Abstract
Protein phosphatases Z that are unique to the fungal kingdom have been associated to resistance to high salt concentration, cell wall integrity, cell cycle regulation, and oxidative stress in fungi. In Aspergillus fumigatus it was shown that PHZA is under the control of the transcription factor Skn7 and is only involved in the control of the oxidative stress. Accordingly, the ΔphzA mutant showed a defect in virulence in an experimental model of corneal infection in immunocompetent animals and that the impact on susceptibility to cell wall drugs is only secondary.
Keywords: Oxidative stress, Phosphatase, Virulence, Aspergillus fumigatus, Corneal infection
1. Introduction
Protein phosphatases Z (Balcells et al., 1997) are found exclusively in yeast and filamentous fungi (Arino, 2002). These phosphatases are composed of a highly conserved COOH-terminal catalytic domain similar to the type 1 S/T phosphatases while the unstructured NH2-terminal regulatory domain contains a N-myristoylation site and a Arg/Ser rich motif that are unique to these fungal proteins (Arino, 2002; Leiter et al., 2012; Posas et al., 1993). Ppz proteins have been extensively characterized in Saccharomyces cerevisiae, Schizzosaccharomces pombe and Candida albicans and shown to be involved in salt tolerance, cell wall integrity, cell cycle regulation, and oxidative stress tolerance (Balcells et al., 1997; Kovacs et al., 2010; Posas et al., 1993, 1995). However, although the Neurospora crassa or Aspergillus nidulans and Aspergillus fumigatus PPZ are able to complement Δppz deletion mutants of S. cerevisiae, Ppz proteins of filamentous fungi were shown to be exclusively involved in resistance to oxidative stress (Leiter et al., 2012; Vissi et al., 2001). In contrast to yeasts, the ΔppzA deletion mutant obtained in A. nidulans was dispensable for the salt stress response and cell integrity signaling.
Due to the essential role of reactive oxygen species (ROS) in the killing of A. fumigatus following infection of the lung (Ben-Ami et al., 2010) and the cornea (Leal et al., 2012), it was of interest to construct a Δppz mutant of A. fumigatus to investigate the role of this protein in the virulence of this opportunistic fungal pathogen. Even though a Δppz mutant was constructed in A. nidulans it was worth constructing an A. fumigatus mutant since it has been repeatedly shown in the past that the deletion of orthologous genes in A. fumigatus and A. nidulans often give different growth phenotypes in the respective mutants (Jimenez-Ortigosa et al., 2012; Takeshita et al., 2005). Moreover, it was attractive to test the effect of cell wall inhibitors on these mutants since the Δppz1 mutants of S. cerevisiae have shown an exquisite sensitivity to caspofungin (Parsons et al., 2006) and a cooperative effect with neutrophils, which are the major ROS producers in fungal infections.
Using a ΔphzA mutant of A. fumigatus we were able to show that the primary function of the Ppz protein was to protect against oxidative stress and that the impact on susceptibility to cell wall drugs was only secondary. PHZA was shown to be under the control of the transcription factor skn7 and is involved in the fungal virulence.
2. Material and methods
2.1. Strains and culture conditions
A. fumigatus strains were grown at 37 °C in either minimal medium AMMC (Cove, 1966) containing 1% glucose and 5 mM ammonium tartrate, Sabouraud medium or 2% Malt agar. Conidia were obtained from agar media plates after 6 days of growth at 37 °C, using 0.05% aqueous solutions of Tween 20.
2.2. Development of ΔphzA knock-out and reverting strains
The ΔphzA deletion mutant was constructed in CEA17 ΔakuBKU80 background using the β-rec/six site-specific recombination system as described earlier (Hartmann et al., 2010; Jimenez-Ortigosa et al., 2012). The PHZA replacement cassette containing the recyclable marker module flanked by 5′ and 3′ homologous regions was generated and cloned in the pUC19 vector. The CEA17ΔakuBKU80 parental strain was transformed with the PHZA replacement cassette by electroporation. Transformants obtained were verified by PCR and southern blot analysis (Supplemental Fig. 1). Complementation of the ΔphzA mutant was obtained by reintroduction in the mutant of the WT copy of the gene flanked by the hygromycin resistance cassette and a 3′ flanking region as described in the supplemental Fig. 2. After cultivation of the ΔphzA mutant on minimal medium containing 10% xylose to induce the excision of the deletion cassette, the complementation cassette obtained by cloning was transformed into the resulting excised ΔphzA mutant. The presence of the WT copy of the gene at the PHZA locus was confirmed Southern Blot analysis.
