregates upon exposure to a collagenase enzyme blend was first demonstrated using the RIN-m5F cell line. Aggregates of hESC-derived pancreatic progenitor cells were then assessed for viability, aggregate size, and aggregate recovery and compared to aggregates grown in suspension culture. The recovered aggregates were subjected to a second enzyme treatment to obtain single cells, which were then used to evaluate the progenitor state by expression of key transcription factors associated with endocrine differentiation by flow cytometry. This study demonstrates that encapsulation in a MMP-sensitive PEG hydrogel is a promising platform for hESC derived pancreatic precursors and an improvement over suspension culture. Overall, the hydrogel maintained pancreatic progenitor markers similar to those in suspension culture, but led to improved long-term viability up to several weeks and maintained aggregate size, while allowing for the facile recovery of encapsulated aggregates at prescribed times through a cytocompatible enzymatic degradation of the hydrogel.
2. Materials and Methods
2.1 Macromolecular monomer (macromer) synthesis
Poly(ethylene glycol) (PEG) tetranorbornene was synthesized following previously established protocols [21,22]. Briefly, 4-arm PEG-NH2 (5000 Da, JenKemUSA) was dissolved in a sparing amount of dimethylformamide (Sigma) and combined with a 6 M excess of 5-Norbornene-2-carboxylic acid (Sigma) in the presence of 3 M excess of 2-(1H-7-Azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate methanaminium (AKSci) and N,N-Diisopropylethylamine (Sigma). The product, PEG tetranorbornene, was recovered by precipitation in ice-cold diethyl ether, dialyzed against de-ionized H2O, sterile filtered, and lyophilized. Functionalization of PEG with norbornene was determined using 1H NMR (Fig. S1) and confirmed to be ~100% (i.e., each arm of a PEG molecule was functionalized with a norbornene) by comparing the area under the alkene peaks for norbornene (6, 6.2 ppm) to the area under the peaks of the methyl groups in PEG (3.6 ppm).
2.2 Hydrogel formation and characterization
A precursor solution was prepared with 10% (g/g) PEG tetranorbornene and a bis-cysteine crosslinker (CVPLSLYSGC) (GenScript) at a 1:1 thiol:ene ratio, along with 0.05% (g/g) photoinitiator (Irgacure 2959), and 0.25 mg/ml rat tail collagen type 1 (Life Technologies) in phosphate buffer saline (PBS, at pH 7.4). Hydrogels were formed by polymerizing this solution with long-wave UV light (352 nm) at ~6 mW/cm2 for 6 minutes into cylinders that were ~2 mm in height and 4.5 mm in diameter. The tangent compressive modulus was determined for acellular hydrogels after a 24 hour free swelling period by subjecting samples to unconfined compression at a rate of 0.5 mm/min up to 15% strain (Synergie 100, 10 N; MTS). The mass swelling ratio was determined by measuring the mass of hydrated gels and dividing by the mass of the same sample after freeze-drying. Hydrogels were characterized by their degradation in a solution of 1.3 Wunsch units of Liberase TL (Roche) per mL PBS. Hydrogel wet weights were measured at 10 minute intervals until reverse gelation (i.e., solubilization of the gel). Distribution of collagen within the hydrogels was analyzed after embedding in freezing medium and sectioning without further processing. Sections (~40 μm) were incubated with a rabbit anti-rat collagen I (Abcam, 1:100) followed by incubation in goat anti-rabbit Alexa Fluor® 488 (1:300, Life Technologies). Images were acquired by confocal microscopy (Zeiss LSM 5 Pascal).
2.3 RIN-m5F cell culture, encapsulation and recovery
A rat insulinoma cell line RIN-m5F (ATCC® CRL-11605™) was cultured in RPMI 1640 media (Life Technologies) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologics), l-glutamine (1X) (Life Technologies), and penicillin streptomycin (1%) (Corning). RIN-m5f cells were cultured to ~90% confluency, aggregated on an orbital shaker (60 rpm) overnight in non-tissue culture treated plates, encapsulated in PEG hydrogels as described in section 2.2, cultured for 24 hours, and then released with Liberase TL at a concentration of 1.3 Wunsch units/mL until reverse gelation, which occurred in 45–60 minutes. Encapsulated and recovered cells were stained with the Live/Dead® Cell Viability Assay (Life Technologies) and imaged on a confocal microscope.