2.3. Phenotypic analysis of ΔphzA deletion strain
The radial growth of the ΔphzA mutant, and ΔphzA∷PHZA reconstituted strains were measured on malt agar and minimal plates after 48 h of incubation at 37 °C or 45 °C. Conidia were then harvested to estimate the conidiation rate by counting with haemocytometer. Conidial germination was analyzed kinetically on sabouraud agar medium for up to 9 h. Sensitivity of ΔphzA mutant to various stresses were tested: pH 5 to pH 9, 10–50 μg/ml caffeine, 0.4–1.5 M NaCl, 10–30 mM LiCl, 12–100 μg/ml caspofungin, 12–75 μg/ml congo red, 10–50 μg/ml calcofluor white 0.001–0.005% SDS, 1.2–2 mM diamide, 2–10 mM menadione, 0.6–3 mM H2O2 and 0.4–1.25 mM tBOOH on minimal medium agar plates. Plates were spotted with conidial suspension calibrated at 1 × 105 condia/ml and grown for 72 h at 37 °C. To analyze the expression of PHZA in the presence or absence of 4 mM of t-BOOH in the WT, Δyap1 Δskn7 and ΔphzA∷PHZA strains, fungus was grown for 16 h in AMMC liquid medium at 37 °C and supplemented with or without t-BOOH (4 mM) for 1 h. Total RNA were extracted and DNAse treated; two micrograms of total RNA was reverse-transcribed using Superscript II Reverse Transcriptase (Invitrogen, Cergy Pontoise, France). Quantitative PCR assays were performed according to Bio-Rad manufacturer's instructions using 96-well optical plates. Each run was assayed in triplicates in a total volume of 25 μl containing the DNA template at an appropriate dilution, 1× AbsoluteSYBR green Fluorescein (Thermo Scientific) and 100 nM of each primer. The primers used for the amplification of EF1α as internal control and PHZA are listed in the supplemental Table 1. PCR conditions were: 95 °C/15 min for one cycle; 95 °C/30 s and 55 °C/30 s for 40 cycles. Amplification of one single specific target DNA was checked with a melting curve analysis (+0.5 °C ramping for 10 s, from 55 °C to 95 °C). The expression ratios were normalized to EF1α expression, and calculated according to the ΔΔCt method (Livak and Schmittgen, 2001). Three independent biological replicates were performed.
2.4. Sensitivity to ROS neutrophils
Whole-blood samples collected from four healthy donors after written consent were obtained from Hôpital Pitié Salpêtrière through the Etablissement Français du Sang (EFS), Paris, France, after approval for the use of this material by the ethics committees of INSERM and the EFS: convention 12/EFS/079. Human PMNs were isolated as previously described (François et al., 2003) and growth inhibition of the ΔphzA and WT mutants after contact with PMN was investigated using 96-well plate (Greiner Bio One, Cell Star). Each well received 100 μl of RPMI 1640 medium supplemented with 4% (v/v) fetal calf serum (FCS) containing 5 × 105 conidia and 1 × 105 PMNs. A. fumigatus was also cultured alone or with Amphotericin B (2 μg/ml, Sigma) as positive and negative control respectively. After 4 h of incubation at 37 °C in a 5% CO2 incubator culture, PMNs were lysed with cold sterile water. Conidia were then collected, plated on Sabouraud agar medium and incubated further for 16 h at 37 °C for the counting of the colony forming unit to calculate percentage of viability.
2.5. Pathogenicity of the ΔphzA deletion mutant
For infection assays, OF1 Male mice (26–28 g and 7 weeks old) were immunosuppressed with cortisone acetate (Sigma) and cyclophosphamid monohydrate (Sigma) injected intraperitoneally on day –3 and –1 (112 mg cortisone acetate per kg of mouse and 200 mg cyclophosphamide monohydrate per kg of mouse) before intranasal inoculation of conidia (day 0) for in vivo experiments. Each mouse was inoculated intranasally with either 106 conidia (or 4 × 107 conidia resuspended in 1 ml of water plus 0.05% Tween 20). After 2 days post-infection, mice were euthanized by asphyxiation (CO2). Infected lung DNAs were extracted by using phenol–chloroform method and resuspended in RNase/DNase free water. Q-PCR method was used to estimate the proportion of the wild type (WT) versus ΔphzA mutant strains in the infected lung. The abundance of the two strains was assessed by using specific PCR primers amplifying PHZA and HPH genes for the WT and the ΔphzA mutant strains respectively. Efficiency standard curves and specificities of primers were tested with serial DNA dilutions of each strain and non-infected lung by using cycling conditions below: 95 °C/15 min for one cycle; 95 °C/30 s and 60 °C/30 s for 40 cycles. Amplification of one single specific target DNA was checked with a melting curve analysis (+0.5 °C ramping for 10 s, from 55 °C to 95 °C). Mean values of triplicate qPCR analysis of the same DNA extracts from three different mice are presented as average and correspond to the quantity of fungal DNA of each strains present in 100 ng of infected lung DNA.