2.4 Differentiation of human embryonic stem cell (hESC) in to pancreatic precursor cells
Human embryonic stem cell (hESC)-derived pancreatic progenitors were generated in a monolayer format directly from hESCs grown on mouse embryonic fibroblasts (MEFs) (Nostro et al., submitted). The hESC line used was MEL1 PDX1:GFP (gift from Dr. Elefanty). To generate definitive endoderm, hESCs on MEFs were induced with 100ng/ml ActivinA (R&D Systems) and 1 μM CHIR 99021 for 1 day in RPMI supplemented with 2 mM glutamine (Gibco-BRL) and 4.5×10−4 M MTG (Sigma), followed by 100 ng/ml ActivinA, 1 μM CHIR 99021, and 2.5 ng/ml bFGF (R&D Systems) for 1 day in RPMI supplemented with glutamine and MTG as described previously, and with 0.5 mM ascorbic acid (Sigma). The medium was exchanged with 100 ng/ml ActivinA and 2.5 ng/ml bFGF for an additional day in RPMI supplemented with glutamine, MTG, and ascorbic acid. The day 3 endoderm population was next patterned for two days by culture in the presence of 50 ng/ml FGF10 and 250 nM KAAD-cyclopamine (Toronto Research Chemicals, ON, Canada) in RPMI supplemented with glutamine, MTG and 1% vol/vol B27 supplement (Invitrogen). At this stage, pancreatic progenitors were induced with 50ng/ml noggin, 50 ng/ml FGF10, 250 nM cyclopamine, 2 μM retinoic acid, and 50 ng/ml exendin4 for two days in DMEM supplemented with glutamine, ascorbic acid and B27. Following induction, the population was cultured in the presence of 50 ng/ml noggin, 50 ng/ml EGF, 1.2 μg/ml Nicotinamide, and 50 ng/ml exendin4 DMEM supplemented with glutamine, ascorbic acid and B27 to promote the development of PDX-1+NKX6.1+ progenitors (Nostro et al. submitted). The culture medium was changed every other day. Single cells were counted with a hemocytometer, reaggregated at day 13 of differentiation and shipped overnight to the University of Colorado.
2.5 hESC encapsulation and culture
Upon arrival aggregates were directly encapsulated in PEG based hydrogels at ~2×107 cells/mL of hydrogel precursor solution (approximately 800,000 cells per hydrogel). Cell number was estimated from pre-aggregated cultures as described in section 2.4 – with the assumption that the number of cells was equivalent to the number of single cells prior to aggregation – and referred to as encapsulated. Each hydrogel was immediately transferred to an individual well in a 24 well non-tissue culture treated plate with fresh media. Unencapsulated aggregates were transferred to a new well at a similar density of ~2×107 cells/mL of media (approximately 800,000 cells per well) in non-tissue culture treated plates and are referred to as - unencapsulated. This time point is referred to as “Day 0”. Medium consisted of high glucose DMEM (Life Technologies) with l-glutamine and pyruvate and supplemented with B27 (1X) (Life Technologies), additional l-glutamine (final concentration 6 mM) (Life Technologies), ascorbic acid (50 μg/ml) (Sigma), and penicillin streptomycin (1%) (Corning). Medium was exchanged every 2 days for up to four weeks.
2.6 hESC viability and aggregate size
At day 0, day 14, and day 28, encapsulated and unencapsulated aggregates were stained with the Live/Dead® Cell Viability Assay and imaged on a confocal microscope. Live cells were stained with calcein-AM, which is converted to green fluorescent calcein by intracellular esterases while dead cells with compromised cell membranes have nuclei labeled with red fluorescent ethidium homodimer. Images of entire aggregates were acquired using the same settings for all conditions. Images were acquired at 15 μm intervals within aggregates and compressed to a 2D image, resulting in some cases an appearance of over exposure. Due to the high cellularity of the aggregates, diffusion of calcein-AM dye into the central region of the aggregates was limited in some cases. After 14 and 28 days in culture, samples were harvested for DNA quantification. In brief, samples were placed in lysis buffer with 2-mercaptoethanol and stored at −80°C until further processing. Samples were thawed and homogenized. DNA was isolated using a DNA isolation kit (E.Z.N.A DNA/RNA Kit) and quantified using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Life Technologies) for double stranded DNA. Aggregate size was measured initially prior to encapsulation and at 14 and 28 days. For the encapsulated aggregates, hydrogels were digested with Liberase TL in media at a concentration of 1.3 Wunsch units/mL and aggregates recovered by centrifugation. Representative brightfield images were acquired of unencapsulated aggregates and those recovered from the hydrogels. An aggregate effective diameter was estimated using NIH image J software from aggregate area determined from the circumference of the aggregate measured at maximum width and assuming a circle.
2.7 Flow Cytometry
At day 14 and day 28, aggregates were released with Liberase TL as described in section 2.3. Unencapsulated aggregates and those aggregates harvested from the hydrogel were further dissociated into single cell suspensions by incubating in trypsin EDTA (Life Technologies) for 10 minutes followed by centrifugation at 2000 rpm for 2 minutes and a PBS rinse. Single cell suspensions were fixed in 10% buffered formalin for 30 minutes at room temperature followed by 90% methanol for 20 minutes on ice. Samples were incubated with mouse anti-human Nkx6.1 (BCBC Antibody Core) (1:1000) for 30 minutes and rat-anti mouse Alexafluor 647 (Life Technologies) (1:100) for 30 minutes prior to being analyzed on a BD FACScan System. The percentage of Pdx1 positive cells were determined using a GFP inserted the Pdx1 locus.