2.6. Murine model of corneal infection
The murine model of fungal keratitis has been described (Leal et al., 2012). Parental and mutant A. fumigatus strains were cultured in 2% Malt agar with 6% potassium chloride in 25 cm2 tissue culture flasks. Dormant conidia were disrupted with a bacterial L-loop and harvested in 5 ml PBS. Pure conidial suspensions were obtained by passing the culture suspension through PBS-soaked sterile gauges placed at the tip of a 10 ml syringe. Conidia were quantified using a hemocytometer and a stock was made at a final concentration of 2 × 104 conidia/μl in PBS. Mice were anaesthetized by intraperitoneal (IP) injection of 0.6% Tribromoethanol, 1.2% tert-butyl alcohol, and PBS. Corneal epithelium was abraded with a 30 gauge needle. Through the abrasion was inserted a 33-gauge Hamilton needle and a 2 μl injection containing 4 × 104 conidia was released into the corneal stroma. Mice were examined daily under a stereomicroscope for corneal opacification, ulceration, and perforation. At set time points, animals were euthanized by CO2 asphyxiation, and eyes were either placed in 10% formalin and embedded in paraffin and sectioned at 5 μm intervals, or excised and placed in 1 ml of sterile saline and homogenized for quantitative culture. MetaMorph imaging software was used to quantify the percent area of opacity and the integrated corneal opacity as described in Leal et al., 2012. All animals were bred under specific pathogen-free conditions and maintained according to institutional guidelines.
2.7. Statistical analysis
At least three biological replicates were performed per experiment. The statistical significance of the results was evaluated by a one-way variance analysis and means comparison by Student's t-test (p < 0.05) using the JMP software (SAS Institute, Cary, NC, USA). In the figures and table 1, values followed by different stars (*) are significantly different with the p-value at least p < 0.01.
Table 1.
Conidiation rate of the ΔphzA mutant. The conidiation rate of the different strains was measured by counting after 48 h of incubation at 37 °C or 45 °C on malt agar medium.
| Malt medium 48 h | 37 °C | 45 °C |
|---|---|---|
| WT | 3.82 × 106 ± 0.10 | 1.87 × 106 ± 0.11 |
| ΔphzA | 3.77 × 106 ± 0.13 | 0.44 × 106 ±0.16* |
| ΔphzA∷PHZA | 3.43 × 106 ± 0.52 | 1.45 × 106 ± 0.19 |
The definition has been done in the mat et meth section
3. Results and discussion
3.1. Phenotypic characterization of the ΔphzA mutant strain
The genome of A. fumigatus, A. nidulans and C. albicans contains a single putative PPZ gene, whereas the yeast S. cerevisiae and S. pombe contain two PPZ genes (Leiter et al., 2012). The PHZA gene of A. fumigatus (AFUA_2G03950) has a predicted amino acid sequence composed of three motifs characteristic of the Ppz phosphatase family (Supplemental Fig. 2): at the N terminus, a myristoylation motif that allows its membrane localization; a short Arg/Ser rich motif which was described to be important for salt tolerance but not for cell wall integrity in Debaryomyces hansenii and S. cerevisiae and (iii) the C-terminal catalytic region (about 300 aa) of PhzA similar to PP1 subfamily (Minhas et al., 2012).
The ΔphzA deletion mutant was constructed as described in Supplemental Fig. 1. The ΔphzA strain did not show any difference in growth rate with the WT and the reconstituted strains after incubation for 48 h at 37 °C or under heat shock at 45 °C on malt or minimal medium agar plates (Fig. 1A). However, the colonies of the ΔphzA deletion mutant were fluffy at 45 °C associated to a slight reduction of conidiation in the mutant as compared to the WT strain (Table 1). One week old conidia from the ΔphzA mutant were viable and able to germinate on solid agar medium with a rate similar to the parental strain (Fig. 1B).
Fig. 1.
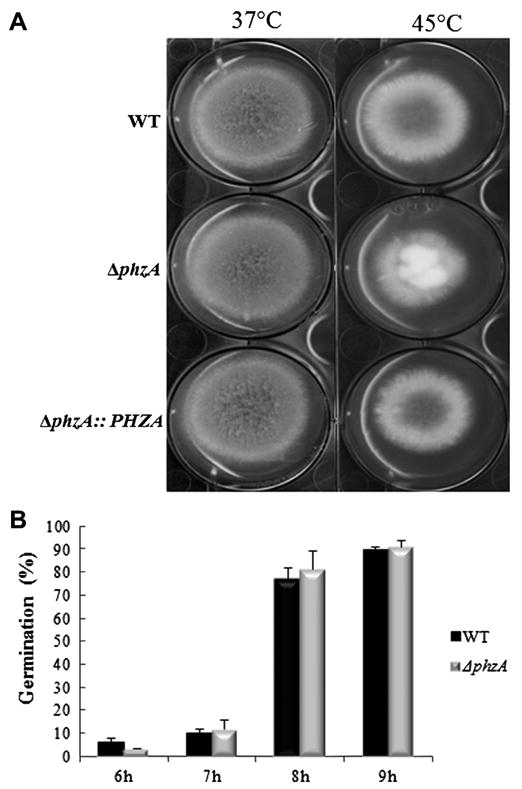
Fungal growth and kinetic of germination of the ΔphzA mutant strain. The fungal growth of the ΔphzA ΔphzA∷PHZA and the WT strains was estimated after 48 h at 37 °C or 45 °C on malt agar medium (A). Kinetic of germination of the ΔphzA and the WT strains were performed on sabouraud agar medium (B).
In yeast, Ppz proteins were described as involved in pH homeostasis, salt tolerance, cell wall integrity, and oxidative stress (Clotet et al., 1996; Leiter et al., 2012; Posas et al., 1993, 1995). The susceptibility of the ΔphzA strain to different pH, KCl, NaCl, and LiCl or SDS and caffeine was identical to the parental strain (Supplemental Fig. 3). No growth difference were seen between the ΔphzA mutant and WT strains in presence of calcofluor white and caspofungin, while a slight sensitivity to congo red for the mutant was observed (Supplemental Fig. 4).
In contrast, the ΔphzA mutant showed an increased susceptibility to reactive oxidants such as menadione, H2O2 and ter-butyl peroxide (t-BOOH) (Fig. 2). The combination of congo red and calcofluor white compounds with the t-BOOH increased the susceptibility of the ΔphzA mutant to oxidative stress (Fig. 3). In contrast, the combination of caspofungin, a specific inhibitor of β-1.3 glucan synthesis that has a very low MIC in the Δppz mutant in S. cerevisiae (Parsons et al., 2006), in combination with t-BOOH did not increase the susceptibility of the mutant strain to oxidative stress. PPZs from Aspergillus and Neurospora were able to complement the Δppz1 deletion mutant of S. cerevisiae and S. pombe regarding to salt tolerance and sensitivity to caffeine supporting the assumption that PHZA is a true functional ortholog and is able to perform the same function in filamentous fungi and yeast (Leiter et al., 2012; Vissi et al., 2001). The results obtained in A. fumigatus suggested that the main function of PhzA is to be involved in tolerance to oxidative stress and that the sensitivity of the ΔphzA mutant to these cell wall inhibitors is only a secondary down-stream effect of the deletion. The CW perturbations and especially changes in permeability due to the Δppz deletion are more important in yeast than Aspergillus. Alterations of the ROS metabolism and especially dysfunction of the mitochondria has been linked to cell wall defects (Qu et al., 2012; Singh et al., 2012).
Fig. 2.
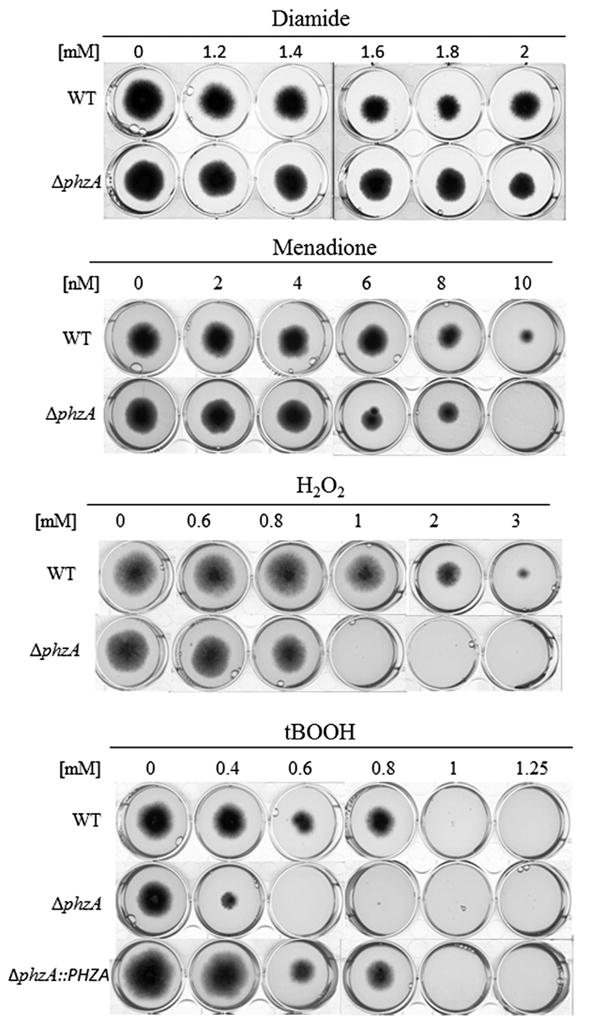
Oxidative stress sensitivity of the ΔphzA mutant strain. The sensitivity of the different strain to diamide (1.2–2 mM), menadione (2–10 nM), H2O2 (0.6–3 mM) and tBOOH (0.4–1.25 nM) were observed after 72 h at 37 °C on minimal medium.