2.8 Statistical Analysis
All data are from three to five independent experiments and presented as the mean with standard deviation as error bars (n=3–5) in the figures or parenthetically in the text as mean (standard deviation). Each independent experiment was performed with two to three technical replicates. Statistical analysis was performed using a two-tailed student’s t-test. Where indicated, two-way analysis of variance with an α=0.05 was performed with encapsulation and culture time as the factors. P-values are provided in the text and p-values less than 0.10 are reported in the figures.
3. Results
3.1 Hydrogel Characterization
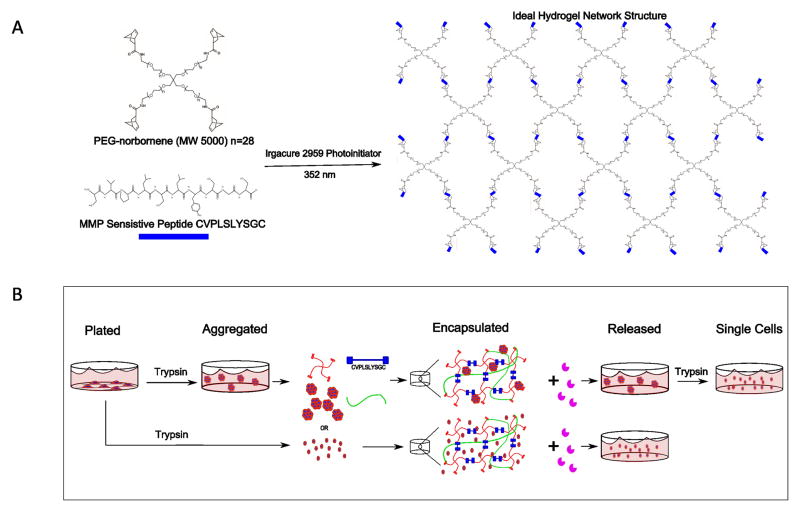
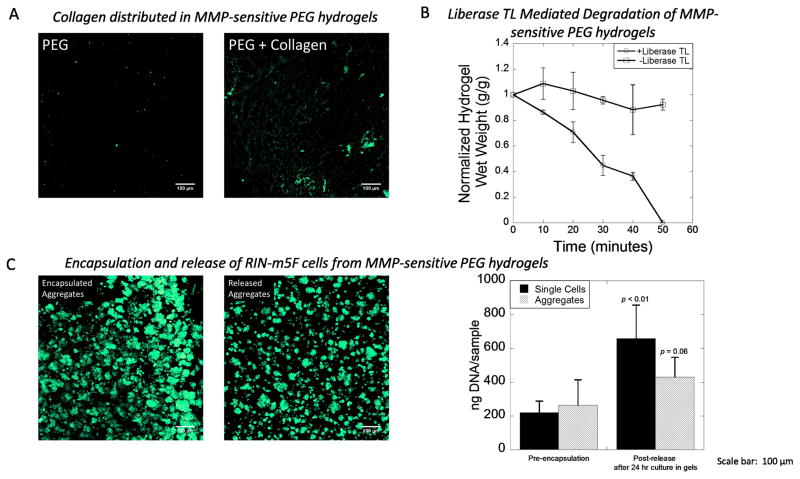
PEG hydrogels containing collagen type 1 and MMP-sensitive crosslinks were produced from a photoclickable thiol-ene polymerization (Fig. 1A) and used as a platform for encapsulation and subsequent release of single cells and aggregates (Fig. 1B). Collagen type 1 within the hydrogel was confirmed by immunostaining and confocal microscopy (Fig. 2A). Acellular hydrogels had an average mass swelling ratio of 11.7 and an average modulus of 35.3 kPa after 24 hours swelling. Hydrogel treatment with 1.3 Wunsch units/mL Liberase TL resulted in hydrogel degradation by proteolytic cleavage of the peptide crosslinks as measured by changes in wet weight and complete dissolution of the hydrogel within ~50 min (Fig. 2B). In contrast, hydrogels cultured without Liberase TL showed no change in wet weight over the same time period (Fig. 2B). RIN-m5F cells were used as a model cell to test the compatibility of this MMP-sensitive PEG hydrogel system for encapsulation of single cells and cell aggregates and their subsequent release. Single cells or cell aggregates were encapsulated and cultured for 24 hours in the hydrogels. Aggregates retained their viability and morphology after 24 hours in the hydrogel shown by predominantly live cells with no visible dead cells (Fig. 2C). The encapsulated cells and aggregates were released by treatment with Liberase TL. Immediately after being released, aggregates retained their viability and morphology (Fig. 2C). DNA quantification was utilized as a measure of cell number and determined immediately before encapsulation and immediately after release from the hydrogels (i.e., after being cultured for 24 hours in the hydrogel and then released by treatment with Liberase TL). DNA content was higher for single cells (p = 0.002) and aggregates (p = 0.06) after being released from the hydrogels indicating that the cells survive the encapsulation and continue to proliferate.