Fig. 3.
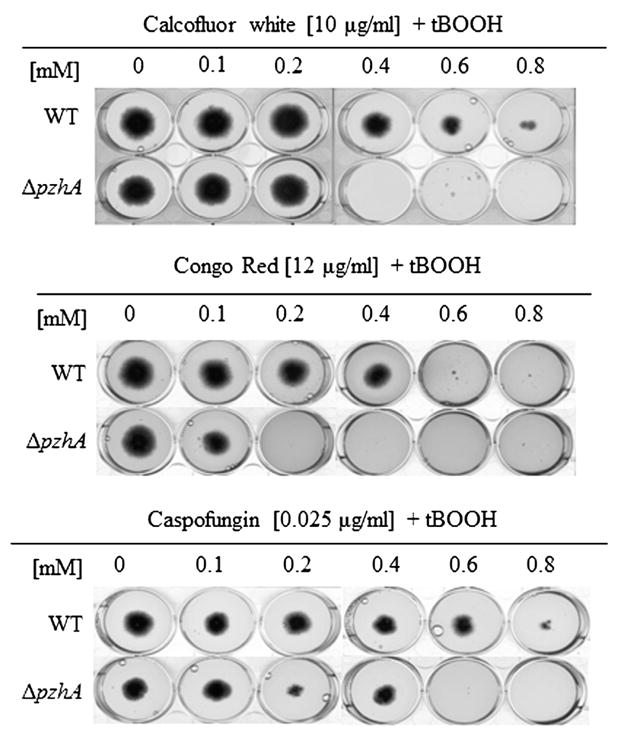
Sensitivity of the ΔphzA mutant to oxidative stress in combination with cell wall disturbing compounds. The sensitivity of the different strains was observed in presence oxidative stress in combination with cell wall disturbing compounds disturbing compound after 72 h of incubation at 37 °C on minimal medium.
The transcription factors Skn7 and Yap1 play a major role in regulating the response to the oxidative stress in vitro and in vivo (Lamarre et al., 2007; Qiao et al., 2008). A cooperative role between Skn7 and Yap1 in the transcriptional regulation of genes in response to oxidative stress has been shown. Thus, deletion mutants of the Skn7 and Yap1 in A. fumigatus as well as S. cerevisiae were described to be highly sensitive to reactive oxidants, in particular t-BOOH and H2O2 (Lamarre et al., 2007; Qiao et al., 2008). To determine if the expression of the PHZA gene was increased during oxidative stress and if it was under the control of Yap1 or Skn7, PHZA expression was measured in Δyap1 and Δskn7 mutants and parental strains before and after oxidative stress induced by t-BOOH (Fig. 4). Quantitative RT-PCR data showed that the expression of PHZA was increased 16-fold in the presence of t-BOOH in the WT, reverting, and Δyap1 strains. In contrast, the expression level of PHZA was 4-fold reduced in the Δstrain in terms of sensitivity to ROS mirrored skn7 mutant in the presence of t-BOOH compared to the WT. These results suggested that Skn7 regulate the PHZA gene during oxidative stress. Moreover, the phenotype of the ΔphzA strain in terms of sensitivity to ROS mirrored completely the Δskn7 mutant (Lamarre et al., 2007). This result suggested that PHZA is the first gene identified as under the exclusive control of Skn7. Earlier studies have suggested that DNM1 and OLA1 were under the strict control of Skn7 in S. cerevisiae but this result was invalidated later when it was shown that Yap1 and Skn7 interact together during oxidative stress (Mulford and Fassler, 2011).
Fig. 4.
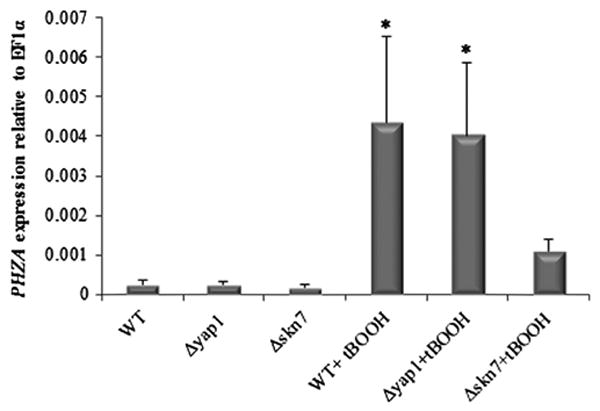
Expression levels of PHZA in WT, Δyap1 and Δskn7 strains in presence of t-BOOH. The expression level of PHZA in the different strains was estimated after 16 h of culture at 37 °C and after induction of oxidative stress by additional incubation of 1 h at 37 °C in presence of 0.4 mM of t-BOOH. The expression of PHZA was related to the EF1α as internal control and experiments were done in biological triplicates.