Figure 1.
A) Reaction scheme depicting the photoclickable polymerization between PEG tetra norbornene macromers and a bis-cysteine peptide (CVPLSLYSGC) crosslinker in the presence of a photoinitiator (Irgacure 2959) and 352 nm light to form a water swollen polymer network with MMP-sensitive crosslinks. B) Schematic of cellular encapsulation in and release from MMP-sensitive PEG hydrogels using Liberase TL for both single cells and aggregates.
Figure 2.
A) Visualization by confocal microscopy of entrapped collagen type 1 (green) within MMP-sensitive PEG hydrogels. No collagen was detected in PEG hydrogels without collagen. B) Hydrogel degradation in the presence and absence of Liberase TL as measured by hydrogel wet weight in the absence of cells. Data are presented as the mean with standard deviation as error bars, n = 3 technical replicates) C) Rat insulinoma (RIN-m5F) cells were used as a model cell demonstrating the encapsulation of single cells and cell aggregates and their subsequent release. Encapsulated aggregates were stained with a Live/Dead membrane integrity assay (live cells fluoresce green and dead cells fluoresce red) after a 24 hour encapsulation period. Release aggregates were stained with a Live/Dead membrane integrity assay immediate after release. DNA content was used as a measure of cell number for both single cells and aggregates before encapsulation and after a 24 hour encapsulation period and subsequent release. Data are presented as the mean with standard deviation as error bars, n = 6 technical replicates). Scale bar = 100 μm. P-values are compared to pre-encapsulation.
3.2 Viability of hESC derived pancreatic progenitor aggregates
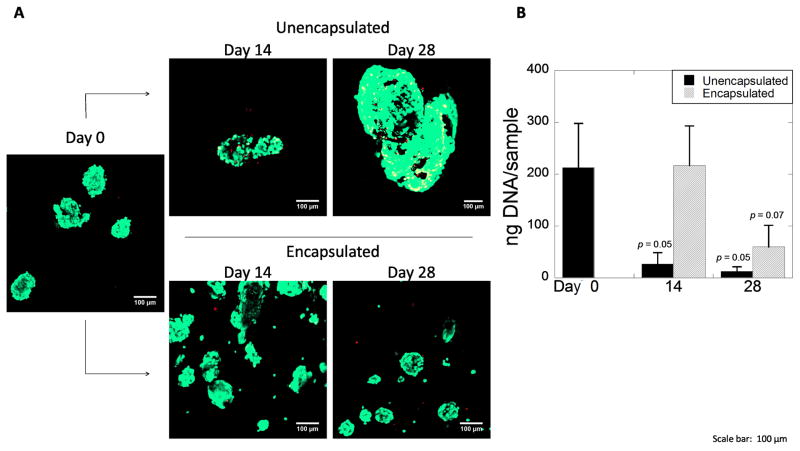
hESC derived pancreatic progenitors were either placed in suspension culture (i.e., unencapsulated) or encapsulated in the MMP-sensitive PEG hydrogels. At days 0, 14, and 28, viability and morphology of unencapsulated and encapsulated aggregates were qualitatively assessed using a membrane integrity assay (Fig. 3A). Initially, aggregates were predominantly viable. By day 14, unencapsulated aggregates formed larger mostly viable aggregates. By day 28, there appeared to be more dually stained cells at the periphery of the aggregates. In contrast, encapsulated aggregates appeared mostly viable at days 14 and 28. Cell number was quantified by total DNA content (Fig. 3B). Culture time (p=0.002) and encapsulation (p=0.018) affected total DNA content as determined by a two-way ANOVA. Total DNA content of the aggregates was initially 212 (85) ng per condition. For the unencapsulated aggregates, DNA content decreased (p = 0.055) by 88% at day 14 and decreased (p = 0.053) by 95% at day 28 when compared to day 0. On the contrary, DNA content at day 14 for the encapsulated aggregates was similar (p = 0.95) to day 0, but by day 28 dropped (p=0.07) by 75% when compared to day 0. Thus, 14 day encapsulated cells survive better than aggregates in suspension culture (p = 0.041), indicating that encapsulation benefits cell viability. For both conditions, a drop in overall cell number was observed by day 28.
Figure 3.
A) Confocal microscopy images of aggregates of hESC-derived pancreatic precursor cells stained with the Live/Dead membrane integrity assay prior to encapsulation at day 0 (left) and after 14 and 28 days for unencapsulated (top) and encapsulated (bottom) conditions. Scale bar = 100 μm. B) DNA content as a measure for cell number at days 0, 14, and 28 for unencapsulated and encapsulated aggregates. Data are presented as the mean with standard deviation as error bars for n=3 independent experiments. P-values are compared to day 0.
3.3 Maintenance of aggregate size
Aggregate size was quantified in the unencapsulated and encapsulated conditions by their distribution represented in histograms (Fig. 4A–C) and by an average effective diameter (Fig. 4D). Inset brightfield images show the appearance and morphology of the aggregates (Fig. 4A–C). For the encapsulated condition, the aggregates were first released from the hydrogel with Liberase TL and then characterized. At day 0, the average effective aggregate diameter was 82 (15) μm. Over the course of 28 days in suspension culture, the average effective diameter increased (p = 0.047) to 195 (38) μm and fewer total number of aggregates were present. However in the encapsulated condition, aggregates maintained a similar average effective diameter of 83 (8) μm at day 14 and 100 (10) μm at day 28 with a similar size distribution (Fig. 4D). There appeared, however, to be fewer aggregates at day 14 and day 28, when compared to the aggregates prior to encapsulation. Overall, aggregate size was maintained by encapsulation while unencapsulated aggregates further agglomerated leading to larger (p = 0.015) aggregates over time as determined by two-way ANOVA.
Figure 4.
Histograms (bin size = 10 μm) of aggregate size as measured by effective diameter at A) day 0 pre-encapsulation, B) day 28 for the unencapsulated aggregates, and C) day 28 for the encapsulated aggregates. Inset images are from brightfield microscopy. Scale bar = 100 μm. D) The average aggregate effective diameter at days 0, 14, and 28. Data are presented as the mean with standard deviation as error bars for n=3–4 independent experiments. P-values are compared to day 0.
3.4 Pancreatic progenitor transcription factors of hESC aggregates
The differentiation state of the aggregates was evaluated by flow cytometry to quantify the percentage of Pdx1+, Nkx6.1+, and Pdx1+/Nkx6.1+ cells in the unencapsulated and encapsulated aggregates (Fig. 5A). Initially, there were 59 (26)% Pdx1+ cells, 51 (19)% Nkx6.1+ cells and 39 (19)% Pdx1+/Nkx6.1+ cells. Overall, culture time and encapsulation did not affect the percentage of Pdx1+, Nkx6.1+, and Pdx1+/Nkx6.1+ cells as determined by two-way ANOVA. There was, however, a reduction (p = 0.03) in Nkx6.1+ and a reduction (p=0.01) in Pdx1+/Nkx6.1+ number of cells in the unencapsulated condition at day 28 when compared to day 0. There were no changes over 28 days in the levels of these markers in the encapsulated condition. Therefore, encapsulation preserved the identity of the pancreatic progenitors.
Figure 5.

The fraction of cells staining positive for the pancreatic precursor transcription factors as measured by flow cytometry analysis for A) PDX1+, B) NKX6.1+, and C) PDX1/NKX6.1+ cells at days 0, 14, and 28. Data are presented as the mean with standard deviation as error bars for n=3–5 independent experiments. P-values are compared to day 0.
4. Discussion
In this study, we demonstrate that a MMP-sensitive PEG hydrogel with the extracellular matrix protein, collagen type 1, is a promising 3D platform for culturing aggregates of hESC derived pancreatic progenitors, which maintains viable aggregates, aggregate size and key markers of endocrine differentiation, specifically Pdx1 and Nkx6.1. Pdx1 has been shown to directly regulate the activation of genes essential for endocrine specification and development, and ultimately β-cell production [23], while Nkx6.1 is activated downstream of Pdx1 and is also required for β-cell development [24]. Thus, the co-expression of Pdx1 and Nkx6.1 is indicative of a pancreatic progenitor cell, which were preserved in this study, and is required for β-cell maturation [25]. This platform also enabled the release of aggregates following a mild treatment with Liberase TL, an enzyme that is commonly used to release islets from pancreatic tissue. The use of Liberase TL to release aggregates from protease sensitive gels represents an advantage over previous works that have employed chymotrypsin [20], which can be cytotoxic [26] – as Liberase TL is commercially manufactured using GMP and has very low endotoxin levels [27]. The Liberase TL concentration and digestion times for hydrogel dissolution are similar to those used to isolate islets [27], though faster release could be achieved with increased concentrations. Aggregates that were cultured in suspension became larger concomitant with a rapid reduction in cell number. The aggregates that did survive in suspension, however, maintained key markers of Pdx1 and Nkx6.1 similar to those that were cultured in the MMP-sensitive PEG hydrogels.