3.2. ΔphzA sensitivity to human neutrophils and fungal virulence on different experimental models
To test whether ROIs are involved in the killing of conidia by human PMN, conidia of the WT strain, the ΔphzA and Δskn7 mutant strains were co-incubated for 4 h with freshly isolated human PMN (Fig. 5). After 4 h of co-incubation, the counting of the CFU (colony forming unit) showed that the ΔphzA and Δskn7 mutant strains displayed higher sensitivity to human PMN than the parental strain. Indeed, whereas ∼50% of the WT conidia were still alive after 4 h of co-incubation, only ∼25% of the ΔphzA and Δskn7 mutant conidia were alive after the same incubation time.
Fig. 5.
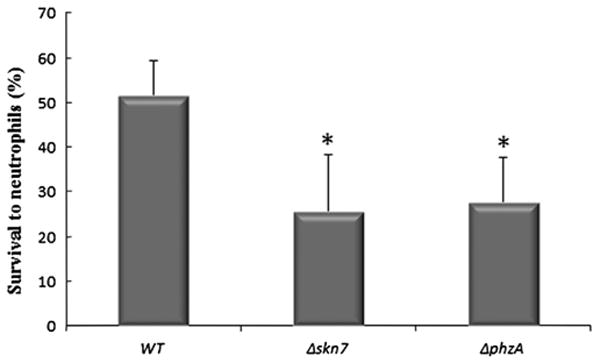
Conidial killing of ΔphzA and Δskn7 mutant strains by human neutrophils. After 4 h of co-incubation with PMN, the percentage of viability of the parental strain, ΔphzA and Δskn7 mutant strains were established. Viable conidia were quantified by plating after osmotic lysis of PMN and the percentage of viable WT or mutant conidia was calculated in relation to controls without PMN.
The virulence of the ΔphzA mutant strain was investigated in two different murine models of infection. Firstly, the virulence of the ΔphzA mutant was estimated in a mixed-infection with the WT and reconstituted strains in an immuno-compromised murine model of pulmonary aspergillosis. Immunosuppression was performed by treatment of mice with corticoids and cyclophosphamide producing global repression of the immune response (Latge, 2001). The quantification of the DNA proportion of each strain in the infected lung tissue of three mice was measured by qPCR analysis. After mixed infections of ΔphzA with WT or ΔphzA∷PHZA with WT, no significant difference was found in the quantity of DNA estimated between strains in infected lungs of the immune-compromised mice since the PHZA/HPH ratio obtained was 0.92 ± 0.13 for the WT/ΔphzA and 0.96 ± 0.17 for the WT/ΔphzA∷PHZA mixed infections. This result suggested that in an immunocompromised murine model of aspergillosis the mutant and wild type strain have the same pathogenicity.
The virulence of the ΔphzA mutant strain was also examined in a well-established immuno-competent murine model of corneal infection (Fig. 6). Conidia from WT, ΔphzA and reconstituted strains were injected into the corneal stroma of mice, and corneal disease and fungal survival were examined (as described by Leal et al., 2012). As shown in Fig. 6A, B, corneas infected with WT conidia developed severe opacification after 24 h, which is consistent with prior studies. In contrast, corneas infected with the ΔphzA mutant had significantly less corneal opacity than the WT and reconstituted strains. To quantify fungal growth/survival, infected eyes were homogenized and CFU was examined. We found significantly less CFU in corneas infected with the ΔphzA mutant when compared with corneas infected with either the WT or the reconstituted strain (Fig. 6C). Histological examination shows multiple hyphae in the mice corneal stroma infected with WT or reconstituted strain, but few hyphae in corneas infected with the ΔphzA mutant (Fig. 6D). Together, these data indicate that PhzA is a virulence factor in this model of Aspergillus infection.
Fig. 6.