The nature of the hydrogel crosslinks physically immobilizes each aggregate within a relative dense polymer network. This relatively stiff (~30 kPa) hydrogel formulation was chosen to spatially confine the aggregates, minimize cell proliferation, and prevent spreading in 3D. In particular, cell spreading can lead to differentiation towards a mesenchymal phenotype, which is undesirable in this application [28]. It is important to note that other studies have shown that soft substrates (0.1 kPa) improve aggregation and insulin secretion in MIN 6 cells (a β-cell line) and dissociated aggregates [29] suggesting that stiffness may play a role, but which was not investigated in this study. Despite the relative stiffness of the hydrogel, the progenitor state of the cells was maintained. The initial mesh size of the hydrogels used in this work is estimated to be ~19 nm, which is significantly smaller than the size of the aggregates (~80 μm in diameter) at the time of encapsulation. Despite the presence of MMP-sensitive crosslinks, no macroscopic signs of degradation were observed over the course of 28 days. This observation may be attributed to the presence of low levels of secreted active MMPs (e.g., MMP2 and MMP9, which cleave this peptide [30]) and/or the relatively tight crosslinking of the hydrogel, which impedes degradation due to the high number of crosslinks that have to be cleaved in order to degrade the hydrogel. As a result, the hydrogel spatially confines each aggregate, preventing agglomeration of aggregates as observed in suspension culture and preserving the original aggregate size for at least up to four weeks as measured in this study.
The MMP-sensitive PEG hydrogels also maintained viable aggregates and the number of hESC derived pancreatic progenitors for up to two weeks. On the contrary, hESC derived pancreatic progenitors cultured in suspension continued to agglomerate as evident by a doubling in aggregate size concomitant with a large reduction in the total number of cells. This agglomeration is a common issue that has been observed with the longer-term culture of hESC in suspension and has been shown to lead to cell death by hypoxia and oxidative stress [31,32]. It is therefore possible that larger aggregates may have formed in the suspension culture, which did not remain viable due to nutrient consumption by cells along the periphery of the aggregate, resulting in cell death and leading to the observed large reduction in cell number. In support of this observation, embryoid bodies greater than 100–300 μm in diameter have been shown to be more apoptotic than smaller aggregates [33], and as well, large islets are known to suffer from poor nutrient diffusion [34]. The hydrogels, therefore, may have led to improved viability of encapsulated aggregates in part due to the presence of smaller aggregates (and therefore fewer cells per aggregate), which will improve nutrient and oxygen transport throughout the aggregates. Although the hydrogel itself may hinder nutrient diffusion particularly for large molecules, the relatively high degree of swelling and large mesh size of the hydrogels are expected to have minimal effect on transport of smaller molecules, such as oxygen and glucose.
Another benefit of encapsulation in this hydrogel platform, beyond maintenance of aggregate size and viable aggregates, is the retention of cell-matrix contacts with collagen type 1. Islets in the pancreas are surrounded by a basement membrane which contains numerous ECM molecules including collagen type 1 [35,36]. Studies have shown that islets cultured on collagen type 1 in 2D have improved survival and insulin secretion compared to islets on tissue culture polystyrene in 2D [37,38]. Additionally, the inclusion of collagen type 1 within a PEG hydrogel of similar stiffness has previously been shown to improve the survival of rat embryonic stem cell derived pancreatic precursor cells [12]. It is interesting to note, that viability at the periphery of the aggregates was improved when cultured in the hydrogels compared to the suspension culture. This observation may be attributed to the presence of cell-matrix interactions with collagen type 1; although additional studies are necessary to test this hypothesis. Furthermore, the encapsulated progenitors were able to maintain their expression of PDX1 and NKX6.1. Thus the hydrogel environment better maintains cell viability while not adversely affecting the progenitor state of the cells.
By day 28, however, cell number eventually dropped in the MMP-sensitive PEG hydrogels although not to the same degree as in the suspension culture. This observation suggests that additional cues may be needed to maintain viability. In this study, defined media was utilized, which may not provide sufficient cell survival cues for long-term culture. It is likely that additional factors, such as basic fibroblast growth factor, may be needed for the long-term culture of hESC derived precursors [39]. Only recently, and for the first time, have studies demonstrated the ability to obtain glucose-responsive insulin producing cells derived from hESCs [17,18]. A complex milieu of factors were required to promote survival and differentiation, which may be necessary to enhance cell survival in the MMP-sensitive PEG hydrogel platform and ultimately produce hESC derived functional β-cells. Nonetheless, the hydrogel platform was a significant improvement over suspension culture in maintaining viable aggregates and maintaining the progenitor state.
One practical benefit of the hydrogel system is the ability to culture hESC derived pancreatic precursors in aggregates for extended periods of time, which can subsequently be easily released via a simple and mild enzymatic degradation of the hydrogel. While we did not control for the initial aggregate size, the hydrogel platform can easily be combined with other technologies that can precisely control the initial aggregate size, such as microcarriers [40] or microwells [41]. This platform may also serve to help screen select small molecules or other signaling factors aimed at promoting β-cell differentiation and maturation in controlled aggregates. The releasable feature of this hydrogel enabled us to assess individual cells by flow cytometry and obtain quantifiable measures of the progenitor state of each cell. This releasable feature also has clinical benefits where aggregates can easily be released and then implanted following clinically established protocols for islet transplantation.
5. Conclusion
We demonstrate that a MMP-sensitive PEG hydrogel containing collagen type 1 maintains viable aggregates and aggregate size of hESC derived pancreatic progenitors. This system allows for easy recovery of aggregates through an enzyme mediated degradation of the hydrogel and a further enzyme treatment to achieve single cells for subsequent analysis. Additionally, the progenitor fate of encapsulated aggregates is maintained evident by the fraction of cells that stained positive for both Pdx1 and Nkx6.1, the key markers required for β-cell maturation. While viability was reduced after four weeks, the fraction of doubly positive cells was maintained. Nonetheless, this hydrogel when combined with the appropriate cues has the potential to be a promising in vitro 3D culture platform for the survival, differentiation, and maturation of hESC derived pancreatic progenitors into functional β-cells.
Supplementary Material
Figure 1S – Functionalization of PEG-Norbornene was determined using 1H NMR by comparing the area under the alkene peaks associated with norbornene (6, 6.2 ppm) to the area under the methyl groups in PEG (~3.6 ppm). Conjugation was approximately 100%, each PEG arm was functionalized with 1 norbornene group.
Table 1.
Primers used for real-time RT-PCR
| Gene | Primer sequence | Efficiency |
|---|---|---|
| Tbp | F: 5′-GCGCAAGGGTTTCTGGTTTGCC-3′ R: 5′-AGGGATTCCGGGAGTCATGGC-3′ |
1.93 |
| Nkx6.1 | F: 5′-GCTCTGTCTCCGAGTCCTG-3′ R: 5′-TCGTTGGGGATGACAGAGA-3′ |
1.98 |
| Pdx1 | F: 5′-TACTGGATTGGCGTTGTTTGTGGC-3′ R: 5′-AGGGAGCCTTCCAATGTGTATGGT-3′ |
1.91 |
Acknowledgments
This work was supported by the Beta Cell Biology Consortium, The National Institute of Diabetes and Digestive and Kidney Diseases Grant DK089561, a U.S. Department of Education GAANN fellowship to LA, and an NSF CAREER award to SJB (0847390). No competing financial interests exist. The authors would like to thank Dr. Mariah Mason for initiating the collaboration between the University of Colorado and the University of Toronto.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Luke D. Amer, Email: Luke.Amer@Colorado.EDU.
Audrey Holtzinger, Email: Aholtzin@uhnres.utoronto.ca.
Gordon Keller, Email: gkeller@uhnresearch.ca.
Melissa J. Mahoney, Email: Melissa.Mahoney@Colorado.EDU.
References
- 1.Alison A. Biology’s new dimension. Nature. 2003:424. [Google Scholar]
- 2.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubashkin M, Ou G, Weaver V. Deconstructing signaling in three dimensions. Biochemistry. 2014;53:2078–90. doi: 10.1021/bi401710d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell Ja, Lutolf MP, et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454–64. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405–12. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Tian X-F, Heng B-C, Ge Z, Lu K, Rufaihaha J, Fan VT-W, et al. Comparison of osteogenesis of human embryonic stem cells within 2D and 3D culture systems. Scand J Clin Lab Invest. 2008;68:58–67. doi: 10.1080/00365510701466416. [DOI] [PubMed] [Google Scholar]
- 7.Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney DJ, Clements Ja. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010;28:125–33. doi: 10.1016/j.tibtech.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Siti-Ismail N, Bishop AE, Polak JM, Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29:3946–52. doi: 10.1016/j.biomaterials.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Gerecht S, Burdick Ja, Ferreira LS, Townsend Sa, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraehenbuehl TP, Langer R, Ferreira LS. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods. 2011;8:731–6. doi: 10.1038/nmeth.1671. [DOI] [PubMed] [Google Scholar]
- 11.Amer L, Mahoney M, Bryant S. Tissue Engineering Approaches to Cell Based Type 1 Diabetes Therapy. Tissue Eng. 2014 doi: 10.1089/ten.teb.2013.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason MN, Arnold CA, Mahoney MJ. Entrapped Collagen Type 1 Promotes Differentiation of Embryonic Pancreatic Precursor Cells into Glucose-Responsive beta-Cells When Cultured in Three-Dimensional PEG Hydrogels. Tissue Eng Part A. 2009;15:3799–808. doi: 10.1089/ten.tea.2009.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amer LD, Mahoney MJ, Bryant SJ. Tissue engineering approaches to cell-based type 1 diabetes therapy. Tissue Eng Part B Rev. 2014;20:455–67. doi: 10.1089/ten.teb.2013.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara M, Kimura H, Montagne K, Komori K, Fujii T, Sakai Y. Combination of microwell structures and direct oxygenation enables efficient and size-regulated aggregate formation of an insulin-secreting pancreatic β-cell line. Biotechnol Prog. 2013;30:178–87. doi: 10.1002/btpr.1837. [DOI] [PubMed] [Google Scholar]