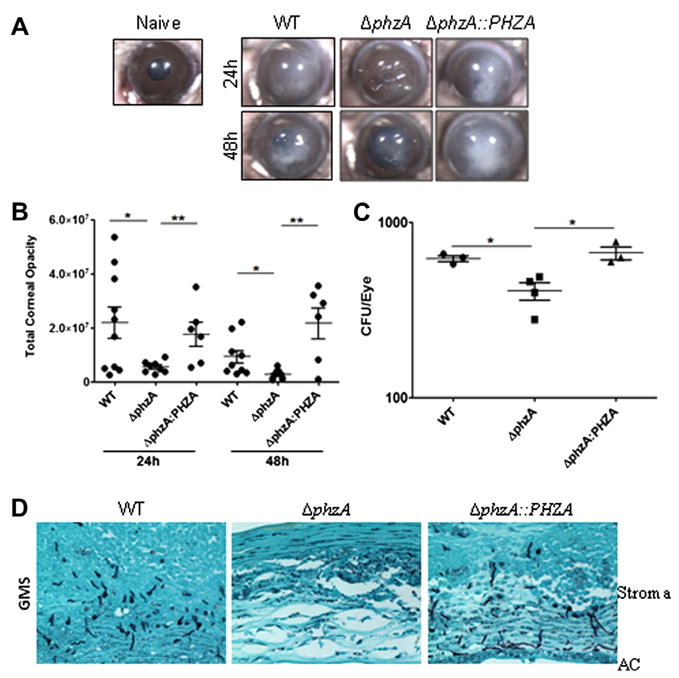
Pathogenicity of the ΔphzA mutant in mice corneal infection model. C57BL/6 mice infected with conidia isolated from the WT, ΔphzA and reconstituted strains. (A) Representative corneas 24 h and 48 h after infection; a transparent cornea of a naïve mouse is shown for comparison. (B) Quantification of corneal opacification by image analysis. (C) CFU 48 h after infection. (D) Representative GMS strains of 5 μm sections of corneas 48 h post-infection showing hyphae in the corneal stroma. (B and C: data points represent individual corneas). Experiments were repeated twice with similar results.
These results pinpoint again the critical choice of the experimental aspergillosis model to investigate the virulence of mutants of A. fumigatus. In heavily immunocompromised animals (after cyclophosphamide and cortisone treatment), none of the mutants that display a ROS sensitivity defect consecutive to the deletion of superoxide dismutase, catalase, or the transcription factors Yap1 and Skn7 and the phosphatase PhzA showed a difference in virulence compared to the parental strain (Lamarre et al., 2007; Lambou et al., 2010; Lessing et al., 2007; Paris et al., 2003). These results suggested that in immunosuppressed murine models, the amount of ROS produced is too low to see a virulence defect in ROS scavenging mutants compared to wild type. In contrast, assays in an immunocompetent host such as models of fungal keratitis or cutaneous aspergillosis, mutants with a defect in ROS scavenging enzymes were less virulent than the parental strain (Ben-Ami et al., 2010; Leal et al., 2012).
Even if Ppz phosphatases are well conserved in evolutionarily distantly related fungi, it appears clearly that Ppz proteins from filamentous fungi do not display the same function than in yeast. While Ppz phosphatases from yeasts play an important role in the salt/stress tolerance and cell wall integrity pathways, these functions were not controlled by Ppz in filamentous fungi such as Aspergillus nidulans and A. fumigatus. PPZ genes from these two species are however able to complement the Δppz mutants from S. cerevisiae. This result also suggested that the genes under the control of PhzA and its upstream regulator Skn7 are different in yeast and moulds. However, like C. albicans, PhzA is important for the fungal virulence of A. fumigatus in immuno-competent mice model, demonstrating the essential role of PhzA in the resistance against reactive oxygen intermediates produced by the immune system.
Supplementary Material
Footnotes
Appendix A. Supplementary material: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fgb.2014.02.009.
References
- Arino J. Novel protein phosphatases in yeast. Eur J Biochem. 2002;269:1072–1077. doi: 10.1046/j.0014-2956.2002.02753.x. [DOI] [PubMed] [Google Scholar]
- Balcells L, Gomez N, Casamayor A, Clotet J, Arino J. Regulation of salt tolerance in fission yeast by a protein-phosphatase-Z-like Ser/Thr protein phosphatase. Eur J Biochem. 1997;250:476–483. doi: 10.1111/j.1432-1033.1997.0476a.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ami R, Lewis RE, Leventakos K, Latge JP, Kontoyiannis DP. Cutaneous model of invasive aspergillosis. Antimicrob Agents Chemother. 2010;54:1848–1854. doi: 10.1128/AAC.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotet J, Posas F, de Nadal E, Arino J. The NH2-terminal extension of protein phosphatase PPZ1 has an essential functional role. J Biol Chem. 1996;271:26349–26355. doi: 10.1074/jbc.271.42.26349. [DOI] [PubMed] [Google Scholar]
- Cove DJ. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- François S, El Benna J, Dang PMC, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2003;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Dumig M, Jaber BM, Szewczyk E, Olbermann P, Morschhauser J, Krappmann S. Validation of a self-excising marker in the human pathogen Aspergillus fumigatus by employing the beta-rec/six site-specific recombination system. Appl Environ Microbiol. 2010;76:6313–6317. doi: 10.1128/AEM.00882-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Ortigosa C, Aimanianda V, Muszkieta L, Mouyna I, Alsteens D, Pire S, Beau R, Krappmann S, Beauvais A, Dufrene YF, Roncero C, Latge JP. Chitin synthases with a myosin motor-like domain control the resistance of Aspergillus fumigatus to echinocandins. Antimicrob Agents Chemother. 2012;56:6121–6131. doi: 10.1128/AAC.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L, Farkas I, Majoros L, Miskei M, Pocsi I, Dombradi V. The polymorphism of protein phosphatase Z1 gene in Candida albicans. J Basic Microbiol. 2010;50(Suppl. 1):S74–S82. doi: 10.1002/jobm.200900434. [DOI] [PubMed] [Google Scholar]