- 15.Azarin S. Effects of 3D microwell culture on growth kinetics and metabolism of human embryonic stem cells. Biotechnol Appl Biochem. 2012;59:88–96. doi: 10.1002/bab.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo TX, Hebrok M. Stem Cells to Pancreatic beta-Cells: New Sources for Diabetes Cell Therapy. Endocr Rev. 2009;30:214–27. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014 doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 18.Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of Functional Human Pancreatic β Cells In Vitro. Cell. 2014;159:428–39. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–66. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Lin C-C, Raza A, Shih H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials. 2011;32:9685–95. doi: 10.1016/j.biomaterials.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts JJ, Bryant SJ. Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials. 2013;34:9969–79. doi: 10.1016/j.biomaterials.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv Mater. 2009;21:5005–10. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver-Krasinski JM, Kasner MT, Yang J, Crutchlow MF, Rustgi AK, Kaestner KH, et al. The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J Clin Invest. 2009;119:1888–98. doi: 10.1172/JCI37028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor BL, Liu F-F, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic Beta cells. Cell Rep. 2013;4:1262–75. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin Cell Dev Biol. 2012;23:701–10. doi: 10.1016/j.semcdb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letko G, Falkenberg B, Wilhelm W. Effects of trypsin, chymotrypsin, and uncoupling on survival of isolated acinar cells from rat pancreas. Int J Pancreatol. 1989;4:431–41. doi: 10.1007/BF02938478. [DOI] [PubMed] [Google Scholar]
- 27.Yesil P, Michel M, Chwalek K, Pedack S, Jany C, Ludwig B, et al. A new collagenase blend increases the number of islets isolated from mouse pancreas. Islets. 2009;1:185–90. doi: 10.4161/isl.1.3.9556. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee M, Virtanen I, Palgi J, Korsgren O, Otonkoski T. Proliferation and plasticity of human beta cells on physiologically occurring laminin isoforms. Mol Cell Endocrinol. 2012;355:78–86. doi: 10.1016/j.mce.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Nyitray CE, Chavez MG, Desai T. Compliant 3D Microenvironment Improves β-Cell Cluster Insulin Expression through Mechanosensing and β-Catenin Signaling. Tissue Eng Part A. 2014;20:1–34. doi: 10.1089/ten.tea.2013.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson J, Hubbell Ja. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–45. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 31.Dang S, Gerecht-Nir S, Chen J. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–82. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 32.Son M-Y, Kim H-J, Kim M-J, Cho YS. Physical passaging of embryoid bodies generated from human pluripotent stem cells. PLoS One. 2011;6:e19134. doi: 10.1371/journal.pone.0019134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valamehr B, Jonas S. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc Natl Acad Sci. 2008;105:14459–64. doi: 10.1073/pnas.0807235105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann R, Zuellig Ra, Kugelmeier P, Baenninger PB, Moritz W, Perren A, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 35.Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius a, Thornell L-E, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51:1181–91. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 36.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DWR, Johnson PRV. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81:423–6. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 37.Pinkse GGM, Bouwman WP, Jiawan-lalai R, Terpstra OT, Bruijn JA, De Heer E. Integrin Signaling via RGD Peptides and Anti-1 Antibodies Confers Resistance to Apoptosis in Islets of Langerhans. Diabetes. 2006;55:1–6. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- 38.Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human beta-cells: effect on insulin content, secretion, and gene transcription. Diabetes. 2006;55:2723–9. doi: 10.2337/db06-0120. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsui H, Valamehr B, Hindoyan A, Qiao R, Ding X, Guo S, et al. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun. 2011;2:167. doi: 10.1038/ncomms1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie Y, Bergendahl V, Hei D. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang Y-S, Chung BG, Ortmann D, Hattori N, Moeller H-C, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S – Functionalization of PEG-Norbornene was determined using 1H NMR by comparing the area under the alkene peaks associated with norbornene (6, 6.2 ppm) to the area under the methyl groups in PEG (~3.6 ppm). Conjugation was approximately 100%, each PEG arm was functionalized with 1 norbornene group.