- Lamarre C, Ibrahim-Granet O, Du C, Calderone R, Latge JP. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet Biol. 2007;44:682–690. doi: 10.1016/j.fgb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Lambou K, Lamarre C, Beau R, Dufour N, Latge JP. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol Microbiol. 2010;75:910–923. doi: 10.1111/j.1365-2958.2009.07024.x. [DOI] [PubMed] [Google Scholar]
- Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–389. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- Leal SM, Jr, Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, Pearlman E. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest. 2012;122:2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter E, Gonzalez A, Erdei E, Casado C, Kovacs L, Adam C, Olah J, Miskei M, Molnar M, Farkas I, Hamari Z, Arino J, Pocsi I, Dombradi V. Protein phosphatase Z modulates oxidative stress response in fungi. Fungal Genet Biol. 2012;49:708–716. doi: 10.1016/j.fgb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Lessing F, Kniemeyer O, Wozniok I, Loeffler J, Kurzai O, Haertl A, Brakhage AA. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot Cell. 2007;6:2290–2302. doi: 10.1128/EC.00267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Minhas A, Sharma A, Kaur H, Rawal Y, Ganesan K, Mondal AK. Conserved Ser/Arg-rich motif in PPZ orthologs from fungi is important for its role in cation tolerance. J Biol Chem. 2012;287:7301–7312. doi: 10.1074/jbc.M111.299438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulford KE, Fassler JS. Association of the Skn7 and Yap1 transcription factors in the Saccharomyces cerevisiae oxidative stress response. Eukaryot Cell. 2011;10:761–769. doi: 10.1128/EC.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Wysong D, Debeaupuis JP, Shibuya K, Philippe B, Diamond RD, Latge JP. Catalases of Aspergillus fumigatus. Infect Immun. 2003;71:3551–3562. doi: 10.1128/IAI.71.6.3551-3562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons AB, Lopez A, Givoni IE, Williams DE, Gray CA, Porter J, Chua G, Sopko R, Brost RL, Ho CH, Wang J, Ketela T, Brenner C, Brill JA, Fernandez GE, Lorenz TC, Payne GS, Ishihara S, Ohya Y, Andrews B, Hughes TR, Frey BJ, Graham TR, Andersen RJ, Boone C. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 2006;126:611–625. doi: 10.1016/j.cell.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Posas F, Casamayor A, Arino J. The PPZ protein phosphatases are involved in the maintenance of osmotic stability of yeast cells. FEBS Lett. 1993;318:282–286. doi: 10.1016/0014-5793(93)80529-4. [DOI] [PubMed] [Google Scholar]
- Posas F, Camps M, Arino J. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J Biol Chem. 1995;270:13036–13041. doi: 10.1074/jbc.270.22.13036. [DOI] [PubMed] [Google Scholar]
- Qiao J, Kontoyiannis DP, Calderone R, Li D, Ma Y, Wan Z, Li R, Liu W. Afyap1, encoding a bZip transcriptional factor of Aspergillus fumigatus, contributes to oxidative stress response but is not essential to the virulence of this pathogen in mice immunosuppressed by cyclophosphamide and triamcinolone. Med Mycol. 2008;46:773–782. doi: 10.1080/13693780802054215. [DOI] [PubMed] [Google Scholar]
- Qu Y, Jelicic B, Pettolino F, Perry A, Lo TL, Hewitt VL, Bantun F, Beilharz TH, Peleg AY, Lithgow T, Djordjevic JT, Traven A. Mitochondrial sorting and assembly machinery subunit Sam37 in Candida albicans: insight into the roles of mitochondria in fitness, cell wall integrity, and virulence. Eukaryot Cell. 2012;11:532–544. doi: 10.1128/EC.05292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Yadav V, Prasad R. Comparative lipidomics in clinical isolates of Candida albicans reveal crosstalk between mitochondria, cell wall integrity and azole resistance. PLoS ONE. 2012;7:e39812. doi: 10.1371/journal.pone.0039812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita N, Ohta A, Horiuchi H. CsmA, a class V chitin synthase with a myosin motor-like domain, is localized through direct interaction with the actin cytoskeleton in Aspergillus nidulans. Mol Biol Cell. 2005;16:1961–1970. doi: 10.1091/mbc.E04-09-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissi E, Clotet J, de Nadal E, Barcelo A, Bako E, Gergely P, Dombradi V, Arino J. Functional analysis of the Neurospora crassa PZL-1 protein phosphatase by expression in budding and fission yeast. Yeast. 2001;18:115–124. doi: 10.1002/1097-0061(20010130)18:2<115::AID-YEA653>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


